Extensive Research and Evaluation of Electro-Organic Synthesis of Nanomaterials
G. Malathi1 , C. Thillaiyadi Valliammai1*, Rahul Ratnakar Mahamuni2, Anthati Sreenivasulu3, J. Madhusudhanan4 and M.I. Niyas Ahamed5
, C. Thillaiyadi Valliammai1*, Rahul Ratnakar Mahamuni2, Anthati Sreenivasulu3, J. Madhusudhanan4 and M.I. Niyas Ahamed5
1Department of chemistry, A.V.V.M. Sri pushpam college (Autonomous ), Poondi-613503, Thanjavur,(Affiliated to Bharathidasan University, Tiruchirappalli-24), Tamil Nadu, India.
2Department of Environmental Science, S.B.E.S. College of Science, Aurangabad, Maharashtra.
3Department of Chemistry, Nagarjuna Government Degree College (Autonomous), Nalgonda, Mahatma Gandhi University, Nalgonda, Telangana.
4Department of Biotechnology, Anand Institute of Higher Technology, Kalasalingam Nagar, OMR, Kazhipattur, Chennai, Tamilnadu.
5Department of Biochemistry, Sacred Heart College (Autonomous), Tirupattur- 635601, Tamilnadu.
Corresponding Author E-mail: thillaiyadivalli@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380511
Article Received on : 21 Feb 2022
Article Accepted on :
Article Published : 29 Sep 2022
Reviewed by: Dr. Subramaniam N.P
Second Review by: Dr. Geetha Suresh
Final Approval by: Dr. S. A. Iqbal
Electricity is being used more directly and artificially than before. Working in a lab with a stronger synthetic emphasis enables the deployment of fresh ideas as well as ones that have been revived from earlier attempts in a wider range of situations. The amount of waste is decreased by using only electrons as reagents. Regenerating stoichiometric reagents in the correct molecular ratio can help electro catalytic catalysis. While minimizing waste is important, doing so also results in quicker and easier processes, gentler transitions, and the availability of more options, such as structural entities and IP space. Regenerative electricity can be used to give a terminal oxidizer or reducing agent that is extremely sustainable, which makes it a very alluring technology. Future electricity will be variable and plentiful, which will be very advantageous for value-added chemicals. The efficient conversion of renewable bio-based feedstocks serves as the first example of how contemporary electro-organic technologies can replace complex conventional processes. A new wave of sustainable chemistry will emerge if these obstacles are removed. This article takes a look at some recent developments in electrochemical synthesis that will undoubtedly affect how the discipline develops in the future.
KEYWORDS:Bio-based feedstocks; Electro catalytic; Electro-organic; Footprint; Electro-organic technologies; Stochastic
Download this article as:| Copy the following to cite this article: Malathi G, Valliammai C. T, Mahamuni R. R, Sreenivasulu A, Madhusudhanan J, Ahamed M. I. N. Extensive Research and Evaluation of Electro-Organic Synthesis of Nanomaterials.Orient J Chem 2022;38(5). |
| Copy the following to cite this URL: Malathi G, Valliammai C. T, Mahamuni R. R, Sreenivasulu A, Madhusudhanan J, Ahamed M. I. N. Extensive Research and Evaluation of Electro-Organic Synthesis of Nanomaterials.Orient J Chem 2022;38(5). Available from: https://bit.ly/3UO3S84 |
Introduction
The current social revolution is merely the beginning. Anger about climate change and the need to take action rises when major natural catastrophes receive broad media attention. The scientific community has already recommended several solutions to help reduce greenhouse gas emissions. As fossil fuels are depleted, so becomes the urgency to decrease our environmental impact. Another procedure that came out of this was electro-organic synthesis, which is now becoming increasingly common. For decades, synthetic organic chemists have ignored this methodology even though it has been widely used for inorganic transformations, such as the production of basic chemicals like chlorine and sodium hydroxide in a chlor-alkali electrolysis process and the Hall-Héroult process for aluminium production2-5.
