Thermodynamic Properties of Binary Liquid Mixtures of Furfural with Toluene and Nitro Benzene at Varying Temperatures
Revathi Uthirapathi1 , Uma Sivakami Krishnamoorthy2 and Rose Venis Ambrose1*
, Uma Sivakami Krishnamoorthy2 and Rose Venis Ambrose1*
1Department of Chemistry, St. Joseph’s College (Autonomous), Affiliated to Bharathidasan University, Tiruchirappalli, India.
2Department of Chemistry, Cauvery College for Women, Affiliated to Bharathidasan University, Tiruchirappalli, India.
Corresponding Author E-mail: rosevenis@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380423
Article Received on : 12 Jul 2022
Article Accepted on :
Article Published : 18 Aug 2022
Binary liquid mixtures of furfural with toluene and nitrobenzene were examined for ultrasonic velocity, viscosity and density at a temperature of 308.15 and 318.15 K in various mole fractions. The calculated thermodynamic properties from density, viscosity, ultrasonic velocity and some excess parameters like Excess Volume(VE), Deviation in Isentropic Compressibility(∆KS), Deviation in Viscosity(∆η), Deviation in Intermolecular Free Length(∆LF), Deviation in Intermolecular Free Volume(∆VF) and Deviation in Acoustic Impedance(∆Z) were determined and found the proper coefficients for a polynomial equation of the Redlich - Kister type, from which the theoretical values were calculated. The interaction ability of the binary liquid mixtures was investigated, as well as the deviations of the binary liquid mixtures from their ideal behavior were studied based on the experiment and theoretical values.
KEYWORDS:Binary liquid mixtures; Density; Molecular interaction; Redlich- Kister equation; Viscosity
Download this article as:| Copy the following to cite this article: Uthirapathi R, Krishnamoorthy U. S, Ambrose R. V. Thermodynamic Properties of Binary Liquid Mixtures of Furfural with Toluene and Nitro Benzene at Varying Temperatures. Orient J Chem 2022;38(4). |
| Copy the following to cite this URL: Uthirapathi R, Krishnamoorthy U. S, Ambrose R. V. Thermodynamic Properties of Binary Liquid Mixtures of Furfural with Toluene and Nitro Benzene at Varying Temperatures. Orient J Chem 2022;38(4). Available from: https://bit.ly/3CeMkeu |
Introduction
Pharmaceutical industries have undergone a major transformation in recent years thanks to ultrasonic analysis of binary liquid mixtures. Utilizing ultrasonic technology is a potent method for understanding the molecular interactions of liquid mixtures1. Complex developments in liquid mixtures have been interpreted as excess thermodynamic properties. These discrepancies were explained as being caused by strong or weak interactions. The thermodynamic and transport characteristics of pure liquid and liquid mixtures can be used to investigate the nature of molecular interactions (either intermolecular or intra molecular) between the mixing liquids as reported by Sekhar et al2. Physical properties like density, viscosity, excess volume, isentropic properties, free length, free volume and acoustic impedance are studied to better understand the environment and strength of intermolecular and intra molecular interactions in multi-component liquid mixtures. Engineering process design and operation also heavily reply on the thermodynamic and transport features of liquid mixtures3. For estimating thermodynamic properties, there exist numerous prediction equations. In this study experimental results are used in studying thermodynamic properties of binary liquid mixtures.
The definition of “biomass” includes dedicated energy plants and trees, aquatic plants, animal waste, agricultural food and feed crop residues, timber, wood residues and other waste resource4. Furfural is one of the byproducts of the pyrolysis of biomass containing lignocelluloses. It is utilized as a feedstock for the creation of different resins in the pharmaceutical and agrochemical sectors. Furfural is also used or created in the pulp, paper and food sectors as reported by Muhammad et al5. Nitrobenzene is a versatile solvent that is commonly used in synthetic and electrochemical research, a crucial raw material in the production of explosives was reported by Uma et al6. At 308.15K and 318.15K, the transport and thermodynamic properties of prepared liquids of binary mixtures were investigated over the entire range of compositions7. Research of their binary liquid mixtures, which gain increasing significance because it more accurately simulates complex real time molecular interaction, is the main objective of this work. Thus the binary liquid mixtures of furfural + toluene, furfural+ nitrobenzene and toluene + nitro benzene were measured for ultrasonic velocity, viscosity, density and other estimated excess thermodynamic properties at 308.15K and 318.15K.
