Evaluation of Antidiarrheal Activity of Bryophyllum Pinnatum Lam. (Oken) Leaves Extracts and Isolation of Active Constituent.
1Faculty of Pharmacy, IFTM University, Moradabad, India, 244102.
2Faculty of Pharmacy, Pharmacy Academy, IFTM University, Moradabad, India, 244102
3Faculty of Pharmacy, School of Pharmaceutical Sciences, IFTM University, Moradabad, India, 244102
Corresponding Author E-mail: priyankagautamyadav@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380504
Article Received on : 30 July 2022
Article Accepted on : 08 Sep 2022
Article Published : 28 Sep 2022
Reviewed by: Dr. Tanay Pramanik
Second Review by: Dr. Kasta Gurning
Final Approval by: Dr. Malinee Sriariyanun
Bryophyllum pinnatum Lam. (Oken) (Crassulaceae) are commonly known as miracle plant. Traditionally, this plant is used to heal wounds and to treat diarrhoea. For this reason, the main aim of the present study is to evaluate the antidiarrheal activity using castor oil-induced diarrhea model. Loperamide drug (3 mg dose,) was used as standard drug. The EAE and EE at 200mg dose and 400 mg dose, respectively produced dose-dependent protection of mice with significant P value. Both extracts of plant produced dose-dependent and significant P-value towards antimotility effect compared with negative control (castor oil) group in model. EE (400 mg dose) possess better antidiarrheal potential in mice groups due to presence of flavone. 5-methyl-4,5,7- trihydroxy flavone was isolated from the ethanol extract. Finally, it can be concluded that, Bryophyllum pinnatum Lam. (Oken) leaves can be used ethnomedicinally to treat, counterbalance, bulwark, and control diarrhea.
KEYWORDS:Antidiarrheal activity; Bryophyllum pinnatum Lam.(Oken); Ethanol extract; Ethyl acetate extract
Download this article as:| Copy the following to cite this article: Yadav P, Mishra A. K, Singh H. Evaluation of Antidiarrheal Activity of Bryophyllum Pinnatum Lam. (Oken) Leaves Extracts and Isolation of Active Constituent. Orient J Chem 2022;38(5). |
| Copy the following to cite this URL: Yadav P, Mishra A. K, Singh H. Evaluation of Antidiarrheal Activity of Bryophyllum Pinnatum Lam. (Oken) Leaves Extracts and Isolation of Active Constituent. Orient J Chem 2022;38(5). Available from: https://bit.ly/3D0kbrq |
Introduction
In the past few decades, exploration towards the health care system is on the rise, it is due to an increase in the ailments. Novel ailments demand highly efficacious drugs at lower doses and also minimal effort in production. Herbal medicine has a long history of well-recorded and well-practiced practices in India. Researchers seeking to discover new drugs have a unique opportunity in India as it is home to a diverse culture, traditions and natural biodiversity1. Herbal remedies are commonly employed in today’s world and people’s trust in natural cures is growing. Traditional medicines are of great choice, as they have predefined working mechanisms with fewer standardized products. Scientists, all around the world have started to sight traditional medicine for primary health care2. Natural compounds extracted from higher plants and microbes are used to develop clinically useful medications3.
Each year, diarrhoea kills an estimated 2.2 million people worldwide. The majority are infants and children below the age of five4. When someone has diarrhoea, their intestines release semisolid or liquid waste three or more times in one day5. Gastric discomfort, wet stool weight, fluid secretion, and absorption all rise along with the fluidity, mass/volume, and turns of bowel movements decreasing6. Water and electrolytes are lost8. The goal of diarrhoea therapy, which is sometimes non-specific, is to lessen the discomfort and inconvenience associated with frequent bowel motions. Undernourishment is the primary cause of diarrhea among children in developing countries9. In order to eliminate diarrhea, the leading cause of death in developing countries8.The World Health Organization (WHO) has introduced a Control Diarrheal Disease program. This program combines learning about traditional medicinal applications with assessment and prevention of health education perspectives10.