In most cases, organic devices require nano-structuring to enhance performance or expand functionality. New technology that creates nanostructures that reduce the size of organic transistors and helps produce ultrathin panel displays is an excellent example. Solar cells made from the nanocomposite of poly (alkylthiophene) and a soluble fullerene derivative showed outstanding energy conversion efficiency because of their nanoscale heterojunctions. Nanoparticles improve the sensors’ response and sensing rates because they have a very large specific surface area6.
Because of their ability to produce high-purity particles with simple methods, electrochemical techniques hold great promise because they allow for better fine-tuning of particle size and higher yields in less time. We’ve made it possible to get a wide range of particle sizes and shapes. Since no reductor chemicals are used, this method is not only good for the environment, but it is also free of dangerous chemicals. 7-9. Electrochemical techniques, such as those using electrostatic and steric stabilisers, use organic monomers and polymeric molecules. It is possible to maintain and sustain electrolyte solutions using inorganic ions in ionic organic compounds without the need for additional chemicals. To their credit, several authors claim that steric stabilisers provide more stability to nanoparticles than electrostatic stabilisers. However, this claim is debatable. Polymer chain lengths can be used to alter the size and form of nanoparticles, as can chemical reduction methods 10-12.
If wind, solar, or hydroelectric power is used to generate electricity, electro-organic processes will become more important. Many renewable energy sources are now available, making it possible to store more electricity and produce more valuable fine chemicals at the same time, which might help this process. For electroorganic synthesis, the development of molecular-level pathways is achieved using electroreduction, the cation-pool method, and bio electrochemistry, which was first proposed. The approaches outlined above have attracted a great deal of attention from industry as well as opened up new options for innovation. One may obtain a better understanding of current electroorganic techniques by looking at them from this aspect, identifying a general pattern, and opening up future possibilities13, 14.
New Frontiers in Electro-organic Conversions
Electro-organic chemists are paving the way for the future of organic chemistry, and this is attributable to the increasing number of these scientists. While early-career researchers may not have to deal with the limits of “green chemistry” in their work, it is crucial to keep this in mind.
Reproducibility as Key Parameter in Process Development
In order to properly conduct electro-organic synthesis in a laboratory, there are a number of essential parameters that must be met. There are two distinct traits indicated in this criterion that are critical. Reproducibility of experiments is one of the factors that affect the reaction (Figure 1).16, 17.
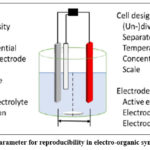 |
Figure 1: Crucial parameter for reproducibility in electro-organic synthesis (batch-type). |
Electro-organic chemistry is still in its infancy and must cope with a mountain of data until massive databases are made available. This multidisciplinary topic is often plagued by people who are unfamiliar with the most important electrolysis parameters. As a result, the method becomes much more difficult to use. Beaker cells are widely used in laboratories, making them an ideal choice for a standardised data set because they are easy for beginners to use and inexpensive for experts. For electro-organic synthesis in flow reactors, a screening system and a packaging system are made up of an undivided or split cell with up to eight processes and a flow reactor.
It is better to start with the correct electro-organic systems. Simple batteries used as an electric source in amateur handmade flasks with no set parameters don’t provide proper response control and discourage reproducibility. Due to their ease of usage, basic batteries can be used in electro-organic installations. The distance between the electrodes is not specified for many electrochemical processes, but it is important to get the desired outcome. In order to tell the difference between simple demonstrations of electrolysis and the application of results towards a useful laboratory technique for synthesis, it is imperative that the literature be described accurately. Electro-organic synthesis in education would enable this to be accomplished.
Novel Electrode Materials beyond the Limits
Electro-organic conversions get a performance boost because of advancements in electrode materials. Carbon-based materials, such as carbon nanotubes, can be used to prevent the corrosion of boron-doped diamond (BDD) electrodes (CNTs). The unique selectivity, chemical and physical robustness, resistance to fouling processes, and self-cleaning qualities of electrode materials used in electro-organic chemistry are important considerations when selecting electrode materials. Because of this, complex molecular structures, like licarin A, can be made to work well with good yields.