Experiment method
Chemical: Furfural (SRL), Toluene (Molychem) and Nitrobenzene (ACS) used were of high purity (<99%). Table-1 shows the thermo physical characteristics of the investigated components.
The purity was further confirmed by comparing the measured values of ultrasonic velocity, viscosity, density with report in the literature, which showed a satisfactory agreement and shown in Table 2. Binary liquid mixtures of various compositions were prepared by volume by weight method, by mixing a constant quantity of pure liquid in airtight stopper bottle8 of 50ml capability using an analytical balance with a 0.0001g precision.
Table 1: Material description:
|
Chemical name |
Molecular formula |
Molar mass (g.mol-1) |
Stated purity (mol %) |
CAS Number |
|
Furfural |
C5H4O2 |
96.08 |
99 |
98-01-1 |
|
Toluene |
C7H8 |
92.14 |
99.3 |
108-88-3 |
|
Nitrobenzene |
C6H5NO2 |
123.11 |
99 |
98-95-3 |
Table 2: Comparison of experimental Ultrasonic velocity (U), Viscosity (η) and Density (ρ) in pure liquids with journalism value by 308.15 and 318.15K.
|
LIQUIDS |
T(K) |
ρ(g cm-3) |
η(mPa s-1) |
U(ms-1) |
|||
|
Experimental values |
Literature values |
Experimental values |
Literature values |
Experimental values |
Literature values |
||
|
Furfural9, 10 |
308.15 |
1.1447 |
1.1440 |
1.2616 |
1.2600 |
1406.5 |
1403.77 |
|
318.15 |
1.1324 |
1.1330 |
1.0921 |
1.0900 |
1370.5 |
1367.81 |
|
|
Toluene11 |
308.15 |
0.8374 |
0.8378 |
0.5087 |
0.5099 |
1253.5 |
1250 |
|
318.15 |
0.8396 |
– |
0.3824 |
– |
1256 |
– |
|
|
Nitrobenzene12 |
308.15 |
0.7980 |
0.7979 |
2.0081 |
2.0080 |
1218 |
1209 |
|
318.15 |
1.1780 |
1.1773 |
1.2071 |
1.2061 |
1418 |
1412.5 |
|
Densities of pure liquids and liquid mixtures were measured by specific gravity method with 10mL relative density bottle and weighed with an exactness of ± 0.001 kg m-3. Viscosities were determined by Oswald viscometer 10mL capability with an accurateness of ± 0.001 cP. From the measured values of density and flow time ‘t’, viscosity ‘η’ was calculated12. The values of constants were occurred by measuring the flow time with distilled water and pure nitrobenzene as standard liquids. The flow time were measured with electronic stop clock. The ultrasonic velocity values were measured using an ultrasonic interferometer13 (Pico, Chennai, India) with a frequency of 2MHz was calibrated using water and nitrobenzene. The overall accuracy in the measurement is ±0.2%. All the measurements were taken at 308.15 and 318.15K with a temperature accuracy of 0.01K using a digital thermostat. A Perkin Elmer spectrum RX1 was used to record the IR spectra (PerkinElmer, inc., Waltham, MA, USA).
Result and discussion
The following standard formulae are used to calculate the thermodynamic properties based on the investigated data.
Density (ρ)
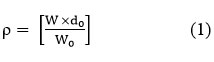
Where ‘w’ is the mass of the liquid or liquid mixtures, ‘w0’ is the mass of the water, and ‘d0’ is the density of the water.
Excess Volume(VE)
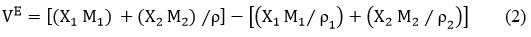
“ρ”- density of the liquid mixtures and X1, M1 and ρ1, X2, M2 and ρ2 – mole fraction, molar mass, and density of pure components 1 & 2 respectively14.
