Phytochemical and Antimicrobial Analysis of Root Extract of Aspargus Adscendens
1Department of applied sciences, Maharaja Surajmal Institute of Technology, New Delhi-110058, India.
2St. Xaviers PG College, Phagi-303005, Jaipur, India.
Corresponding Author E-mail: rekhatripathi@msit.in
DOI : http://dx.doi.org/10.13005/ojc/380505
Article Received on : 23 May 2022
Article Accepted on : 01 Sep 2022
Article Published : 16 Sep 2022
The aim of this study was to characterize the phytochemicals and to investigate the antimicrobial efficacy of the shade dried roots of the Asparagus adscendens. The powder roots were extracted with chloroform and after removal of solvent under reduced pressure was subjected for chromatographic separation. After column chromatography and purification five compounds i.e .hexacosanyltriacontanoate, hexatriacontanone, 2,3,5,7-tetramethoxydi hydrophenanthrene, -sitosterol and stigmasterol were characterized with the help of physical and spectral analysis(IR, 1HNMR, 13CNMR and mass). The crude extract was tested against the selected bacteria and fungi using disc diffusion method. The root extract exhibited significant antibacterial activity with maximum efficacy against E. coli (activity index 0.82 at 1000 µg/disc and 0.73 at 500 µg/disc).
KEYWORDS:Asparagus adscendens roots; Antibacterial activity; Antifungal activity; Spectral analysis
Download this article as:| Copy the following to cite this article: Tripathi R. Sharma A. Phytochemical and Antimicrobial Analysis of Root Extract of Aspargus Adscendens. Orient J Chem 2022;38(5). |
| Copy the following to cite this URL: Tripathi R. Sharma A. Phytochemical and Antimicrobial Analysis of Root Extract of Aspargus Adscendens. Orient J Chem 2022;38(5). Available from: https://bit.ly/3R3VAWg |
Introduction
Asparagus genus belongs to Family Liliaceae is a perennial plant. The young shoots are used as vegetable or salad. This genus possess various types of biological properties e.g. antioxidants, anti-inflammatory, anti-hepatotoxic anti-oxytocic, immunostimulant, antibacterial and reproductive agents1-2. The genus asparagus includes about 300 species around the world. The tuberous roots of Asparagus are main source as the drug shatavar or shatavari. The drug in crude form is used in increase secretion of milk and also improves appetite in lactating women. Tuberous roots of Asparagus currillus after mixing with honey is given in case of diarrhea, diabetes and dysentery. One of the important species is Asparagus adscendens known as yellow musli is mainly grown in Asian countries and also in Garhwal valley. Traditionally it is recommended as nerve tonic and also used for memory impairment. In ayurvedic system of medicine it is helpful in treatment of female disorders3-6. Asparagus polysaccharides also exhibited health benefits against tumor cells7. In India Asparagus adscendens is mainly distributed in Garhwal (Himalayan) hills, Punjab, Madhya Pradesh, Uttar Pradesh, Himachal Pradesh, Bihar, West Bengal, Orissa, Jammu and Kashmir and lower Himalayan Hills. It is a shrub with tall, sub erect, smooth stem having ascending branches and white tuberous roots. Asparagus ascendens is a rich source of nutritious starch with low calorie, sodium values and good source of vitamins 8-10.
Asparagus adscendens is mostly used as indigenous medicine. On the basis of literature survey the plant contains 3-b-O-{b-O-glucopyranosyl}-stigmasterol, 3-b-O-{b-O-glucopyranosyl (1-2)-a-L-arabinophyranosyl}-stigmasterol11, 3-heptadecanone, 8-hexadecanoic acid, tritriacontane, palmitic, stearic acid12, oligofurastanosides, spirostanosides13, b-sitosterol-b-D-glucoside, spirostanol glycosides and furostanol glycosides14,15. The plant is known for its antioxidant, antiamnesic activities, used in diarrhea, dysentery, leucorrhoea, nutritive values, tonic, beneficial in stress management, inflammatory conditions and general debility16-18.
