Solar Photocatalytic Degradation of Rhodamine-B Dye Using Lettuce Extracted TiO2 Nanoparticle
A. Motcha Rakkini , L. Mary Arul Rosaline
, L. Mary Arul Rosaline ,K. Lucy Keller, S. Nagalakshmi and J. Amala Infant Joice*
,K. Lucy Keller, S. Nagalakshmi and J. Amala Infant Joice*
Holy Cross College (Autonomous), Affliated to Bharathidasan University, Tiruchirappalli-620002, Tamilnadu India.
Corresponding Author E-mail: drshanmugaselvan@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380509
Article Received on : 25 Jun 2022
Article Accepted on : 25 Sep 2022
Article Published : 29 Sep 2022
Reviewed by: Dr. Wendimagegn T. Dinbore
Second Review by: Dr. Jigmet Ladol
Final Approval by: Dr. Charanjit Kaur
The present study describes the green synthesis of TiO2 nanoparticles using Sesbania Grandiflora and Solanum nigrum leaf extracts. The characterisation of synthesised nanoparticles is carried out using XRD and FTIR. The photo catalytic degradation of Rhodamine-B dye is carry out under sunlight irradiation by using UV- Vis spectrophotometer. Among the synthesized catalysts, SG/TiO2 was found to be the best for the photocatalytic degradation of dye. The degraded dye water is also tested for the water quality parameters and the results are discussed.
KEYWORDS:Advanced Oxidation Process; Photocatalytic degradation; Rhodamine B; Sesbania grandiflora; Solanum nigrum
Download this article as:| Copy the following to cite this article: Rakkini A. M, Rosaline M. A, Keller K. L, Nagalakshmi S, Joice J. A. I. Solar Photocatalytic Degradation of Rhodamine-B Dye Using Lettuce Extracted TiO2 Nanoparticle. Orient J Chem 2022;38(5). |
| Copy the following to cite this URL: Rakkini A. M, Rosaline M. A, Keller K. L, Nagalakshmi S, Joice J. A. I. Solar Photocatalytic Degradation of Rhodamine-B Dye Using Lettuce Extracted TiO2 Nanoparticle. Orient J Chem 2022;38(5).. Orient J Chem 2022;38(5). Available from: https://bit.ly/3Cf8nkB |
Introduction
Water being the base of existence of life on earth, without which our land would be barren. This life saving liquid is also a universal solvent that also makes it easier to get polluted 1,2. As surface water resources such as ponds and revers are the main target to get polluted by effluents and it turns to be a headache for chemists to find a suitable solution. As these water resources get polluted could barren the land, leading to infertility, demolish the aquatic life and may even cause death to humans when come across directly or indirectly. This polluted water then goes to water bodies, which is harmful to the life kingdom of plants as well as animals 3. Dyes are chemical compounds and exhibiting colour with high molecular weights. It is chemically bonded to the substrate. Most of the natural dyes derived from animals, minerals, plant sources such as roots, barks, berries, woods, leaves, fungi and lichens. Synthetic dyes are derived from organic or inorganic compounds. Synthetic dyes are offered a wide range of colours and are man-made from petrochemicals. Pigments are a coloured substance which is not chemically bound to the materials. Usually, dyes are soluble in water but pigments are insoluble. In modern technical world, dyes are broadly used in the textile industry, food industry, photochemical cells, hair colouring, and leather tanning industry, paper production and cosmetics 4.