Isentropic Compressibility(KS)
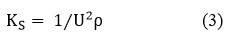
Where, “U” – speed of sound and “ρ” – density of the liquid mixtures.
Deviation in Isentropic Compressibility(∆KS)
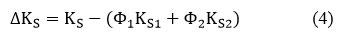
Where “∆KS” denotes the mixtures isentropic compressibility, Φ1,K1,S1 and Φ2, K2 S2 denotes the volume fraction and isentropic compressibility of pure components 1 & 2, respectively.
Viscosity (η)
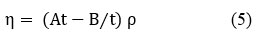
Where “ρ” denotes the density of a pure liquid or a mixture of liquids, “t” denotes the time flow in seconds and A & B characteristic constants at specified temperature.
Excess Viscosity (∆η)
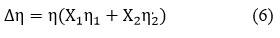
Where, η1 & η2 are the corresponding pure component 1 & 2 viscosity values15.
Table 3: Binary liquid mixtures of furfural, toluene and nitrobenzene with physical and thermodynamic properties at 308.15 and 318.15K.
|
308.15K |
318.15K |
|||||||||||||||
|
X1 |
ρ (g cm-3) |
η (mPa.s-1) |
U (ms-1) |
∆VE (cm3mol-1) |
∆Ks (Tpa-1) |
X1 |
ρ (g cm-3) |
η (mPa.s-1) |
U (ms-1) |
∆VE (cm3mol-1) |
∆Ks (Tpa-1) |
|||||
|
Furfural + toluene |
Furfural + toluene |
|||||||||||||||
|
0.0000 |
0.8539 |
0.5093 |
1268 |
0.0000 |
0.0000 |
0.0000 |
0.8407 |
0.3771 |
1255 |
0.0000 |
0.0000 |
|||||
|
0.1365 |
0.8866 |
0.6374 |
1304 |
-0.0928 |
-33.6016 |
0.1365 |
0.8731 |
0.4868 |
1286 |
-0.0784 |
-31.9250 |
|||||
|
0.1941 |
0.9009 |
0.6893 |
1318 |
-0.1296 |
-44.1411 |
0.1941 |
0.8875 |
0.5354 |
1298 |
-0.1119 |
-42.0773 |
|||||
|
0.2999 |
0.9285 |
0.7815 |
1341 |
-0.2020 |
-57.7428 |
0.2999 |
0.9150 |
0.6220 |
1318 |
-0.1879 |
-55.8189 |
|||||
|
0.4302 |
0.9642 |
0.8895 |
1365 |
-0.2730 |
-65.6184 |
0.4302 |
0.9508 |
0.7235 |
1339 |
-0.2557 |
-64.0679 |
|||||
|
0.5172 |
0.9891 |
0.9580 |
1378 |
-0.2879 |
-65.5631 |
0.5172 |
0.9756 |
0.7880 |
1349 |
-0.2684 |
-64.1010 |
|||||
|
0.6013 |
1.0138 |
1.0198 |
1387 |
-0.2718 |
-61.2051 |
0.6013 |
1.0005 |
0.8453 |
1356 |
-0.2572 |
-59.7283 |
|||||
|
0.7133 |
1.0481 |
1.0973 |
1397 |
-0.2171 |
-50.4220 |
0.7133 |
1.0349 |
0.9183 |
1362 |
-0.1974 |
-48.6961 |
|||||
|
0.7895 |
1.0725 |
1.1468 |
1401 |
-0.1670 |
-40.0863 |
0.7895 |
1.0594 |
0.9669 |
1365 |
-0.1466 |
-38.4659 |
|||||
|
0.8917 |
1.1066 |
1.2098 |
1405 |
-0.0927 |
-22.5566 |
0.8917 |
1.0938 |
1.0282 |
1366 |
-0.0783 |
-21.5308 |
|||||
|
1.0000 |
1.1447 |
1.2685 |
1406 |
0.0000 |
0.0000 |
1.0000 |
1.1324 |
1.0921 |
1366 |
0.0000 |
0.0000 |
|||||
|
Furfural + nitro benzene |
Furfural + nitro benzene |
|||||||||||||||
|
0.0000 |
1.1863 |
1.6194 |
1439 |
0.0000 |
0.0000 |
0.0000 |
1.1780 |
1.2071 |
1418 |
0.0000 |