Experimental
Roots of Asparagus adscendens were collected from Tehri Garhwal, Uttarakhand, India in the month of September 2020 and confirmed from the herbarium of Botany Dept of H.N.B. Central University Srinagar, Garhwal (U.K.).
Shade dried and powdered roots (2 kg) were extracted with chloroform on a steam bath for 36 hrs then the extract was filtered hot and concentrated under reduced pressure.
Isolation and Identification of Phytochemicals
110g crude extract of roots of Asparagus adscendens was eluted with the solvents of increasing polarity over a column of silica gel. On elution five compounds were isolated, purified and characterized by co-TLC, mixed m.p. and spectral analysis (IR, 1HNMR, 13CNMR and mass spectral data).
Compound 1: Hexacosanyl triacontanoate
On elution with petroleum ether white powdered compound hexacosanyl triaconanoate, m.p. 68ºC was obtained. The IR spectrum exhibited characteristic absorption at 2900, 2835, 1735, 1705 (C=O), 1260, 725, 710 cm-1.The mass spectrum (m/z) showed M+ at 816, 447, 435, 381 etc.
Compound 2: Hexatriacontanone
Further Elution with petroleum ether-chloroform (9:1) yielded hexatriacontanone, crystallized from acetone as a white powder, m.p.76ºC. The spectral data were obtained as; IR(KBr) 2910, 2850, 1720 (C=O), 1130, 720, 715 cm-1, MS; 520(M+).
Compound 3: 2,3,5,7-tetramethoxyphenanthrene
Eluting the column with Chloroform yielded 2,3,5,7-tetramethoxyphemanthrene as white powder, m.p. 122-23ºC. Spectral data observed as IR (KBr) 1760, 1590, 1460, 1470, 1372, 1280 cm-1etc. MS (m/z) 301(M+H)+, 300(M)+, 286, 285, 258, 228, 226, 181, 150, etc.
Compound 4: Stigmasterol
Petroleum ether-chloroform (1:1) yielded stigmasterol as colorless solid powder, m.p.167-68ºC. Its spectral data are as: IR (KBr) 3410-3220 (OH), 1467 (C=C bonding), 1380, 1362, 1260, 1055,965 etc.MS m/z 412 (M+), 399, 384, 370, 369, 314, 302, 273 etc.
Compound 5: b-sitosterol
Further elution of column with chloroform-ethylacetate (80:20) yielded b-sitosterol after crystallization with methanol as white powder, m.p. 134-135ºC. The spectral data areas: IR(KBr) 3505, 3420, 2960, 2920,1595,1462,1380,1050 etc, MS(m/z)414(M+),397,396,383,369, 255, 213 etc.
Antimicrobial Analysis
The crude chloroform extract of roots of Asparagus adscendens was screened against Escherichia coli, Staphylococcus aureus, Proctus vulgaris and Salmonella paratyphi B. for bactericidal efficacy and against Aspergillus flavus, Aspergillus niger, Fusarium moniliforme and Rhizoctonia bataticola for fungicidal activity by using Disc diffusion method19.
Result and Discussion
Phytochemical Analysis
Compound (1) was isolated as white powder. The mass spectrum exhibited [M+] at 816 corresponding to molecular formula C56H112O2. IR spectrum (KBr) of the compound 1 indicated the presence of ester group by showing characteristic absorption at 1735cm-1. The other characteristic absorption were located at 2900, 2835cm-1 for C-H stretching and 1260cm-1 for C-O stretching of the ester group. 1HNMR spectrum showed two triplets for two protons each at d 3.90(C-1’) and d2.16(C-2) corresponding to the methylene group attached to ester oxygen and ester carbonyl group. The terminal methyl group was observed at 0.85 for six protons.
 |
Scheme 1 |
In 13CNMR spectrum of compound 1 the carbonyl carbon of ester
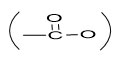
group appeared at d174.4. Thus compound 1 was characterized as hexacosanyl triacontanoate20,21.