There are many techniques for the removal of industrial dyes from water or any other sources. It is cost-effective, inefficient and inflexible which again to be treated. Processes of advanced oxidation (AOP), was a set of chemical treatment to remove organic and inorganic materials in wastewater by oxidation reaction with hydroxyl group (.OH). It is formed by the combination of free radicals. Micro-pollutant was treated with the help of UV radiation with ozone gas, Drinking water containing organic pollutants where treated with systems 5. The Photo catalysis process was environmentally beneficial approach it’s carried out with light, in which light energy converted to chemical energy. The development of novel treatment methods for converting organic pollutants (dye effluents) to non pollutant materials.TiO2 is a naturally occurring mineral. It acts as a semiconductor, highly stable and non –toxic. The other name of titanium dioxide is titanium (IV) oxide, Titanium white, pigment white or titania. Illuminate, rutile and anatase are the different phases of titanium 6. Green synthesis of TiO2 catalyst prepared by using the plant of Solanum nigrum andSesbania grandiflora leafs extract which is used as reducing agent to convert the precursor of Titanium Isopropoxide into nanoparticles. Solanum nigrum is commonly known as blackberry nightshade or Manathakkali in Tamil. Solanum nigrum naturally occurs in Africa and is used as food as well as a medicinal plant. It acts as capping agent. It belongs to the family of Solanaceae 7. Sesbania grandiflora is a Agati keerai in Tamil and humming bird tree in English. It is omnipotent spinach and is capable of curing the psychological problems of human beings. Sesbania grandiflora leaves with β–carotene undergo changes upon baking, thus influencing the color characteristics of bread. The brightness and yellowness of the bread may be decreased due to the enzymatic browning. 8-12.
Rhodamine-B is a fully synthetic dye. It is a thiazine dye. It was first prepared by German chemist Heinrich Caro in 1876. The INN (International Non-proprietary Name) of Rhodamine-B is otherwise called methylthioninium chloride, is a medication and dye. It is a dark green colour powder but dissolved in water it appear in blue color. Methylthioninium chloride mainly used to treat methemoglobinemia. In malaria treatment Rhodamine-B used is found by Paul Guttmann and Paul Ehrlich in 1891. Rhodamine-B used in textile industries and leather industries 13. In our present work, we have synthesized TiO2 nanoparticles by using Sesbania grandiflora, Solanum nigrum as a natural precursors for the degradation of Rhodamine B. The characterization of catalyst was carried out using FT-IR and XRD. The photocatalytic degradation of Rhodamine B was carried out under sunlight irradiation 14.
Materials and Methods
Chemicals and reagents
Leaves of the plant Solanum nigrum, Sesbania grandiflora are collected from Dindigul District. Ethanol and Double distilled water is used in the Synthesis process. In this study, Rhodamine -B dye is used as a model pollutant. Titanium isopropoxide is used as a precursor.
Preparation of Plant Extract
The fresh Leaves of Solanum nigrum, Sesbania grandiflora were washed with tap water and distilled water to remove the dust particles on their surface separately. The collected leaves were shade dried at room temperature for one week in a normal atmosphere. The leaves were cut into pieces, grained to get the fine powder. 100 ml of ethanol added to the 10g of leaf powder then mixed well and heated for one hour at 50oC. The ethanoic leaf extract was obtained by filtering using Whatman No.1 filter paper. The ethonoic leaf extract was used for the biosynthesis of TiO2 Nanoparticles.
Green Synthesis of TiO2 Nanoparticles
25ml of Titanium isopropoxide solution was prepared for the synthesis of TiO2 nanoparticles. 25ml of Titanium isopropoxide solution was added to the 100 ml of ethonoic leaf extract it reacted under stirring at 50oC. After four hours of continuous stirring, titanium dioxide nanoparticles was formed. The Nanoparticles was filtered with Whatman No:1 filter paper and the crude washed with ethanol solution. The separated TiO2 nanoparticles were dried and ground using a mortar then calcined at 500oC in a muffle furnace for about five hours. The as synthesized TiO2 nanoparticles were used for further studies 15.
Degradation of Dye by Photo catalytic action
2ml of Rhodamine-B dye is added as a component in 100 ml of pure distilled water, 100 mg of biodegradable synthesized TiO2 nanoparticles are mixed with 100 ml of Rhodamine dye. A control is continued without the addition of TiO2. To make the working solution of equilibrium, we need to stir for 30 minutes in order to receive the reaction suspension by mixing. The above must be done before exposing to irradiation. Afterwards, it should be kept under sunlight from morning till evening monitored. At intervals of a few hours, the aliquots of 2 ml of suspension should be filtered and monitored. It is used to evaluate the photosynthetic decay of the dye 16.