The capacity of several medicinal plants to prevent, treat, and manage diarrhoea has been studied by researchers 11,12. Balanites rotundifolia, Boscia coriacea, Amaranthus caudatus, Plumbago zeylanica, Solanum hastifolium, Ecballium elaterium, Mentha longifolia, Rhamnus cathartica, and Teucrium polium are a few examples of medicinal plants with antidiarrheal properties. Thus, the WHO has inspired work related to the treatment and anticipation of diarrheal diseases using traditional medical applications13,14. The plant is also popular as an air plant, cathedral bells and curtain plant, good luck. The plant requires tropical, subtropical, moist, and warm temperatures to grow efficiently. Due to this reason, the cultivation of the plant is well suited in the environmental conditions of America, China, India and Australia15. India, a country of different climates has suitable climatic conditions for the easy growth of fleshy herbs. The excessive prevailing Bryophyllum pinnatum Lam. (Oken) also has an identification of garden plant15,16. The Bryophyllum pinnatum has advantage to manage a variety of ailments also such as conjunctivitis, edema, piles, wounds, eczema, chickenpox, and fever. Burns, rheumatoid arthritis, antiseptic, blisters, and gastrointestinal discomforts are all commonly treated with different parts of Bryophyllum pinnatum Lam. (Oken)16,17. In Southeast Nigeria, slightly heated leaves are used for superficial skin illness and also used for placenta dropping, acting as a tocolytic agent to prevent premature labor17. In the present study, the antidiarrheal activity of Bryophyllum pinnatum Lam. (Oken) was investigated.
Materials and Methods
Preparation of plant extracts
The leaves of Bryophyllum pinnatum Lam. (Oken) are of great importance. The leaves required for the study were collected from a Ranch of Hari Singh in Lucknow, (Uttar Pradesh), India. Lucknow’s National Botanical Research Institute (NBRI) identified and authenticated the plant. The weight of the leaves was around 2.7 kg. The leaves were a dark green hue and also were rough in texture.
 |
Figure 1: Collected plant and leaves of Bryophyllum Pinnatum. |
After the collection, the first step was to make the leaves free from the dirt. This process was carried out by dusting and followed by cleaning with the help of pure cotton cloth. Once the leaves were clean the leaflets were stalled by the shade drying process. For the reduction of size utilization of grinder delivered the sufficient size powder material of leaves. The extraction of the leaves using Soxhlet is the step underway for further study on leaves. The Soxhlet extraction process was carried out successively for elution of chemical constituent from the plant part (leaves) with the help of the solvents (Petroleum Ether 60-80˙ C Ethyl acetate and 70% Ethanol)18.
Drying of extracted solutions
The extracted solutions were stored in different round bottom flask. They were dried using rotary evaporator.The dried extracts EAE and EE were kept in dessicator to prevent microbial contamination19.
Phytochemical screening
There are many ways to identify the plant’s origin. One such way is the identification of phytochemicals present by different biochemical tests on the plant extracts20,21. The results were shown in Table 1.
Ethical considerations
In this study, all experimental conditions and protocol were maintained in accordance with the Institutional Animal Ethics Committee received regulation (Reg. No-1088/PO/Re/S/07) under the rules 5(a) of the “Breeding of and experiments on animal control and supervision rules 1998”.
Experimental animals
The study of antidiarrheal activity was performed on Swiss albino mice, weighing 17-35gm of either sex. The set of 5 mice was divided into seven groups of positive control, negative control, standard (Test 1-4) treated groups. All were housed under standard conditions of temperature (25 ± 2 °C) along with sawdust as beddings, the regular pellet diet and continuous supply of water ad-libitum, 12 h/12 h circadian rhythms to familiarize the mice with the room conditions and storage conditions. The acclimatization process on animals continued for a week before the experimental procedures. Before the commencement of experiments. The animals were fasted for 16 hours, they had access to tap water during that period22.