As a result of the long-term and short-term economic benefits of carbon allotrope materials compared to transition metals, these materials will have a significant influence on society because of their sustainable nature. Cathodic corrosion can be stopped by using leaded bronze, which is an alloy, instead of lead as the cathode in metal electrodes (22, 23).
 |
Scheme 1: Performance of boron-doped diamond anodes in electro-organic synthesis. Click here to View scheme |
The increased mechanical properties and reduced toxicity of this substance made it considerably simpler to handle. Foams and meshes, which are three-dimensionally structured electrodes, can speed up the electro-organic transformation process by increasing the active electrode surface. Many organisations use reticulated vitreous carbon as an electrode material because it is inert and long-lasting (RVC). Graphite felt and foam materials like nickel are often replaced by these more functional alternatives because of their superior performance. Adsorption of gases at the electrode’s lower active surface is problematic since many electro-organic reactions create gases as a by-product. Adding gaseous reagents to inhibit anodic hydrogen evolution can be accomplished using gas diffusion electrodes that are currently in use in the chloralkaline process to prevent cathodic hydrogen evolution. The performance, sustainability, and cost of new electrodes must all be taken into account. In cathodic activities, the counter anode must focus on maintaining the counter electrode at the lowest possible cost, thereby reducing technical barriers.
Robust Processes for Broad Applications
Electro-organic transformations can be highly valuable, but their usage in organic chemistry is limited due to the lengthy reaction times. Applications that demand flexibility and are more resistant to variable power consumption and response parameters benefit from the ability to operate across a wide current density range. 27). The Kolbe electrolysis and the Baizer process are two examples of electro-organic processes that work at high current densities. Using current densities of 100 mA/cm greatly shortens the electrolysis time.
The decreased viscosity and microdomains in the HFIP-methanol mixture support an order of magnitude higher current density. Because of the selective reactions, it is possible to separate valuable elements from waste products using galvanostatic electrolysis. Observing that reactions are not sensitive to substrate over-conversion can result in a number of undesirable byproducts and a time-consuming workup process 29). Significant adjustments to parameters will not lead to worse outcomes or failure if only dependable electro-organic techniques are utilised as examples in the development process. For researchers who desire to use them, electroorganic conversions speed up the start of the study process without causing any issues. 30, 31.
Paired Electrolysis
New processes are encouraged by considerations of long-term viability and low financial impact. Despite the fact that electrode reactions generate high-quality products, few individuals are familiar with the entire electrolysis process. This could lead to breakthroughs in processes that result from this. In order for the hydrogen evolution process to be recognised as a separate entity, it must be explicitly included. Due to the utilisation of electricity in tandem with chemical and energy use, as well as resource use, this approach is particularly sustainable. Refining metals and creating chlorine and sodium hydroxide in the chlor-alkali process may also use the same idea on industrial scales. Even basic systems without separate chambers can be used for electrolysis aqueous (Scheme 2) 32, 33.
 |
Scheme 2: Different types of paired electrolysis. |
In p-tertiary benzaldehyde dimethyl acetal paired electrolysis, Se achieved 100% atom and 180 percent current efficiency.Only if the chemicals in question can be readily distilled, crystallised, and filtered, is this a viable method of preparing a sample. Convergent electrolysis of glyoxal and oxalic acid produces glyoxylic acid when the oxidation potentials are precisely equal. 34, 35 As a result of the electrolysis process, a variety of chemicals such as glucose, gluconic acid, and even sorbitol are produced. This complicated technology, which is based on electrolysis, only needs a single electrode to work because it doesn’t need any other substrates.
The redox reagent produced by the counter electrode aids in the conversion of the substrate to the final product. Using an Fe2+/Fe3+ mediator system with linear paired electrolysis, it is able to produce D-arabinose with a current efficiency of 127%. As a result of the oxidation process, the anodic transformation of direct peroxide to direct peroxide and the cathodic transformation of indirect peroxide to indirect peroxide are both involved. As a result, it’s vital to deal with a variety of reaction kinetics while making sure that accurate reaction optimization is achieved. 36). A different idea is the domino synthesis, in which a substrate is first changed at the electrode and then changed at the counter electrode 37.