0.0000 |
|||||
|
0.1247 |
1.1818 |
1.5642 |
1430 |
0.0228 |
2.5496 |
0.1247 |
1.1731 |
1.1859 |
1408 |
0.0137 |
2.1643 |
|||||
|
0.1915 |
1.1792 |
1.5361 |
1426 |
0.0340 |
3.8905 |
0.1915 |
1.1704 |
1.1747 |
1403 |
0.0230 |
3.2813 |
|||||
|
0.2797 |
1.1758 |
1.5011 |
1420 |
0.0480 |
5.4050 |
0.2797 |
1.1667 |
1.1603 |
1396 |
0.0350 |
5.0073 |
|||||
|
0.3983 |
1.1711 |
1.4564 |
1414 |
0.0613 |
6.8765 |
0.3983 |
1.1615 |
1.1429 |
1388 |
0.0486 |
6.4193 |
|||||
|
0.5048 |
1.1667 |
1.4187 |
1410 |
0.0674 |
7.0300 |
0.5048 |
1.1567 |
1.1298 |
1383 |
0.0558 |
6.5400 |
|||||
|
0.5958 |
1.1629 |
1.3879 |
1407 |
0.0639 |
6.5103 |
0.5958 |
1.1525 |
1.1199 |
1379 |
0.0513 |
6.0733 |
|||||
|
0.6835 |
1.1592 |
1.3604 |
1406 |
0.0536 |
5.3596 |
0.6835 |
1.1485 |
1.1121 |
1376 |
0.0409 |
4.9573 |
|||||
|
0.7911 |
1.1544 |
1.3274 |
1404 |
0.0377 |
3.6358 |
0.7911 |
1.1433 |
1.1039 |
1372 |
0.0251 |
3.3456 |
|||||
|
0.8793 |
1.1504 |
1.3018 |
1403 |
0.0225 |
2.3018 |
0.8793 |
1.1389 |
1.0983 |
1369 |
0.0122 |
2.0129 |
|||||
|
1.0000 |
1.1447 |
1.2685 |
1401 |
0.0000 |
0.0000 |
1.0000 |
1.1324 |
1.0912 |
1365 |
0.0000 |
0.0000 |
|||||
|
Toluene + Nitro benzene |
Toluene + Nitro benzene |
|||||||||||||||
|
0.0000 |
1.1863 |
1.6216 |
1439 |
0.0000 |
0.0000 |
0.0000 |
1.1780 |
1.2071 |
1418 |
0.0000 |
0.0000 |
|||||
|
0.1379 |
1.1403 |
1.5431 |
1436 |
-0.1422 |
-27.0357 |
0.1379 |
1.1304 |
1.1521 |
1408 |
-0.1186 |
-24.4083 |
|||||
|
0.2540 |
1.1019 |
1.4673 |
1430 |
-0.2661 |
-46.2203 |
0.2540 |
1.0910 |
1.1085 |
1400 |
-0.2404 |
-42.6919 |
|||||
|
0.3655 |
1.0650 |
1.3959 |
1420 |
-0.3596 |
-60.2163 |
0.3655 |
1.0533 |
1.0674 |
1390 |
-0.3374 |
-57.2814 |
|||||
|
0.4608 |
1.0333 |
1.3262 |
1409 |
-0.4025 |
-68.8240 |
0.4608 |
1.0210 |
1.0301 |
1380 |
-0.3804 |
-66.1278 |
|||||
|
0.5797 |
0.9937 |
1.2131 |
1391 |
-0.4176 |
-73.8138 |
0.5797 |
0.9807 |
0.9491 |
1363 |
-0.3978 |
-71.6304 |
|||||
|
0.6612 |
0.9665 |
1.1100 |
1375 |
-0.3961 |
-72.2344 |
0.6612 |
0.9530 |
0.8705 |
1348 |
-0.3711 |
-69.9959 |
|||||
|
0.7477 |
0.9375 |
0.9828 |
1354 |
-0.3335 |
-65.0348 |
0.7477 |
0.9236 |
0.7562 |
1329 |
-0.3063 |
-62.3279 |
|||||
|
0.8382 |
0.9070 |
0.8213 |
1329 |
-0.2269 |
-50.2404 |
0.8382 |
0.8927 |
0.6161 |
1304 |
-0.1933 |
-46.4829 |
|||||
|
0.9234 |
0.8785 |
0.6568 |
1303 |
-0.1142 |
-30.1785 |
0.9234 |
0.8640 |
0.4819 |
1281 |
-0.0843 |
-26.8109 |
|||||
|
1.0000 |
0.8530 |
0.5087 |
1272 |
0.0000 |
0.0000 |
1.0000 |
0.8385 |
0.3728 |
1255 |
0.0000 |
0.0000 |
|||||
Table 4: Binary liquid mixtures of furfural, toluene and nitrobenzene with thermodynamic parameters at 308.15 and 318.15K.