The absorption of the dye was recorded by using UV – Visible spectrophotometer. From the UV – Visible absorption spectra, the λ max of Rhodamine B dye was found to be at 554 nm.
% degradation of Rhodamine B dye = (I.C – F.C) / I.C x 100 ———–(1)
Where,
I.C– Initial Absorbance of Rhodamine B (Blank)
F.C– Sample Absorbance
Results And discussion
Fourier transform infrared Spectroscopy (FTIR)
FTIR was used to identify the chemical properties of the green synthesized titanium dioxide nanoparticles of Solanum nigrum and Sesbania grandiflora leaves extract. The functional group of the green synthesised Titanium dioxide nanoparticles was identified by using FTIR.
Figure-1 shows the FTIR of sample SG /TiO2. The strong peak is appeared 669 cm-1 due to crystal lattice vibrations of Ti-O-Ti band, this showed the anatase morphology of TiO2 nanoparticles. 1271 cm-1 is due to C-O-C stretching, 1384 cm-1 is due to C-O stretching, 1630 cm-1 is due to C=O stretching vibration and 3416cm-1 OH stretching vibrations.
 |
Figure 1: FTIR spectra of sample SG/ TiO2 |
Figure-2 shows the FTIR of SN/ TiO2 nanoparticles. The strong peak is obtained around 680 cm-1.1628cm-1is for C=O stretching, 2121cm-1 for O=C=O stretching. 3411cm-1 to 3768cm-1 is showed the OH stretching vibration 17-19.
 |
Figure 2: FTIR spectra of sample SN/ TiO2 |
X-ray diffraction analysis
The 2θ peaks at 25.30° and 48.01° confirm its anatase structure. The intensity of XRD peaks of the sample reflects that the formed nanoparticles are crystalline and broad diffraction peaks indicate very small size crystallite. JCPDS card no. 21-1272. Both SG/ TiO2 SN/ TiO2 gives almost same values in d-spacing and 2⍬ values.
Table 1: XRD peak list of TiO2
|
2⍬ |
⍬ |
FWHM |
Size |
d-spacing |
|
25.270 |
12.635 |
0.109 |
75 |
3.52149 |
|
36.910 |
18.455 |
0.108 |
78 |
2.43337 |
|
37.771 |
18.885 |
0.122 |
69 |
2.37986 |
|
38.528 |
19.264 |
0.128 |
66 |
2.33482 |
|
48.010 |
24.005 |
0.107 |
81 |
1.89350 |
|
53.849 |
26.924 |
0.122 |
73 |
1.70114 |
|
55.037 |
27.518 |
0.114 |
79 |
1.66720 |
|
62.073 |
31.036 |
0.137 |
68 |
1.49402 |
|
62.649 |
31.324 |
0.130 |
72 |
1.48107 |
 |
Figure 3: XRD Pattern of SG/ TiO2 Click here to View figure |
 |
Figure 4: XRD Pattern of SN/ TiO2 |
Photocatalytic studies of plant extracted TiO2 catalysts
Photocatalytic degradation of Rhodamine B dye over SG/ TiO2 catalyst
UV- Visible spectrum was recorded to study the photodegradation of Rhodamine B dye shown in Figure-5 by using SG/ TiO2 photocatalyst at a time interval of 30 minutes. The degradation of Rhodamine B was carried out, using sun as the main source of light from 0 to 360 minutes with SG/TiO2. For the purpose of comparison, dark experiment was also carried out in the absence of sunlight and the results reveals only less than 1% degradation was observed. The results showed that the degradation of Rhodamine B dye was increased with respect to time. The dye was irradiated under sunlight with 100 mg of SG/TiO2 catalyst. The dye was degraded in the presence of sunlight till 360 minutes. The intensity of the peak decreased with increased in exposure time shown in Figure -5. Thereby the absorbance of the dye was decreased with increased in time.
 |
Figure 5: UV-Visible absorption spectra of Rhodamine B dye over SG/ TiO2catalyst. |
The percentage of degradation of Rhodamine B dye over SG/TiO2 was calculated. From Figure-6 it has been observed that the percentage of degradation of Rhodamine B dye was gradually increasing as the time increases in the presence SG/TiO2 catalyst when they are irradiated under sunlight. The catalyst SG/TiO2 shows complete degradation of (100 %) at 360 minutes.