Compound 2 was obtained as white powder and the molecular formula was determined as C36H72O by mass spectroscopy. The molecular ion observed at m/z 520.4(M+) with other important ions at m/z 464, 463, 435, 85 etc. In the proton NMR spectrum (d ppm, CDCl3) four protons appeared at 2.27 (J=6.5Hz) as a triplet for the two methylene group attached to carbonyl (C=O) group. A triplet for six protons was observed at d0.83 for methyl groups. A broad singlet at d1.25 showed the presence of remaining sixty two protons. In 13CNMR spectrum absorbance at d210 confirmed the presence of carbonyl carbon.IR spectrum (KBr) indicated the presence of carbonyl group at 1720cm-1.Thus the compound 2 was identified as hexatriacontanone22.
 |
Scheme 2 |
Compound 3(white powder) was determined as C18H20O4 with 301(M+H)+,300(M+). Other prominent ions were observed at m/z 286, 285, 258, 228, 227, 226, 182, 181, 150, 105 etc. In IR spectrum (KBr) showed characteristic absorptions at 1760, 1590, 1450, 1470, 1370, 1285, 1225 etc. In 1HNMR spectrum (dppm, CDCl3) of compound (3) exhibited four aromatic protons as singlet at d7.90(1H), 6.70(1H) and 6.42(2H). Complete assignment of 1HNMR and 13CNMR is given in table I, thus compound (3) was identified as 2,3,5,7-tetramethoxydihydrophenanthrene23-25.
 |
Scheme 3 Click here to View Scheme |
Table 1: Spectral data of 2,3,5,7-tetramethoxydihydrophenanthrene.
|
H/C |
1H |
13C |
|
1 |
6.70 (s) |
111.0 |
|
2 |
– |
146.8 |
|
3 |
– |
146.0 |
|
4 |
7.90 (s) |
112.0 |
|
4a |
– |
125.2 |
|
4b |
– |
116.2 |
|
5 |
157.6 |
|
|
6 |
6.42(s) |
98.0 |
|
7 |
– |
158.4 |
|
8 |
6.42(s) |
104.8 |
|
8a |
– |
139.6 |
|
9 |
2.70(m, 4H) |
31.0 |
|
10 |
2.70(m,4H) |
28.6 |
|
10a |
– |
129.8 |
|
2-OCH3 |
3.90(s, 6H) |
56.0 |
|
3- OCH3 |
3.80(s, 6H) |
55.8 |
|
5- OCH3 |
3.85(s, 3H) |
55.4 |
|
7- OCH3 |
3.80(s, 3H) |
55.0 |
Compound (4) was obtained as colorless solid after crystallization from methanol. On the basis of spectral data the molecular formula was assigned as C29H48O. The molecular ion was observed at m/z 412(M+) with the other prominent ion at 399, 384, 369, 302, 273, 255 etc in the mass spectrum. The IR (KBr) spectrum displayed characteristic absorptions at 3410-3220 cm-1 indicating the presence of O–H stretching and 1467Cm-1 for C=C bending vibrations. Absorptions at 1380, 1362, 1055, 955 etc. are characteristic for steroids.
In 1HNMR spectrum (CDCl3, dppm) showed a pair of double doublets at 5.10(J=15.3Hz) and 5.18(J=15.3Hz) for olefinic protons at C-22 and C-23. The large J values indicated the trans geometry of protons. A broad triplet at 5.30 was accounted for the proton present at C-6 position. A multiplet observed at 3.45 for one proton accounted for C-3 methine.
 |
Scheme 4: Stigmasterol. |
Stigmasterol
The presence of six methyl groups in compound (5) was observed and their positions has been given in parenthesis i.e. 0.71(s,3H,C-18),0.84(t,3H,C-29),0.94(s,3H,C-19),1.00(d,3H,C-21) and 1.15(d,6H,C-26 and C-27). Remaining 26 protons here observed from 1.24 to 2.26 and characterized as stigmasterol26,27.