Electro-organic Synthesis on a Larger Scale in Flow Cells
Scaling up electro-organic technologies for industrial usage remains a hurdle even though they have been developed successfully. Even while electro-organic reactions have a number of limitations, the most significant one is their inability to withstand long-term use in the real world. The potentiostatic technique is also commonly used in electrochemical operations. Electrolysis progress is slowed by longer response times and a complex three-electrode arrangement caused by decreasing substrate in the process 38. In the same way as with conventional transforms, a number of scale-up parameters must be handled. Volume-to-surface ratios decrease in larger batch reactors due to the larger electrodes. Effective mixing is required for the bulk transport of chemicals following the conclusion of electrolysis. 39, 40.
With the heat generated by electrolysis occurring mostly at the electrodes, the electrode surface is made harder to cool. Large-scale substrate conversion requires the development of flow-electrolysis cells, which eliminate the need for a costly and complicated electrical device. Continuous manufacturing of value-added products is economically possible with flow electrolysis. These issues can be addressed by using a cluster of flow-electrolysis cells organised in a linear fashion. When items are constantly being disposed of, the danger of oxidative deterioration is minimised.
However, micro-flow setups have a restricted electrode surface and poor economic productivity, despite the development of several flow-electrolysis approaches. It is only suitable for operations requiring high current densities, as long-channel flow cells do not enable gas release (single pass conversions). In order to increase production, flow cells must be numbered and flow cells must be swapped in order to provide the best electrolysis conditions. In contrast, studies have shown that flow cells with higher surface-to-volume ratios, smaller electrode gaps, and better temperature control can provide even greater benefits. Narrow-gap electrolysis cells can work without an electrolyte support as long as there is still some conductivity, such as a small amount of water.
Electrochemistry in the Synthesis of Natural Products and APIs
Total synthesis procedures get more complex when electrical transformations are used with diverse syntheses. With electro-organic synthesis, metal contamination is avoided thanks to carbon allotrope electrodes, unlike traditional transformations. Electro-organic synthesis has many great features, such as easy reaction conditions, shorter pathway lengths, efficient use of atoms and money, and a big drop in the amount of dangerous chemicals (42, 43).
Although complex compounds are small in number, electro-organic procedures typically employ single redox-active components as substrates since this is the most common approach. Aside from these difficulties, small sizes also make it difficult for complete synthesis applications to take place. Intricate molecular structures like those seen in dixiamycin B can only be created using electricity and other non-conventional synthesis techniques. When N-isopropylacrylamide is present, xiamycin B ester molecules are formed.
 |
Figure 2: Natural products and APIs with electro-organic key transformations. |
An example of late stage functionalization in electro-organic synthesis is alliacol A.’s cyclization approach. Bonding two molecules, one of which was a protected enol and the other a furan, resulted in the formation of intramolecular carbon-carbon bonds. A wide variety of alkaloids may be found in both nature and medicine. Because of the reduced synthesis methodologies utilised, the synthesis of kopsidine A, (–)-thebaine, and (–)-oxycodone is significant 44. The oxidation of the bridging nitrogen at the conclusion of the kopsidine A synthesis produced the intramolecular C–O link. Trioxygenated laudanosine derivatives produced (–)-Thebaine and (–)-Oxycodone via anodic C–C bond formation in certain regions and diastereoselectively. A straightforward synthesis of finnerenone, an API used to treat heart disease, uses the racemization reaction substrate to create the other enantiomer (Scheme 3) 45..
 |
Scheme 3: Electro-organic racemisation for an API by Bayer. |
Stereoselective Electro-organic Conversions
For the synthesis of natural chemicals, chiral information must be installed. Standard asymmetric catalysis is seldom used for enantioselective conversions, because the majority of electro-organic conversions are incompatible with enantiomerically excessive conditions. Asymmetric electrolysis methods were established by scientists and researchers for their previous work, which employed chiral electrolytes, solvents, and asymmetric electrode functionalization. These days, chiral mediators are used in asymmetrical electrolysis procedures. In the framework of electrons as an environmentally friendly reagent, electrocatalytic asymmetric synthesis leverages asymmetric organic synthesis. Use chiral auxiliaries, which are electro-auxiliaries, to pre-functionalize stereophonic information (Scheme 4) 46.