A plot of Absorbance vsTime in minutes shown in Figure-6 increasing the reaction time, the absorbance value gradually decreases as the time increases and after 6 hours the sample shows nearer to zero absorbance.
 |
Figure 6: Absorbance Vs Time plot of Rhodamine B dye over SG/TiO2 catalyst. |
A plot of % degradation Vs Time in minutes is shown Figure-7. It has been confirmed that as the reaction time increases, the percentage of degradation of the dye also increases and it reaches the maximum degradation of 100 % at 360 minutes for the dye over SG/TiO2 catalyst.
 |
Figure 7: Decolourization Vs Time plot of Rhodamine B dye over SG/TiO2 catalyst. |
Photocatalytic degradation of Rhodamine B dye over SN / TiO2 catalyst :
The decolorization of Rhodamine B dye was carried out by the reaction using SN/ TiO2 photocatalyst at a time interval of 30 minutes. The degradation of Rhodamine B was recorded from 0 to 420 minutes with SN/ TiO2. The photocatalytic degradation efficiency of SN/TiO2 catalyst was analysed. The results show that the maximum degradation was observed at 420 minutes. This is evidenced from Figure -8 that the intensity of the lmax at 554 nm was gradually decreases as the reaction time increases and also it shows a slight bathochromic 580nm. The dye was irradiated in the presence of sunlight with 100 mg of SN/TiO2 photocatalyst and 100% decolorization was obtained about 7 hours. The absorbance of the dye was decreased with increased in time.
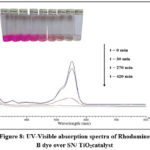 |
Figure 8: UV-Visible absorption spectra of Rhodamine B dye over SN/ TiO2catalyst Click here to View figure |
The percentage of degradation of the Rhodamine –B dye over SN/TiO2 was calculated using equation and it is given in Figure-9. It has been observed that the percentage of degradation of Rhodamine B dye was gradually increases as the time increases in the presence SN/TiO2 catalyst when they are irradiated under sunlight irradiation. The catalyst SN/TiO2 shows a maximum degradation of the dye (100%) at 420 minutes.
A plot of Absorbance Vs Time in minutes is shown Figure-9 increasing the reaction time, the absorbance value gradually decreases as the time increases and after 7 hours the sample shows zero absorbance.
 |
Figure 9: Absorbance Vs Time plot of Rhodamine B dye over SN/TiO2 catalyst. |
A plot of % of degradation Vs Time in minutes shown in Figure-10. It has been confirmed that as the reaction time increases the percentage of degradation of the dye increases and 100 % is the maximum degradation is observed at 420 minutes for the dye over SN/TiO2 catalyst.
 |
Figure 10: Decolourization Vs Time plot of Rhodamine B dye over SN/TiO2 catalyst. |
From the above discussion it is concluded that, 100 % is the maximum degradation for the dye over SG/TiO2 catalyst observed at 360 minutes and for SN/TiO2 catalyst 100 % maximum degradation observed at 420 minutes. Among the catalysts SG/TiO2 catalyst exhibited the maximum degradation of 100% in a shorter time of 360 minutes. Percentage of Degradation of Rhodamine – B over catalyst 24-27.