|
308.15K |
318.15K |
||||||||||
|
∆η(mPa.s) |
∆LF(10-10m) |
∆VF(10-14 m3mol-1) |
∆Z (kgm-3 s-1)
|
∆π(Pa)
|
∆η(mPa.s) |
∆LF(10-10 m) |
∆VF(10-14 m3mol-1) |
∆Z(kgm-3 s-1)
|
∆π(Pa)
|
||
|
Furfural + toluene |
Furfural + toluene |
||||||||||
|
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
||
|
0.0245 |
-69.3918 |
-2.0867 |
1.3139 |
-16.7556 |
0.0121 |
-65.8607 |
-2.9049 |
0.6505 |
-42.45 |
||
|
0.0326 |
-91.2414 |
-2.7789 |
2.3241 |
-24.8084 |
0.0195 |
-86.8810 |
-4.2192 |
1.4168 |
-51.33 |
||
|
0.0444 |
-119.3095 |
-3.0015 |
4.1727 |
-38.0188 |
0.0305 |
-115.6168 |
-5.1773 |
3.4251 |
-65.79 |
||
|
0.0536 |
-135.2528 |
-2.8141 |
6.5523 |
-49.1167 |
0.0388 |
-132.4920 |
-5.0490 |
5.9304 |
-78.96 |
||
|
0.0561 |
-134.3648 |
-2.5303 |
7.4868 |
-52.5884 |
0.0411 |
-131.6366 |
-4.5624 |
6.7039 |
-83.04 |
||
|
0.0540 |
-123.8933 |
-2.1773 |
6.9154 |
-54.8138 |
0.0383 |
-120.9535 |
-3.9004 |
5.9345 |
-87.40 |
||
|
0.0465 |
-100.0258 |
-1.6306 |
5.4095 |
-51.6059 |
0.0312 |
-95.9066 |
-2.8593 |
3.6993 |
-83.95 |
||
|
0.0382 |
-78.3005 |
-1.2233 |
4.0633 |
-44.2965 |
0.0252 |
-74.1736 |
-2.0826 |
2.1980 |
-71.93 |
||
|
0.0236 |
-43.0051 |
-0.6473 |
1.8829 |
-24.0848 |
0.0135 |
-40.2638 |
-0.9713 |
0.5555 |
-47.06 |
||
|
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
||
|
Furfural + nitro benzene |
Furfural + nitro benzene |
||||||||||
|
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
||
|
-0.0115 |
4.3217 |
0.0115 |
-3.7922 |
-1.8701 |
-0.0067 |
2.2743 |
0.0077 |
-2.5937 |
-183.24 |
||
|
-0.0161 |
6.8553 |
0.0157 |
-5.8448 |
-2.7514 |
-0.0102 |
3.7780 |
0.0122 |
-4.0110 |
-270.70 |
||
|
-0.0202 |
9.8032 |
0.0191 |
-8.1359 |
-3.7620 |
-0.0144 |
6.9153 |
0.0159 |
-6.4559 |
-372.14 |
||
|
-0.0232 |
12.7522 |
0.0213 |
-10.2851 |
-4.8025 |
-0.0181 |
9.3357 |
0.0194 |
-8.2624 |
-478.70 |
||
|
-0.0236 |
12.7193 |
0.0227 |
-10.2038 |
-5.3640 |
-0.0188 |
9.0775 |
0.0197 |
-8.0838 |
-537.57 |
||
|
-0.0224 |
11.3152 |
0.0229 |
-9.1052 |
-5.4983 |
-0.0181 |
7.8316 |
0.0187 |
-7.1332 |
-553.30 |
||
|
-0.0192 |
8.5729 |
0.0209 |
-7.0509 |
-5.2508 |
-0.0158 |
5.3490 |
0.0157 |
-5.3099 |
-530.94 |
||
|
-0.0144 |
5.0568 |
0.0173 |
-4.3512 |
-4.3523 |
-0.0115 |
2.5552 |
0.0104 |
-3.0851 |
-441.46 |
||
|
-0.0090 |
3.0487 |
0.0111 |
-2.6407 |
-2.9897 |
-0.0068 |
1.0952 |
0.0045 |
-1.6378 |
-303.99 |
||
|
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
||
|
Toluene + nitro benzene |
Toluene + nitro benzene |
||||||||||
|
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
||
|
0.0750 |
-85.8255 |
-0.9309 |
16.0737 |
0.5906 |
0.0600 |
-77.8833 |
-2.3346 |
6.9317 |
52.66 |
||
|
0.1284 |
-146.2334 |
-1.7002 |
26.5000 |
1.1784 |
0.1134 |
-135.8125 |
-4.3140 |
13.8556 |
110.57 |
||
|
0.1810 |
-189.6352 |
-2.4431 |
32.4099 |
1.8984 |
0.1652 |
-181.2138 |
-6.2184 |
19.9261 |
179.03 |
||
|
0.2174 |
-215.3029 |
-3.0599 |
35.3079 |
2.5604 |
0.2074 |
-207.8299 |
-7.8437 |
23.1242 |
247.74 |
||
|
0.2367 |
-228.5513 |
-3.7575 |
35.5711 |
3.2726 |
0.2257 |
-222.9055 |
-9.6586 |
24.8186 |
313.77 |
||
|
0.2243 |
-221.8295 |
-4.1340 |
32.9354 |
3.5144 |
0.2151 |
-216.1674 |
-10.6917 |
23.1399 |
336.81 |
||
|
0.1932 |
-197.6301 |
-4.3798 |
27.5709 |
3.4791 |
0.1729 |
-190.8078 |
-11.2770 |
18.8687 |
314.22 |
||
|
0.1326 |
-150.5973 |
-4.2016 |
19.4525 |
2.8194 |
0.1083 |
-140.8855 |
-10.7332 |
11.9111 |
236.66 |
||
|
0.0628 |
-88.5151 |
-3.0689 |
11.6661 |
1.5761 |
0.0453 |
-79.7547 |
-7.6340 |
6.7243 |
120.52 |
||
|
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
0.0000 |
||
Table 5: Standard deviation and coefficients of the Redlich – Kister polynomial equation at various temperatures.