Anti Diarrhoeal Activity
Castor Oil Induced Diarrhoea
Swiss albino mice of either sex were initially fasted for 18 h with freely accessible water, and grouped and dosed according to the instructions under grouping and dosing. Group 1 animals (mice) were individually treated with 0.5 ml saline only referred to as positive control (saline group). Group 2 mice were given only inducer i.e., 0.5 ml castor oil orally referred to as negative control. Group 3 mice received 3mg dose Loperamide, an antidiarrheal drug known as a standard group. Group 4-5 animals were individually treated with EE at low selected dose 200mg dose orally and at high selected dose 400mg dose indicated Test 1 –2 respectively. Group 6-7 animals were treated with EAE at low selected dose 200mg dose orally and at high selected dose 400mg dose indicated Test 3 –4 respectively. One hour after treatment of the animals with saline, extract doses and standard drug Loperamide, diarrhoea will be induced by oral administration of 0.5 ml castor oil to each mouse for the presence of predictable diarrheal droppings. In addition to the number of feces and the weight of feces, the time between inducing diarrhoea and excreting the first diarrheal feces was also observed in minutes. In a separate cage with a white paper-lined floor, the animals were then housed in a transparent enclosure6,13. The paper was removed or changed every hour for a total of four hours. The feces were taken and weighed over the entire observation period. The swap in body weight between the preliminary and the post-experiment period was also calculated. Lastly, the percentages of fecal output (% FOP) and diarrheal inhibition (% inhibition of defecation) were calculated23,24.
Percentage inhibition of defecation was calculated by using the following formula
% inhibition of defecation = Negative control means – treated mean/ Negative control mean × 100
Gastrointestinal Motility Test Model
After fasting for 18 h and having free access to water, the mice will be grouped into seven groups and treated as described under the grouping and dosing section. Mice of Group 1 were given only the inducer, i.e., 0.5 ml of castor oil orally, referred to as the negative control. The standard group of mice (Group 2) was given 3mg dose of Loperamide, an antidiarrheal drug. Group 3-4 mice were treated individually with EE at low selected doses 200mg dose orally and at high selected doses 400mg dose indicated in Test 1 – 2. In groups 5 and 6, each animal was given EAE at a low selected dose of 200 mg dose orally and a high selected dose of 400 mg dose orally in Test 3 and 4 respectively One hour after treatment, each animal was given 0.5 ml of castor oil. A 5% activated charcoal suspension was administered to all mice one hour after castor oil administration. After 30 minutes of administering charcoal, the mice will be sacrificed, and the entire length of the intestine (from the pylorus to the cecum) will be removed and placed lengthwise on a white piece of paper4. Table 4 summarizes the length of the intestine traveled by the charcoal meal and the percent inhibition of intestinal transit as well as the distance traveled by the charcoal meal and the length of the intestine.The Peristalsis index can be calculated using the following formula.
Peristalsis index= Distance traveled by the charcoal meal/ Total length of the intestine ×100
Isolation of compounds from EE
In this process, various mesh sizes silica gel is used in the present experiment.The mesh size of silica gel ranges from 250 – 300. The column size is 130 cm x 6 cm. In the present work, column 100 cm height is filled and packed with a slurry of silica gel whereas the ethanol in extracts (8.0 gm) was dissolved in the respective solvent and mixed with 200 gm of silica gel. Both were mixed appropriately and dried till the EE was adsorbed onto the surface of silica gel. This dried silica with EE was loaded on the top of the packed column covered with cotton pluck to avoid reverse flotation of extraction at the time of solvent pouring. Further, a gradient of solvent was flushed from non-polar to polar series. A series of 100% Dichloromethane followed by Ethanol. A total of 130 fractions were collected and concentrated by distillation.The fractions obtained and volume of DCM and ethanol used are shown in Table 5. Each fraction was loaded on a TLC plate and developed in different solvent systems and observed under a UV lamp.
Characterisation of compound M1
The compound M1 was characterised by UV, IR, NMR and Mass Spectroscopy
Data and statistically analysis.
Based on the number of mice in each group (n = 5), The researchers team present these data as mean ± S.D. Based on values acquired from saline-positive control mice, baseline values were established. In all cases of the Castor oil-induced diarrheal model, the results procured from EE and EAE and standard drug groups were compared with those obtained from negative control animal groups. Each data/value was controlled to one-way analysis of variance (ANOVA) using Graph Prism Pad, version 8.0.1; established statistical values of P ≤ 0.001.
Results
Percentage yield of EAE and EE
The % yield of EAE and EE was found to be 2.4 % and 3.03 % respectively.
Phytochemical screening
The secondary metabolites present in PEE, EAE and EE were carbohydrates, glycosides, flavanoids, steroids, amino acids, tannins respectively.The results were shown in Table 1.