 |
Scheme 4: Asymmetric electrolysis modes with significant enantiomeric excess. |
The chiral framework is provided by polymers, organometal complexes, or proteins, with several reusable carbon electrodes (TON). 2-acetylpyridine (4-acetylpyridine) is isometrically symmetric after reduction with (-)-(S)-(-)-phenylalanine methyl ester.Today’s approaches make use of poly-L-valine or spiroxyl motifs to boost coating efficiency and increase enantiomeric excesses. 47, 48. Innovative techniques for chiral electrode surfaces that use inexpensive, readily accessible materials for chiral encoding can yield up to 80% enantiomeric excess.
This process is called electrodeposition, and it involves depositing a metal like Pt or Ni on the asymmetric molecules that are in contact with a liquid crystal. After the template is removed, a mesoporous metal is left with stable chiral cavities. During electrolysis, these pores keep the unwanted enantiomer in check while allowing the co-reagent to attack the substrate. There are no optimal substrate orientations for conventional metal electrodes, which results in racemates. Interest in the use of chiral mediators has risen as the synthesis of the desired stereoisomer has increased. The use of iodoarenes and N-oxyl motifs is widespread. When a chiral amine was added to a few studies, 49, asymmetric electrolysis was seen..
Electro-valorisation of Renewable Bio-based Feedstocks
In the past, fossil fuels were the primary source of chemical synthesis fuels. Efforts to establish sustainable techniques for chemical conversion and the manufacturing of value-added items from biomass, such as lignin, carbohydrates, proteins, terpenes, and lipids, have been hampered by the finite availability of previous fossil fuel supplies. It has been found that electrochemistry with renewable feedstock for making fuels and chemicals works well together.50 Synthetic chemistry’s conversion of greenhouse CO2 to the C1 building block is fraught with difficulty. With the wide variety of potentials accessible, electrochemistry paves the way for further CO2 conversion applications because of CO2’s strong redox potential. Building blocks like carbon monoxide for syngas, formic acid, or ethylene altered depending on the cathode material were discovered throughout the path of advancement. Formic acid is made from lead, mercury, and gold, whereas methane and ethylene are produced by copper and silver, and CO51 is made from silver, gold, and zinc.
Numerous articles take a look at what’s been going on. Papermaking produces cross-linked polyphenolic lignin, the second most common biopolymer. Vanillin can be made from lignin at a lower cost using typical thermally exploitative techniques, yet lignin is still used. It is clear that chemical research has grown tremendously since lignin’s conversion. Because of its large molecular weight, lignin in Kraft pulp is difficult to degrade using conventional chemical methods. The use of potentially harmful reagents and methods is therefore necessary, 52.
Value-added products like vanillin, which may be used to create commodities like vanillin on metals such as gold, copper, nickel, lead oxide, palladium, or dimensionally stable anodes, show higher overall performance in electro-organic processing than other types of products. Electrodes made of Ni/P-foam and Ni/NiOOH have been described, as well as a photoelectrochemical approach utilising Ta2-O5-IrO2-Films and TiO2-Nanotubes. However, new electrodes and materials are currently being developed 58. Electrochemical Kraft lignin degradation can be made easier by the use of many methods that generate fewer complex mixtures, but only a handful of these methods allow for a highly selective transformation. We were able to make the hydrolysate of organosolv lignin stronger by using a combination of direct anodic oxidation, cathodic graphite felt oxidation, and RuO2/Ti-mesh catalytic oxidation.53 Under ambient conditions, native lignin is synthesised by adding a variety of value-added compounds, including photo-and electro-redox catalysis. Electrochemistry was used to get these chemical building blocks, like fatty acids, out of sawdust, which is a common raw material.
Conclusions
Numerous discoveries are made possible by taking into account various components in electro-organic synthesis, but this also results in ever-more discoveries. Future electricity will be variable and plentiful, which will be very advantageous for value-added chemicals. The efficient conversion of renewable bio-based feedstocks serves as the first example of how contemporary electro-organic technologies can replace complex conventional processes. A new wave of sustainable chemistry will emerge if these obstacles are removed.