|
T/K |
a |
b |
c |
σ |
|
Furfural + Toluene |
||||
|
VE (cm3mol-1) |
||||
|
308.15 |
-1.1077 |
-0.0211 |
0.0607 |
0.0052 |
|
318.15 |
-1.0365 |
-1.0365 |
0.0749 |
0.0064 |
|
η (mPa.s) |
||||
|
308.15 |
3.7769 |
0.3818 |
0.8864 |
0.0803 |
|
318.15 |
3.0976 |
0.3608 |
0.7262 |
0.0657 |
|
∆KS(Tpa-1) |
||||
|
308.15 |
-256.2960 |
14.3467 |
1.4753 |
0.4308 |
|
318.15 |
-249.5330 |
13.8062 |
1.0171 |
0.2795 |
|
∆η(mPa.s) |
||||
|
308.15 |
-3.7770 |
0.3818 |
0.8864 |
0.0339 |
|
318.15 |
3.0977 |
0.3608 |
0.0000 |
0.0007 |
|
∆Z (kg m-3 s-1) |
||||
|
308.15 |
27.5603 |
1.1563 |
-2.8911 |
0.2467 |
|
318.15 |
23.7754 |
0.5017 |
-4.2782 |
0.3723 |
|
∆LF (10-10 m) |
||||
|
308.15 |
5.3507 |
0.1338 |
0.0242 |
0.8156 |
|
318.15 |
-0.1712 |
0.2266 |
-0.4349 |
0.0106 |
|
∆VF (10-14 m3mol-1) |
||||
|
308.15 |
1.0478 |
1.1510 |
-0.6172 |
0.9227 |
|
318.15 |
1.8818 |
1.6216 |
-0.1046 |
0.1436 |
|
∆π(10-05 Nm-2) |
||||
|
308.15 |
215.1630 |
-1.1856 |
0.3749 |
0.1808 |
|
318.15 |
-341.1850 |
-1.2568 |
-1.5414 |
0.2334 |
|
Furfural + nitro benzene |
||||
|
VE(cm3mol-1) |
||||
|
308.15 |
0.2603 |
0.0008 |
-0.0119 |
0.0011 |
|
318.15 |
0.2045 |
0.2045 |
-0.0544 |
0.0009 |
|
η (mPa.s) |
||||
|
308.15 |
5.6814 |
-0.1735 |
1.4437 |
0.1278 |
|
318.15 |
4.8017 |
-1.3069 |
3.0813 |
0.0507 |
|
∆KS(Tpa-1) |
||||
|
308.15 |
27.0760 |
-1.2965 |
-1.2180 |
0.0746 |
|
318.15 |
24.7860 |
-2.8112 |
0.8736 |
0.0995 |
|
∆η(mPa.s) |
||||
|
308.15 |
5.6814 |
-0.1735 |
1.4438 |
0.0001 |
|
318.15 |
4.8018 |
-1.3070 |
3.0813 |
0.0002 |
|
∆Z (kg m-3 s-1) |
||||
|
308.15 |
-39.5562 |
1.2791 |
2.3476 |
0.2014 |
|
318.15 |
-30.4981 |
1.5467 |
2.3128 |
0.1832 |
|
∆LF (10-10 m) |
||||
|
308.15 |