Table 1: Phytochemical Parameters of Bryophyllum pinnatum leaves.
|
Phytochemical analyzed |
Test performed |
Results |
||
|
|
PEE |
EAE |
EE |
|
|
Carbohydrates29 |
Molisch test |
– |
+ |
+ |
|
Benedicts test |
– |
+ |
+ |
|
|
Fehling s test |
– |
+ |
+ |
|
|
Alkaloids29 |
Mayer s test |
– |
+ |
+ |
|
Wagner s test |
– |
+ |
+ |
|
|
Dragendrof test |
– |
+ |
+ |
|
|
Hager s test |
– |
– |
+ |
|
|
Saponin Glycosides29 |
Foam test |
– |
+ |
+ |
|
Anthraquinone Glycosides29 |
Bontrager’s test |
– |
+ |
+ |
|
Modified Borntragers test |
– |
+ |
– |
|
|
Flavonoids29 |
Shinoda test |
– |
– |
+ |
|
Ferric chloride |
– |
– |
+ |
|
|
Cardiac Glycosides29 |
Legal test |
– |
+ |
+ |
|
Balget test |
– |
+ |
+ |
|
|
Keller Killani |
– |
+ |
+ |
|
|
Steroids29 |
Libermann Burchard |
+ |
– |
– |
|
Phytosterols |
Salkowskis test |
– |
– |
– |
|
Protein and Amino Acids29 |
Xanthoproteic |
– |
– |
– |
|
Ninhydrin test |
+ |
+ |
+ |
|
|
Millon s test |
– |
+ |
_ |
|
|
Tannins29 |
Ferric chloride |
– |
– |
– |
|
Gelatin test |
– |
+ |
+ |
|
|
Fat/Oil29 |
Sudanreagent test |
+ |
– |
– |
|
Osmic acid test |
+ |
– |
– |
|
(+) indicates the presence of phytoconstituents, (-) indicates the absence of phytoconstituents
PEE-Petroleum Ether Extract, EAE- Ethyl Acetate Extract, EE-Ethanol Extract
Inhibition of Castor oil-induced diarrhea
A mice model of diarrhoea induced by castor oil showed antidiarrheal effects for both EE, EAE. According to observation Table 2-3, EE, EAE decreased fecal output, total number of wet feces and the average weight of feces when compared to the negative (castor oil) control group. The values of percentage inhibition of defecation were also observed in Test 1, Test 2, Test 3 and Test 4. A high dose of ethanol extract (Test-2) group showed a high percentage inhibition of defecation in comparison with the negative control group (castor oil).
Table 2: Parameters observed for inhibition of castor oil-induced diarrhea.
|
Group no. |
Treatment
|
Total no. of Faeces |
Total no. of Wet Faeces |
The average weight of total Faeces(gm) |
Onset of Diarrhoea |
|
(min.) |
|||||
|
Group 1 |
Positive control (0.5 ml Saline) |
7.20+1.02 |
3.40+0.67 |
0.96+0.79 |
100.20+5.54 |
|
Group 2 |
Negative control (0.5 castor oil) |
8.0+1.41 |
7.0+1.69 |
0.89+0.96 |
87.8+2.99 |
|
Group 3 |
Loperamide (3mg dose) |
2.60+0.51 |
2.0+0.82 |
0.41+0.35 |
168.2+8.93 |
|
Group 4 |
Test 1 (EE; 200mg dose) |
6.20+0.73 |
3.80+1.00 |
0.97+0.82 |
88.80+4.69 |
|
Group 5 |
Test 2 (EE; 400mg dose) |
3.20+1.30 |
2.80+0.87 |
0.64+0.74 |
133.6+12.5 |
|
Group 6 |
Test 3 (EAE; 200mg dose) |
7.20+0.99 |
4.20+0.77 |
1.16+1.14 |
90.20+6.33 |
|
Group 7 |
Test 4 (EAE; 400mg dose) |
5.80+0.88 |
3.60+0.95 |
0.84+1.02 |
92.60+6.44 |
All values are expressed as mean ± SD of 05 mice., p value: <0.001 compare the respective group with the (negative castoroil) control group
Table 3: The average weight of wet feces and percentage inhibition of defecation.