Acknowledgements
The authors would like to thank their respective management of their institutes for granting academic liberty for the successful completion of the research work.
Conflicts of interest
The authors declare no conflict of interest.
Funding Sources
References
- D. Feldheim and C. Foss, Metal Nanoparticles: Synthesis, Characterization and Applications, Marcel Dekker, New York, NY, USA, 2002.
- J. Lue, “A review of characterization and physical property studies of metallic nanoparticles,” Journal of Physics and Chemistry of Solids, 2001, 62, 1599–1612,
- N. Faraji, W. Mahomood Mat Yunus, A. Kharazmi, E. Saion, M. Shahmiri, and N. Tamchek, “Synthesis, characterization and nonlinear optical properties of silver/PVA nanocomposites,” Journal of the EuropeanOptical Society, 2012, 7, 12040.
- T. He, C. Wang, X. Pan, and Y. Wang, “Nonlinear optical response of Au and Ag nanoparticles doped polyvinylpyrrolidone thin films,” Physics Letters A, 2009 , 373, 592 -595.
- R. Vadakkekara, M. Chakraborty, and P. Parikh, “Catalytic performance of silica-supported silver nanoparticles for liquid phase oxidation of ethylbenzene,” Industrial and Engineering Chemistry Research, 2012, 51(16), 5691–5698.
- M. Radzig, V. Nadtochenko, O. Koksharova, J. Kiwi, V. Lipasva, and I. Khmel, “Antibacterial effects of silver nanoparticles on gram-negative bacteria: influence on the growth and biofilms formation, mechanisms of action,” Colloids and Surfaces B, 2013, 102, 300–306.
- M. V. Rold´an, A. Frattini, O. de Sanctis, H. Troiani, and N. Pellegri, “Characterization and applications of Ag nanoparticles in waveguides,” Applied Surface Science, 2007, 254 (1), 281–285.
- Q.Wu, H.Cao,Q. Luan et al., “Biomolecule-assisted synthesis of water-soluble silver nanoparticles and their biomedical applications,” Inorganic Chemistry, 2008, 47(13), 5882–5888.
- S. Prabhu and E. Poulose, “Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects,” International Nano Letters, 2012, 2, 32.
- L. M. Liz Marzan and I. Lado-Tourino, “Reduction and stabilization of silver nanoparticles in ethanol by nonionic surfactants,” Langmuir, 1996, 12, 3585–3589.
- M. Guzm´an, J. Dille, and S. Godet, “Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity,” International Journal of Chemical and Biological Engineering, 2009, 2, 104–111.
- G. N. Glavee, K. J. Klabunde, C. M. Sorensen, and G. C. Hadjipanayis, “Borohydride reduction of cobalt ions in water. Chemistry leading to nanoscale metal, boride, or borate particles,” Langmuir, 1993, 9(1), 162–169.
- N. Leopold and B. Lendl, “Anewmethod for fast preparation of highly surface-enhanced raman scattering (SERS) active silver colloids at room temperature by reduction of silver nitrate with hydroxylamine hydrochloride,” The Journal of Physical Chemistry B, 2003, 107(24), 5723–5727.
- P. Jeevanandam, C. Srikanth, and S. Dixit, “Synthesis of monodisperse silver nanoparticles and their self-assembly through simple thermal decomposition approach,” Materials Chemistry and Physics, 2010, 122(2-3), 402–407.
- K. Akhbari, A. Morsali, and P. Retailleau, “Silver nanoparticles from the thermal decomposition of a two-dimensional nanocoordination polymer,” Polyhedron, 2010, 29(18), 3304 – 3309.
- T. Edison and M. Sethuraman, “Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue,” Process Biochemistry, 2012, 47, 1351–1357.
- V. Kotakadi, Y. Rao, S. Gaddam, T. Prasad, A. Reddy, and,D. Sai Gopal, “Simple and rapid biosynthesis of stable silver nanoparticles using dried leaves of Catharanthus roseus. Linn. G. Donn and its anti-microbial activity,” Colloids and Surfaces B, 2013, 105, 194–198.