0.4894 |
-0.0150 |
-0.0358 |
0.0031 |
|
318.15 |
0.0018 |
-0.0252 |
0.0371 |
0.0003 |
|
∆VF (10-14 m3mol-1) |
||||
|
308.15 |
0.2177 |
-0.0006 |
-0.0491 |
0.0084 |
|
318.15 |
0.0629 |
-0.0436 |
0.0659 |
0.0058 |
|
∆π(10-05 Nm-2) |
||||
|
308.15 |
-215.3550 |
-10.3025 |
-2.1602 |
0.1470 |
|
318.15 |
-215.6990 |
-36.1059 |
32.0132 |
0.6720 |
|
Toluene+ nitro benzene |
||||
|
VE(cm3mol-1) |
||||
|
308.15 |
-1.6605 |
-0.0509 |
0.0586 |
0.0032 |
|
318.15 |
-1.5761 |
-1.5761 |
0.0908 |
0.0068 |
|
η (mPa.s) |
||||
|
308.15 |
5.1702 |
-0.5147 |
1.0276 |
0.0773 |
|
318.15 |
4.0199 |
-0.3777 |
0.7297 |
0.0526 |
|
∆KS(Tpa-1) |
||||
|
308.15 |
-288.9300 |
-16.2319 |
-0.2111 |
0.6829 |
|
318.15 |
-278.3900 |
-16.6389 |
3.6765 |
0.3346 |
|
∆η(mPa.s) |
||||
|
308.15 |
5.1702 |
-0.5147 |
1.0277 |
0.0019 |
|
318.15 |
4.0200 |
-0.3777 |
0.7297 |
0.0042 |
|
∆Z (kg m-3 s-1) |
||||
|
308.15 |
143.1650 |
1.3677 |
-0.0900 |
0.0438 |
|
318.15 |
95.5696 |
3.8653 |
-5.0718 |
0.3308 |
|
∆LF (10-10 m) |
||||
|
308.15 |
-9.0176 |
-0.0399 |
0.0102 |
0.0158 |
|
318.15 |
0.1733 |
0.3654 |
-0.5878 |
0.0415 |
|
∆VF (10-14 m3mol-1) |
||||
|
308.15 |
-13.9947 |
-2.1795 |
-1.0539 |
0.1903 |
|
318.15 |
-3.6067 |
-5.5960 |
-2.4651 |
0.3295 |
|
∆π(10-04 Nm-2) |
||||
|
308.15 |
120.0240 |
16.1399 |
-1.8502 |
0.4417 |
|
318.15 |
115.1310 |
13.9889 |
-5.0257 |
0.0602 |
For binary liquid mixtures
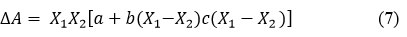
From this theoretical values were calculated16
Using the theoretical values, the relation was used to determine the standard deviation values
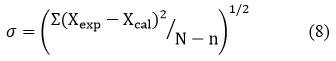
Where, the number of data points is N and the number of coefficients is n.