|
Group No. |
Treatments |
The average weight of wet Faeces(gm) |
%Inhibition of Defecation |
|
Group 1 |
Positive control (0.5 ml Saline) |
0.506+0.04 |
—— |
|
Group 2 |
Negative control (0.5ml castor oil) |
1.06+19.66 |
—— |
|
Group 3 |
Loperamide (3mg dose) |
0.26+0.02 |
75.8945 |
|
Group 4 |
Test 1 (EE; 200 mg dose) |
0.69+0.11 |
34.6516 |
|
Group 5 |
Test 2 (EE; 400mg dose) |
0.37+0.10 |
65.53672 |
|
Group 6 |
Test 3 (EAE; 200mg dose) |
0.80+0.04 |
25.04708 |
|
Group 7
|
Test 4 (EAE; 400mg dose)
|
0.55+0.04
|
48.21092
|
All values are expressed as mean ± SD of 05 mice., p value: <0.001 compare the respective group with the (negative castor oil) control group.
Effects on gastrointestinal motility
As compared to the negative control group (castor oil), the EE significantly reduced the propulsion movement of charcoal meal through the gastrointestinal tract. The gastrointestinal distance travelled by the charcoal meal in the mice lessened by both ethanol and ethyl acetate, but the high dose of ethanol extract (Test 2) group showed reproducible results of percentage peristalsis movements i.e., 33.23% compared to the negative control group as shown in Table 4.
Table 4: Showing Peristalsis index
|
Group No. |
Treatments |
Length of Small Intestine |
Distance travelled by Charcoal Meal |
Peristalsis Index |
|
Group 1 |
Negative control |
57.80+0.87 |
43.20+3.56 |
74.6 |
|
Group 2 |
Loperamide (3mg dose) |
55.80+2.43 |
7.80+1.30 |
13.93 |
|
Group 3 |
Test 1 (EE; 200mg dose) |
58.60+2.60 |
35.20+3.22 |
59.86 |
|
Group 4 |
Test 2 (EE; 400mg dose) |
56.6+1.64 |
18.60+3.16 |
33.23 |
|
Group 5 |
Test 3 (EAE; 200mg dose) |
58.20+1.52 |
39.80+4.35 |
65.91 |
|
Group 6 |
Test 4 (EAE; 400mg dose) |
58.80+1.86 |
37.40+3.73 |
64.14 |
All values are expressed as mean ± SD of 05 mice., p value: <0.001 compare the respective group with the (negative castor oil) control group.
The outcome of antidiarrheal activity was established by the comparison of the test drug with that of the sample. The comparison was made between the test drugs isolated by EE, EAE at the 200mg/kg body weight and 400 mg/kg body weight. The significant effect of EE extracted drug gave the better and was nearest effect to the standard drug.
Spectral data of isolated compound
The outcome of similar TLC (Fractions 30-70) were pooled and Rf value of all of these fractions (30-70) were at 0.6 .The fractions were concentrated to yield compound M1 (21 mg). Compound M-1 in greenish-yellow pigment consisting of 5-methyl 4, 5,7-trihydroxy flavone, obtained from fraction 30-80 of ethanol extract as shown in Table 5. The UV absorption bands which occurred at 263.2 nm was classified as a flavone. Its IR spectrum constituted characteristics absorption bands for ether (1106cm-1 ) hydroxyl (3412cm-1 ), aromatic stretching (1413), carbonyl (1711cm-1 ) functional groups. The 1HNMR spectrum of compound M1 protons at δ8.280 (H-4ʹ, s, 1-OH is attached), δ9.569 (H-7, s, 2-OH attached), δ10.833 (H-5, s, 3-OH attached), δ7.373 (H-3, d, 2H), δ 2.490 (H-5ʹ methyl 3H), four aromatic protons δ6.779, 6.847, 6.912, 6.934 (H-6ʹ,3ʹ,2ʹ,8, d, 4H) respectively are observed. The electron impact mass spectrum of M1 produce a molecular peak at m/z 285.2751 (M+) corresponding to a flavone nucleus having hydroxyl groups with molecular formular C16H13O5 (m/z 285 calculated). The mass spectrum of M1 had intense ion peaks at m/z 284.2720 (M-1) which resulted due to proton migration. The above spectroscopic data suggest that compound M1 was a flavonoid and was identified as 5ʹ- methyl 4ʹ, 5,7-trihydroxy flavone.
 |
Figure 2: NMR spectrum of isolated compound M1. |
Table 5: Fractions obtained and polarity of solvent used Structure compound M1.