Compound (5) after crystallization with ethanol yielded white powder. On the basis of mass spectrum and 1HNMR, molecular formula for compound (5) was established as C29H50O, m/z 414(M+). The other prominent fragments were located at m/z 397, 383, 369, 255 etc. In IR spectrum (KBr) the O-H stretching were observed at 3550-3420cm-l. The presence of carbon – carbon double bond was confirmed by the absorption at 1595cm-1. Absorption at 1060 was located which confirms the C-O stretching.
In 1HNMR (CDCl3, dppm) spectrum the methyl groups were observed at 0.65(s,3H,C-18),0.97(s,3H,C-19),1.23(d,3H,C-21),0.83(d,3H,C-26),0.90(d,3H,C-27),0.94(t,3H,C-29),
 |
Scheme 5: b-sitosterol. |
b-sitosterol
confirming the presence of six methyl groups. A multiplet at 3.48 for one proton confirmed the presence of hydroxyl group at C-3 position. A triplet for one proton at 5.20 with coupling constant J-2.8Hz was due to presence of –OH group at C-3 position. On basis of above data compound (5) was characterized as b-sitosterol28.
Antimicrobial analysis
The test extract exhibited anti bacterial activity against all the test bacteria, the maximum activity was observed against E. coli (activity index 0.82 at 1000 µg/disc and 0.73 at 500 µg/disc).The root extract also showed significant activity against S. aureus (activity index 0.77 at 1000 µg/disc and 0.56 at 500 µg/disc) In case of antifungal activity only F. moniliforme and R. bataticola exhibited some activity.
Table 2: Antibacterial and Antifungal activity of Root extract of Asparagus adscendens.
|
|
Test Bacteria |
Test Fungi |
|||||||
|
Dose |
|
E. coli |
S. aureus |
P. vulgaris |
S.paratyphi B |
A.flavus |
A.niger |
F.moniliforme |
R.bataticola |
|
1000µg/disc |
IZ |
18.0 |
14.0 |
6.0 |
8.0 |
± |
± |
11 |
9 |
|
AI |
0.82 |
0.77 |
0.30 |
0.42 |
0.55 |
0.43 |
|||
|
500 µg/disc |
IZ |
16.0 |
10.0 |
– |
± |
± |
± |
8 |
6 |
|
AI |
0.73 |
0.56 |
0.40 |
0.29 |
|||||
IZ – inhibition zone (in mm) including the diameter of disc (6mm)
AI – activity index ( inhibition zone of sample/ inhibition zone of standard)
Standard – Amikacin = 10 µg/ml (bacteria); Mycostatin = 100 units/disc (fungi)
( ± ) Trace activity: ( -) No activity
Conclusion
Five phytochemicals i.e .hexacosanyltriacontanoate, hexatriacontanone, 2,3,5,7-tetramethoxydi hydrophenanthrene, b-sitosterol and stigmasterol were isolated and characterized with the help of spectral studies from the root extract of Asparagus adscendens. Chloroform extract of roots of A. adscendens possess active compounds which exhibited significant anti bacterial activity against E. coli and S. aureus. It can be natural and harmless alternate of antibiotics for the treatment of many bacterial infections.
Acknowledgement
Authors are thankful to Head, Department of Chemistry, University of Rajasthan for providing lab facilities.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- Karuna, D.S.; Dey P.; Das S.; Kundu A.; Bhakta T, J. Trad. Complement. Med, 2018, 8, 60-65, https://doi.org/10.1016/j.jtcme.2017.02.004.
CrossRef - Sunday, R.M.; Obuotor E. M.; Kumar, A, Trends in Applied Sciences Research. 2019,14,199-204.
CrossRef - Gaur, R. D, The flora of the District Garhwal, North-West Himalaya, Srinagar, Garhwal, 1999, p.170.
- Kirtikar, K. R.; Basu, B. D, Indian Medicinal Plants Editiors: Singh, B., Sing, M.P.; Dehradun India, 1984, p.2499.