Analysis of the degraded dye sample
Among the synthesised catalysts (SG/TiO2, SN/TiO2), SG/TiO2 catalyst is degraded the Rhodamine B dye into more percentage. The decolourised/degraded sample was tested for water quality parameters to confirm the conversion of dye waste water into portable water. Water quality parameters of Rhodamine B dye waste water and degraded water was carried out by experiments on pH, turbidity, total dissolved solids and conductance.
pH
The dye effluents are highly fluctuating in pHand is important in the dyeing step, limiting chemical factor for aquatic life. Presence of concentration of hydrogen ion is measured by pH. The acidity or alkalinity of waste water affects both industrial waste water treatment and the environment. Generally we know that below pH 7 it is acidic. Above pH 7 it is alkaline in nature. pH of 7 is neutral. The universal standard pH for waste water discharge ranges lies between 6 -9. Hence the pH of the Rhodamine B dye sample is 7.13. After complete photo degradation of Rhodamine B sample, have the pH value is reduced to 6.86. From the result it can be concluded that the decolourised water can be used for various purpose.
Total dissolved solids (TDS)
A total dissolved solid (TDS) is a measure of the dissolved combined content of all inorganic and organic substances contain in a liquid. TDS is used to study of water quality for industrial waste water samples. It includes both volatile and non-volatile solids. In Water, TDS concentration can be determined using a digital meter.
Turbidity
Turbidity imparts an enormous problem in waste water treatment. It is a measure of cloudiness of water and measured by turbidity meter. Turbidity meter measures the scattering of light. Normal drinking water should have less than 5 NTU. It affects the growth rate of algae present in micro aquatic plants because increase the turbidity causes a decrease in amount of light for photosynthesis. It also can increase water temperature and block out the light needed by aquatic vegetation.
Conductance
Conductivity is a measure of water’s capability to pass an electrical current. It is directly proportional to the concentration of ions in the water. Dye waste water increased the conductivity because of the presence of inorganic dissolved solids of chloride, sodium, iron, phosphate and nitrate. The conductance value of the decolurized water is decreased 28-31.
 |
Figure 11: Dye water Vs Decolourised water of Rhodamine B dye. |
The water quality parameters were carried out using various techniques such as pH, Total Dissolved Solids (TDS), Turbidity and Conductance. The quality of the degraded water is under permissible limit and hence the treated water can be discharged into water bodies.
Catalytic Recyclability
Recoverability and Recyclability are the advantages of heterogeneous catalysts, especially for commercial and industrial applications. The recyclability test of the SG/TiO2 and SN/TiO₂ were also carried out. The catalysts can be easily separated from the reaction mixture by brief centrifugation and multiple washings with distilled water followed by drying. The recovered catalysts were evaluated for the decolourisation of Rhodamine -B dye. 100% decolourisation was attained even after the catalyst was used five times and it was monitored by UV-visible spectroscopy. This demonstrates the high catalytic activity and photo stability of the photo catalysts. 32-34.
Conclusion
The purpose of the present study was to investigate the degradation of Rhodamine B by SG/TiO2 and SN/TiO2 nanoparticles under solar irradiation. The synthesis of TiO2 nanoparticles with faster and purest in a reliable shorter duration period. TiO2 was synthesized using Sesbania grandiflora and Solanum nigrum leaves. The various functional groups were identified using the FTIR spectrum. XRD showed the crystalline nature of the TiO2 nanoparticles.SEM image shows the morphology of the TiO2. The degradation of Rhodamine B dye was measured using UV-Visible spectrometer. Synthesized TiO2 acts as an active photocatalyst for the degradation of harmful dye as well other as waste water contaminants. The Quality of the degraded dye sample was tested for pH, TDS, Turbidity and Conductance. The recyclability test confirms the stability of green syntitania nano particles.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
Reference
- Amit Kumar Pathak, 2013, ‘Water Pollution and Treatment’ International Journal of Environmental Engineering and Management, Vol.4, No.3, pp.191-198.
- Mehtab Haseenan, Muhammad Faheem Malik, Asma Javed, Sidra Arshad, Nayab Asif, Sharon Zulfiqar and Jaweria Hanif, 2017, ‘Water pollution and human health’ /environmental-risk-assessment-and-remediation, Vol.1, No.3, pp.16-19.
- Samchetshabam Gita and Ajmal Hussan, 2017, ‘Impact of Textile Dyes Waste on Aquatic Environments and its Treatment’ Environment & Ecology, Vol.35, No.3, pp.2v9-2353.