|
S. No. |
Volume of Ethanol (ml) |
The volume of n- DCM (ml) |
Fraction combined |
% Used in column |
Total volume (ml) |
Remarks |
|
1 |
25 |
475 |
1-10 |
5% |
500 |
Blank |
|
2 |
50 |
450 |
10-20 |
10% |
500 |
No spot |
|
3 |
50 |
450 |
20-30 |
10% |
500 |
No spot |
|
4 |
75 |
425 |
30-40 |
15% |
500 |
Greenish yellow spot |
|
5 |
75 |
425 |
40-50 |
15% |
500 |
light greenish yellow spot
|
|
6 |
75 |
425 |
50-60 |
15% |
500 |
light greenish yellow spot |
|
7 |
75 |
425 |
60-70 |
15% |
500 |
Light greenish yellow spot |
|
8 |
75 |
425 |
70-80 |
15% |
500 |
Very Light yellow spot |
|
9 |
125 |
375 |
80-90 |
25% |
500 |
No spot |
|
10 |
175 |
325 |
90-100 |
35% |
500 |
—— |
|
11 |
200 |
300 |
100-110 |
40% |
500 |
Tailing |
|
12 |
250 |
250 |
110-120 |
50% |
500 |
——- |
|
13 |
500 |
120-130 |
100% |
500 |
No spot |
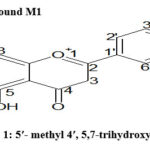 |
Scheme 1: 5ʹ- methyl 4ʹ, 5,7-trihydroxy flavone Click here to View figure |
Discussion
Traditional herbal drugs uses Bryophyllum pinnatum Lam. (Oken) leaves to treat a wide range of ailments, including diarrhoea. Therefore, this study was designed to evaluate Bryophyllum pinnatum Lam. (Oken) leaves antidiarrheal effects in albino mice using antidiarrheal activity test models. Diarrhoea is seeded by various pathophysiologic processes that are increased in luminal osmolarity and chloride secretion or decreased in the absorption of electrolytes and abnormal intestinal motility leads to a reduction in transit time of intestine25.
Castor oil-induced model has been broadly used for induction for evaluation of the antidiarrheal activity. Castor oil produces ricinoleic acid, a hydroxylated fatty acid that induces diarrhea, upon metabolism by intestinal lipase in the gut. It causes diarrhoea through various mechanisms resulting in the release of prostaglandins, via irritation of the gastrointestinal mucosa, stimulating gastrointestinal motility and electrolyte secretion, reducing the absorption of electrolytes from the stomach and colon; these are similar to the pathophysiological processes that lead to diarrhoea. To reduce such pathophysiological changes, antimotility and antisecretory agents are commonly used.
As an extracting solvent, the research team used 70 % ethanol. Hydroalcoholic solvents are customary to be more efficient and systematic in extracting the most beneficial phytoconstituents from the plant material, which is due to their high polarity range. Since solubility dictates the way thus compounds can be extracted with hydroalcoholic solvents, many compounds that are soluble in ethanol but insoluble in ethyl acetate can be extracted with hydroalcoholic solvents.
The outcome of the use of EE as an antidiarrhoeal agent is that, there has been a reduction in intestinal fluid and electrolytes accumulation by both EAE and EE. The efficacy of EE may be related to its ability to reduce the secretion of water content in feces and electrolytes into the intestinal tract while improving absorption and reducing intestinal overload/bloating, which may lead to a reduction in the number of feces and wet feces cases in treated groups, as well as diarrheal turns. This extract has an anticholinergic effect, decreasing secretion and intestinal motility significantly with P value<0.001. Additionally, several parameters relating to EE that showed antidiarrheal actions were analyzed and compared with the negative controls (castor oil group. The two serial doses of EE (Test 1 and Test 2) and EAE (Test 3 and Test 4) were establish to produce a depletion in the frequency of defecation and diarrheal output to the significant level. Moreover, at the higher dose, 400 mg dose, the plant 70% ethanol extract (Test 2) produced better results in each parameter of the model in particular to decreasing number of wet feces, the average weight of wet feces, and delaying the onset of diarrhoea when compared with the negative controls group. As the dose of both extracts was increased, the percentage inhibition in frequency of defecation increased as well. Test 2 showed that the % inhibition defecation produced by the higher dose of ethanol extract (Test 2) was nearer to that produced by the standard drug loperamide. By demonstrating a significant delay in onset of diarrhoea following the (Test 2) ethanol extract high dose combined with an increasing percentage inhibition in the amount of fecal output and diarrheal part, the research team confirm that EE inhibits diarrhoea more effectively at commensurately higher dose.