- K. Shameli, M. Ahmad, E. Al-Mulla et al., “Green biosynthesis of silver nanoparticles using Callicarpa maingayi stem bark extraction,” Molecules, 2012, 17(7) 8506–8517.
- R. Trbojevich, N. Pellegri, A. Frattini, O. de Sanctis, P. J. Morais, and R. M. Almeida, “Preparation and isolation of gold nanoparticles coated with a stabilizer and sol-gel compatible agent,” Journal ofMaterials Research, 2002, 17(8), 1973–1980.
- D. Zhang, X. Liu, X. Wang, X. Yang, and L. Lu, “Optical properties of monodispersed silver nanoparticles produced via reverse micelle microemulsion,” Physica B, , 2011, 406 (8), 1389–1394.
- K. H. Baek, J. H. Kim, K. B. Lee, H. S. Ahnn, and C. S. Yoon, “Surface plasmon resonance tuning of silver nanoparticle array produced by nanosphere lithography through ion etching and thermal annealing,” Journal ofNanoscienceandNanotechnology, 2010, 10(5), 3118–3122.
- M. L. Rodrıguez-Sanchez, M. J. Rodrıguez, M. C. Blanco, J.Rivas, and M. A. Lopez-Quintela, “Kinetics and mechanismof the formation of Ag nanoparticles by electrochemical techniques:a plasmon and cluster time-resolved spectroscopicstudy,”The Journal of Physical Chemistry B, 2005, 109,1183–1191.
- M. M. Wadkar, V. R. Chaudhari, and S. K. Haram, “Synthesisand characterization of stable organosols of silver nanoparticlesby electrochemical dissolution of silver in DMSO,” Journal ofPhysical Chemistry B, 2006, 110(42), 20889–20894.
- J. Zhu, X. Liao, X. Zhao, and H. Chen, “Preparation of silver nanorods by electrochemical methods,” Materials Letters, 2001, 49(2), 91–95.
- M. Mazur, “Electrochemically prepared silver nanoflakes and nanowires,” Electrochemistry Communications, 2004, 6(4), 400–403.
- Y. Yu, S. Chang, C. Lee, and C. Wang, “Gold nanorods: electrochemical synthesis and optical properties,” The Journal of Physical Chemistry B, 1997, 101(34), 6661–6664.
- M. Zhou, S. Chen, S. Zhao, and H. Ma, “RETRACTED: onestep synthesis of Au-Ag alloy nanoparticles by a convenient electrochemical method,” Physica E, 2006, 33, 28–34.
- M. Bordenave, A. Scarpettini, M. Rold´an, N. Pellegri, and A. Bragas, “Plasmon-induced photochemical synthesis of silver triangular prisms and pentagonal bipyramids by illumination with light emitting diodes,” Materials Chemistry and Physics, 2013, 139(1),100–106.
- H. Peng, A. Yang, and J. Xiong, “Green, microwave-assisted synthesis of silver nanoparticles using bamboo hemicelluloses and glucose in an aqueous medium,” Carbohydrate Polymers, 2013, 91, 348–355.
- A. Pal, S. Shah, and S. Devi, “Microwave-assisted synthesis of silver nanoparticles using ethanol as a reducing agent,” Materials Chemistry and Physics, 2009, 114, 530–532.
- H. Yin, T. Yamamoto, Y. Wada, and S. Yanagida, “Largescale and size-controlled synthesis of silver nanoparticles under microwave irradiation,” Materials Chemistry and Physics, 2004, 83(1), 66–70.
- A. Frattini, N. Pellegri, D. Nicastro, and O. de Sanctis, “Effect of amine groups in the synthesis of Ag nanoparticles using aminosilanes,” Materials Chemistry and Physics, 2005, 94(1), 148–152.
- L. Huang, Y. Zhai, S. Dong, and J.Wang, “Efficient preparation of silver nanoplates assisted by non-polar solvents,” Journal of Colloid and Interface Science, 2009, 331(2), 384–388.
- A. Rodr´ıguez-L´opez, A. Paredes-Arroyo, J. Mojica-Gomez et al., “Electrochemical synthesis of magnetite and maghemite nanoparticles using dissymmetric potential pulses,” Journal of Nanoparticle Research, 2012, 14, 993.