- Yaseen D.A, and Scholz .M, 2019, ‘Textile dye wastewater characteristics and constituents of synthetic effluents: a critical review’ International Journal of Environmental Science and a
- Nana L. Gavade, Shivaji University, Kolhapur, K. M. Garadkar, 2015, ‘Green synthesis of TiO2 and its photocatalytic activity’ Journal of Materials Science: Materials in Electronics, Vol.26, No.5, pp.3309-3315.
- Abhilasha Jain and Dipti Vaya,2017, ‘Photocatalytic Activity of TiO2 Nanomaterial’ Journal of Chilean Chemical Society, Vol.62, No.4, pp.1-8.
- Yerukali Sudha Rani, V. Jayasankar Reddy, Shaik Jilani Basha, Mallapu Koshma, G. Hanumanthu and P. Swaroopa, 2018, ‘A review of Solanum Nigrum’, World Journal Of Pharmacy And Pharmaceutical Sciences, Vol.6,No.12, pp. 293-303.
- Pradip Karmakar, Vikas Singh, RB Yadava, B Singh, ‘Rameswar Singh and Motilal Kushwaha,2016, Agathi [Sesbania grandiflora L. (Agast)]: Current Status of Production, Protection and Genetic Improvement’ National Symposium on Vegetable Legumes for Soil and Human Health, pp.12-14.
- Gaikwad Priyanka Subhash, Shete Rajkumar Virbhadrappa, Otari Kishor Vasant, 2010, ‘Spinacia Oleracea Linn: A Pharmacognostic And Pharmacological Overview’ International Journal of Research in Ayurveda & Pharmacy, Vol.1, No.1,pp.78-84.
- Rajani Chauhan, Km.Ruby, Aastha Shori, Jaya Dwivedi (2012) “Solanum Nigrum With Dynamic Therapeutic Role: A Review” “International Journal of Pharmaceutical Sciences Review and Research,Vol 15, No.14, PP-65-71.
- Payal R. Dande, G.M.Sharma, R.M.Sharma, and G.S.Chakaraborthy (2010), ‘Pharmacognostical Studies Of Leaves Of Spinacia Oleracea Linn’ International Journal of Pharmaceutical Science and Research vol.no.9, PP. 41-46.
- Lavanya P, Hari V, Pavithra T and Arunakumari T “Pharmacological review on sesbania grandiflora l.poir” International Journal of Current Advance Research, Vol 6, no. 2, pp. 1903-1907.
- Vidya Kulkarni, Vijayakumar Palled, Sharanagouda Hiregoudar, K. V. Prakash, Devanand Maski2 and Sushilendra, 2019, ‘Bio-Synthesis and Characterization of Titanium Dioxide Nanoparticles
- (TiO2) Using Azadirachta indica Leaf (Neem Leaf) Extract’ International Journal of Current Microbiology and Applied Sciences, Vol.8,No.10, PP.2309-2317.
- Hassanien R1, Abed-Elmageed and Husein 2019, ‘Photocatalytic Study and Anticancer activity of Green-Synthesized Ag Nanoparticles Using Drumstick Leaf Extract’Journal of Nanoscience and Nanotechnology Applications, Vol.3, No.1, PP.1-13.
- M. Vanaja, K. Paulkumar, M. Baburaja, S. Rajeshkumar, G. Gnanajobitha,C. Malarkodi, M. Sivakavinesan, and G. Annadurai, 2014, ‘Degradation of Rhodamine-B using Biologically Synthesized Silver Nanoparticles’ Bioinorganic Chemistry and Applications, Vol.2014, PP.1-8.
- K. Hadjiivanov, J Lamotte, F Mauge, J Saint-Just, M che (1998), “FTIR studty of co interaction with Ru/TiO2 catalyst”, Journal of catalysis, Vol.176, No.02, PP. 415-425.
- Tamara kecskes, Janos Rasco Kiss (2014), “FTIR and mass spectroscopic studies on the interaction of formaldehyde with TiO2 supported Pt and Au catalysts”, Journal of applied catalysis, Vol.1-2, No.273, PP.55-62.