The second model, the gastrointestinal motility test model was also performed on mice. In castor oil-treated mice, EE reduced GI motility as measured by reduced movement of charcoal meal provided in suspension form through GI. As compared with a negative control (castor oil), EE produce a remarkable reduction in gastrointestinal charcoal meal movement at the low dose of 200mg dose and at the high dose of 400mg dose. The value of the peristaltic index was calculated and observed to be close to that of loperamide 3mg dose, a standard drug. In comparison to low dose, higher dose of the extract reduced the gastrointestinal motility considerably. As a result of reduced intestinal motility, fluid is absorbed more readily and the intestinal stay time increases. Therefore, the EE had profound effects on mice and showed antimotility activity.
Concerning the qualitative test for phytochemical screening of ethyl PEE, EAE and EE of Bryophyllum pinnatum Lam. (Oken) leaves. The assessment was found positive for the presence of amino acids, alkaloids, carbohydrates, flavonoids, glycosides and some other secondary constituents in plant extract as it is represented in Table 1. Reports in the literature showed that one of the phytoconstituents, flavonoids are referred as antioxidants which are responsible for suppressing the activation of prostaglandin synthesis enzymes and observed to inhibit intestinal motility and fluid/water and electrolyte secretion too26,27. Correspondingly, the EE contains flavonoids which has played a significant role in showing positive antidiarrheal actions as reported previously28. The outcome of the work proposed that EE produces a remarkable result and highest inhibition in diarrhoea droppings.
Conclusion
In conclusion, this investigation proves that EE of Bryophyllum pinnatum Lam. (Oken) leaves produce antidiarrhoeal effects. The researchers achieved success in isolating a phytocompound 5-methyl 4,5,7-trihydroxy flavone from EE. This study confirms the traditional use of the plant for diarrhoea and may pave the way for its use as new anti-diarrheal medication in the future.
Acknowledgement
The authors want to give gratitude to management, IFTM University for preparing laboratory space for successful holding experiments of proposed research work.
Conflicts of Interest
None.
Funding Sources
There is no funding source.
References
- Dubey, N.; Umre, R.; Ganeshpurkar, A.; Pandey, V. In Vitro, In Vivo and In Silico antiulcer activity of Ferulic acid. F. J. Phar. Sci. 2018, 4, 248-253.
CrossRef - Payyappallimanna, U. Role of Traditional Medicine in Primary Health Care: An overview of perspectives and challenge. Yokohama J. Soc. Sci. 2010, 14, 6.
- Che, C. T.; George, V.; Ljinu, T. P.; Andrae, P. K. Fundamentals, Applications and Strategies. Pharmacog. 2017, 2, 15-20.
CrossRef - Venkatesan, N.; Thiyagarajan, V.; Narayanan, S.; Arul, A.; Raja, S.; Kumar, S. G. V.; Rajarajan, T.; Perianayagam, J. B. Antidiarrheal potential of Asparagus racemous wild root extracts in laboratoire animals. J. Phar. Pharmaceut. Sci. 2005, 8(1), 9–45.
- Hirchhorn, N. The treatment of acute diarrhoea in children. An historical physiological perspective. Am. J. Clin. Nutri. 1980, 33, 637–663.
CrossRef - Mbagwu, H. O. C.; Adeyemi, O. O. Anti-diarrhoeal activity ofthe aqueous extract of Mezoneuron benthamianum Baill (Caesalpinaceae). J. Ethnopharmacol. 2008, 116, 16–20.
CrossRef - Suleiman, M. M.; Dzenda, T.; Sani, C. A. Antidiarrhoeal activity of the methanol stem- bark extract of Annona senegalensis Pers. (Annonaceae). J. Ethnopharmacol. 2008, 116, 125–130.
CrossRef - Fontaine, O. Bacterial diarrhea and treatment. Lancet., 1988, 331, 1234–1235.