- Negi, J. S.;Sing, G.P.;Rawat, M. S.; Bist, V.K, Phcog. Rev. 2010,4, 215-220.
CrossRef - Thakur, S.; Sharma, D.R, International J. Pharm. Sci. and Health Care .2015 ,3, 82-97.
- Guo, Q.; Wang, N.; Liu, H.; Li, Z.; Lu, L.; Wang, C, J. of Functional Foods. 2020,65, 103727, https://www.sciencedirect.com/science/ article/pii/S1756464619306516.
CrossRef - Jadhav, A. N.; Bhutani, K.K, Indian J. Chem .2006 , 45B ,1515-1524.
- Jacqueline, N.M.; Peter, R.F.; Yasser, H. Yasser, Abdel-Wahab, British J. Nutrition .2006, 95, 576-581.
CrossRef - Bansode, F.W.; Arya, K.R.; Singh, R.K.; Narender, T, Pharm. Biol. 2015, 53, 192-200, ttps://www.tandfonline.com/doi/full/10.3109/ 13880209.2014.913295.
CrossRef - Tandon, M.; Shukla, Y.N.; Thakur, R.S, Fitoterapia .1990,61, 473.
- Tandon, M.; Shukla, Y.N.; Thakur, R.S, Phytochemistry .1990, 29, 2957-2959.
CrossRef - Sharma, S. C.;Thakur, N. K, Phytochemistry .1994, 36, 469-471.
CrossRef - Sharma, S. C.; Chand, R.; Sati, O. P, Phytochemistry. 1982, 21, 2075-2078.
CrossRef - Sharma, S. C.; Chand, R.; Bhatti, B.S.; Sati, O. P, Planta Med. 1982, 46, 48-51.
CrossRef - Shinwari, I. M.; Khan, A.M, J. Ethnopharmacol. 2000, 69, 45-56.
CrossRef - Kanwar, A.S.; Bhutani, K. K, Phytother. Res. 2010, 24,1562-1566, https://onlinelibrary.wiley.com/doi/10.1002/ptr.3218.
CrossRef - Bhatt, V. P.; Negi, G. C. S, Indian J. of Traditional Knowledge. 2006, 5, 331-335.
- Gould, J.C.; Bowie, J.H, Ednib.Med.J. 1952, 59, 178.
CrossRef - Garg, S. N.; Siddiqui, M. S.; Agarwal, S. K, J. Nat. Prod. 1992, 55, 1315-1319.
CrossRef - Mahmood, U.; Shukla, Y. N.; Thakur, R. S, Phytochemistry. 1983, 22, 167-170.
CrossRef - Singh,R. S.; Misra, T. N.; Pandey, H. S.; Singh, B. P, Phytochemistry. 1991, 30, 3799-3801.
CrossRef - Kil, Y. S.; Park, J.; Han, A. R.; Woo, H. A.; Seo, E. K, Molecules. 2015, 20, 5965-5974, https://doi.org/10.3390/molecules20045965.
CrossRef - Zhao, G. Y.; Dang, B. W.; Zhang, C. Y.; Cui, Y. D.; Bi, J. Y.; Zhang, G. G, J. Nat. Med. 2018, 72, 246-251, https://europepmc.org/article/ med/ 29063360.
CrossRef - Kshisagar, R. D.; Kamekar, Y. B.; Jagtap, S. D.; Upadhyay, S. N.; Rao, R.; Bhujbal, S. P, International J. Green Pharmacy.2010, 4, 147, https://www.greenpharmacy.info/index.php/ijgp/article/view/135
CrossRef - Venkata, S. P.; Chaturvedula, I. P, International current Pharma. Journal. 2012,1, 239-242.
CrossRef - Sharma, M. C.; Singh, R. K, Herba Polon. 1987, 33, 83-85.
CrossRef - Sharma, A. K.; Sharma, M. C.; Dobhal, M. P, Der Pharmacia letter. 2013, 5, 355-361, http://dx.doi.org/10.7897/2230-8407.1004150.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