- Alonsa, JM Dona Rodriguez, G colon, JH navio, J perez pena, (2009), “ FTIR study of photocatalytic degradation of 2-propanol in gas phase with different Tio2 catalysts ,Vol.1-2, No. 89, pp.204-213.
- Wei Huang, Zhijun Zuo, peide Han, Zhihong Li, Tingdong Zhao, (2009), “XPS and XRD investigation of CO/Pd/TiO2 catalyst by different preparation method”, Journal of electron spectroscopy and related phenomena, Vol.173, pp. 88-95.
- Kheamrutai Thamaphat, Pichet Limsuwan, Boonlaer Ngotawornchai (2008), “ Phase characterization of TiO2 powder by XRD and TEM”, National science Journal, vol.42, pp.357-361.
- M. Iniya Pratheepa (2017), “X-Ray Diffraction Analyses of Titanium Dioxide Nanoparticles,” Vol 3, Issue 11, pp.83- 88.
- Omer Kaygil i(2017) “Sol-Gel Synthesis and characterisation of TiO2 power”. International Journal of Innovative Engineering Applications 1, 2(2017),pp. 38-40.
- Mehmet Hugul, Erol Ercag, Resat Apak,(2002),“Kinetics studies on UV photodegradation of some chlorophenols using TiO2 catalyst”, Journal of environmental science and Health , vol.37, No 03, pp.365-383.
- Wenjun Liang, Jian Li, Yuguan Jin, (2012), “Photocatalytic degradation of gaseous formaldehyde by TiO2/UV, Ag/TiO2/UV and Ce/TiO2/UV”, Vol.51, pp.345-350.
- Olga fonteller, Mario J Munoz- Baticsta, Jose Carlos Conesa (2017), “UV and Visible hydrogen photo production using pt promoted Nb-dopped TiO2 photocatalysis”, Journal of environmental science, vol.206, pp.133-145.
- Hayal Khan, Zhuoran Jiang, Dimitrios Berk, (2018), “Molybdenum dopped graphene/TiO2 hybrid photocatalyst for UV/visible photocatalysts applications”, Solar energy, Vol.162, pp.420-430.
- Khade, G. V. (2015), “Green synthesis of TiO₂ and its photocatalytic activity”, Journal of Materials Science, Materials in Electronics Vol.26,pp.3309-3315
- M. Amini (2016) “Photocatalytic degradation of some organic dyes under solar light irradiation using TiO2 and ZnO nanoparticles” Vol 1(1): pp.79-86,
- Dr. R. Shanmuga Selvan, Effect of Aluminium, Magnesium Doping on Magnetic Nature of Zinc Oxide Nanoparticles Studied by X-Ray Diffraction Method, Oriental Journal of Chemistry, Vol. 38, June 2022, pp-1-13. http://dx.doi.org/10.13005/ojc/380310
- Gao,Y.Yang,(2018) “Biodegradation and Decolorisation of Dye waste water: A Review” .IOP Conference Series: Earth and Environmental Science.Vol.178. pp.012013-012019.
- Kalpesh Sorathiya, and Biswajit Mishra(2016) ”Enhancement in Rate of Photocatalysis Upon Catalyst Recycling”. Vol 6, Article number: 35075, pp.1-9.
- Alberti, S., Caratto, V., Peddis, D., Belviso, C., & Ferretti, M. (2019). “Synthesis and characterization of a new photocatalyst based on TiO2 nanoparticles supported on a magnetic zeolite obtained from iron and steel industrial waste, Journal of Alloys and Compounds. Vol 797, pp. 820-825.
- Bai,X., Shang,X., Wan, H., Che, Y., Yang, B., He, J., & Song, J. (2020). “Sustainable recycling of titanium from TiO2 in spent SCR denitration catalyst via molten salt electrolysis.” Journal of Energy Chemistry. Vol.58, pp. 557-563.

This work is licensed under a Creative Commons Attribution 4.0 International License.