CrossRef - Longanga, O. A.; Vercruysse, A.; Foriers, A. Contribution to the ethnobotanical, phytochemical and pharmacological studies of traditionally used medicinal plant in the treatment of dysentery and diarrhea in Lomela area. J. Ethnopharmacol. 2000, 71(3), 411–423.
CrossRef - Snyder, J. D.; Merson, M. H. The magnitude of the global problem of acute diarrhoeal disease: a review of acute surveillance data. Bulletin of WHO. 1982, 60, 604–613.
- Abdullahi, R.; Haque, M. Preparation of Medicinal Plants: Basic extraction and fractionation procedures for experimental purposes. J. Phar. Bio. Sci. 2020, 12, 1-10.
CrossRef - Adeyemi, O.O.; Akindele, A. J. Antidiarrhoeal activity of the ethyl acetate extract of Baphia nitida” (Papilionaceae). J. of Ethnopharmacol. 2008, 116, 407–412.
CrossRef - Abera, B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J. Ethnobio. Ethnomed. 2014, 10, 40.
CrossRef - Thorat, S. S.; Rutunja, R. S.; Swapnali, A. M.; Naziya, R. P. A review of Bryophyllum pinnatum. I. Res. J. Pharm. 2017, 8 (12), 1-3.
CrossRef - Ojewole, J. A. O. Antinociceptive, anti-inflammatory and antidiabetic effects of Bryophyllum pinnatum (Crassulaceae) leaf aqueous extract. J. Ethnopharmacol. 2005, 99, 13-19.
CrossRef - Eboka, J.C. Effects of aqueous leaf extract of Bryophyllum pinnatum on guinea pig tracheal ring contractility. Niger. J. Physio. Sci. 2010,149-157.
- Pattewar, S.V. Kalanchoe pinnata: Phytochemical and Pharmacological Profile. Int J. Pharm Sci Res. 2012, 3(4), 993-1000.
CrossRef - Jensen, W. B. J. Chem. 2007, 84, 1913-1914.
CrossRef - Thamkaew, G.; Sjoholm, I.; Galindo, F. A review of drying methods for improving the quality of dried herbs. Food Sci. Nutri. 2021, 61, 1763-1786.
CrossRef - Evans, W. C.; Trease. Els. H. Sci. 2009, 16.
- Khandelwal, K. R. Prac Pharmacog. Delhi. 2002, 9, 149-153.
- Fawcett, B. A. A. Animal Research Review: Guidelines for the Housing of Mice in Scientific Institutions, 2016.
- Mekonnen, B.; Assrie, B. A.; Wubneh, B. Z. Antidiarrheal activity of 80% methanolic extract of Justicia schimperiana. Evid. Compl Alt. Med. 2018, 20, 1-10.
CrossRef - Rahman, K.; Barua, S.; Islam, F.; Islam, R.; Sayeed, A. M.; Parveen, S. Studies on the antidiarrheal properties of leaf extract of Desmodium puchellum. Asian Pac. J. Trop. Biomed. 2013, 3(8), 639-643.
CrossRef - Sarker, S. D.; Latif, Z.; Gray, A. I. Methods in biotechnology. Nat. prod. 2006, 269–273.
CrossRef - Mora, A.; Paya, M.; Rios, J,; Alcaraz, M. J. Structure activity relationships of polymethoxy flavones and other flavonoids as inhibitors of nonenzymic lipid peroxiation. Biochem Pharmacol. 1990; 36, 317–32.
- Okunji, C. O., Iwu, M. M. Phytochemical screening of Pyrenacantha staudtii. Inter J. Crude Drug Res. 1988, 4, 246-252.
CrossRef - Rauf, A.; Rashid, U.; Bawazeer, S.; Khan, K.;Aljohani, A. S.M.; Mubarak, M.S.; Khan, H.; Mishra, A. P. In Vivo and In Silico Studies of Flavonoids Isolated from Pistacia integerrima as Potential Antidiarrheal Agents, Am. Chem. Soc. 2021, 6, 15617–15624.
CrossRef - Priyanka, Y.; Arun, K, M.; Harpreet, S.; A Recent Review on Chemistry and Biological Activities of Bryophyllum Pinatum (Lam.) Oken Family: Crassulaceae. Oriental Journal of chemistry, 2, 37.

This work is licensed under a Creative Commons Attribution 4.0 International License.