- C. Y. Cheng, S. Thiagarajan, and S. M. Chen, “Electrochemical fabrication of AuRh nanoparticles and their electroanalytical applications,” International Journal of Electrochemical Science, 2011, 6(5), 1331–1341.
- D. F. Yancey, E. V. Carino, and R. M. Crooks, “Electrochemical synthesis and electrocatalytic properties of Au@Ptdendrimerencapsulated nanoparticles,” Journal of the American Chemical Society, 2010, 132(32), 10988–10989.
- B. Yin, H. Ma, S. Wang, and S. Chen, “Electrochemical synthesis of silver nanoparticles under protection of poly (Nvinylpyrrolidone),” The Journal of Physical ChemistryB, 2003, 107(34), 8898–8904.
- M. Tejmaya, I. R¨omer, R. Merrifield, and J. Lead, “Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media,” Environmental Science and Technology, 2012, 46(13), 7011–7017.
- C. Luo, Y. Zhang, X. Zeng, Y. Zeng, and Y. Wang, “The role of poly (ethylene glycol) in the formation of silver nanoparticles,” Journal of Colloid and Interface Science, 2005, 288(2), 444– 448.
- R. G. Shimmin, A. B. Schoch, and P.V. Braun, “Polymer size and concentration effects on the size of gold nanoparticles capped by polymeric thiols,” Langmuir, 2004, 20(13), 5613–5620.
- A. Shkilnyy, M. Souc´e, P. Dubois, F. Warmont, M. L. Saboungi, and I. Chourpa, “Poly(ethylene glycol)-stabilized silver nanoparticles for bioanalytical applications of SERS spectroscopy,” Analyst, 2009, 134(9), 1868–1872.
- D. Sutton, Electronic Spectra of Transition Metal Complexes, McGraw-Hill, London, UK, 1968.
- H.Wang, X. Qiao, J.Chen, X.Wang, and S. Ding, “Mechanisms of PVP in the preparation of silver nanoparticles,” Materials Chemistry and Physics, 2005, 94, 449–453.
- U. Kreibig and M. Vollmer, Optical Properties of Metal Clusters, Springer, Berlin, Germany, 1995.
- X. K.Meng, S. C. Tang, and S. Vongehr, “A review on diverse silver nanostructures,” Journal of Materials Science & Technology, 2010, 26, 487–522.
- L. Rodr´ıguez-S´anchez,M. C. Blanco, andM. A. L´opez-Quintela, “Electrochemical synthesis of silver nanoparticles,” The Journal of Physical Chemistry B, 2000, 104, 9683–9688.
- R. He, X. Qian, J. Yin, and Z. Zhu, “Preparation of polychrome silver nanoparticles in different solvents,” Journal of Materials Chemistry, 2002, 12, 3783–3786.
- R. Jenkins and R. L. Snyder, Introduction to X-Ray Powder Diffractometry, JohnWiley& Sons, New York, NY, USA, 1996.
- S. Calvin, S. X. Luo, C. Caragianis-Broadbridge et al., “Comparison of extended x-ray absorption fine structure and Scherrer analysis of x-ray diffraction as methods for determining mean sizes of polydisperse nanoparticles,” Applied Physics Letters, 2005, 87, ( ID 233102) , 3 pages.
- K. Kelly, E. Coronado, L. Zhao, and G. Schatz, “The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment, “The Journal of Physical Chemistry B, 2003, 107, 668–677.
- D. Evanoff and G. Chumanov, “Size-controlled synthesis of nanoparticles. 2. Measurement of extinction, scattering, and absorption cross sections, “The Journal of Physical Chemistry B, 2004, 108(37), 13957–13962.
- S. Link and M. A. El-Sayed, “Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods,” The Journal of Physical Chemistry B, 1999, 103, 8410–8426.
- M. Reetz and W. Helbig, “Size-selective synthesis of nanostructured transition metal clusters,” Journal of the American Chemical Society, 1994, 116(16), 7401–7402.

This work is licensed under a Creative Commons Attribution 4.0 International License.