All of the predicted excess parameters were fitted to a polynomial equation of the Redlich -Kister type using17 the least squares methods to get the adjustment parameters a, b and c.
 |
Figure 1: Excess Volume(VE) relative to the mole fraction(X1) of furfural at 308.15K &318.15K for the binary liquid mixtures of furfural (FF) with Toluene (T) and Nitro benzene(NB). |
VE and ∆KS values of Furfural + Toluene are negative (Fig 1 and 2) and Toluene + Nitro benzene are more negative (Fig1 & 2) across the entire composition range. The methyl (electron-donating) group in toluene releases electron toward the benzene ring mainly due to hyper conjugation and partly due to inductive effect18. In toluene hyper conjugation overcomes the inductive effect. As a result, the negative charge on the toluene molecules –CH3 groups is stabilized, allowing the hydrogen atom to have a positive charge. Due to the hyper conjugation nature of the toluene molecule, there may be a contact between the positive charge on the hydrogen atom of the toluene molecule and the negative charge on the O atom of furfural molecule and nitrobenzene molecule in the two liquid mixtures, resulting in a partial H- bond. Two liquid mixtures are excess volume increases the temperature increase. As the temperature increases, molecules gain thermal energy, which affects the breakdown of intermolecular interaction between dissimilar molecules.
 |
Figure 2: Deviation in Isentropic Compressibility (∆KS) relative to the volume fraction(Φ1) of furfural at 308.15K & 318.15K for the binary liquid mixtures of furfural (FF) with Toluene (T) and Nitro benzene(NB). |
VE and ∆KS values of Furfural + Nitro benzene are positive (Fig1& 2), indicating reduced interaction across the entire composition range. Observed excess volume may be broken down into physical, chemical and geometrical effects19. Dispersion forces and non-specific physical interactions make up the majority of physical interaction that contributes positively. Furfural’s oxygen atom deviates from the nitro group’s oxygen atom, involving the forces of Vander Waals in the process. Dispersive forces, which show a weak chemical contact between dissimilar molecules, have been linked in this study to positive excess volume deviations. VE decreases with temperature increases, showing the interaction increases with temperature increases. The molecules are activated by thermal energy, which also speeds up the connection of molecules that are dissimilar to one another. As a result, interacting molecules have more incredible energy when approaching components at higher temperatures.
 |
Figure 3: Deviation in Viscosity(∆η) relative to the mole fraction(X1) of furfural at 308.15K & 318.15K for the binary liquid mixtures of furfural (FF) with Toluene (T) and Nitro benzene(NB). |
 |
Figure 4: Deviation in Intermolecular Free Length(∆LF) relative to the Mole Fraction(X1) of furfural at 308.15K & 318.15K for the binary liquid mixtures of furfural (FF) with Toluene (T) and Nitro benzene(NB). |
∆η values of Furfural + Toluene and Toluene + Nitro benzene are positive (Fig 3) and furfural + Nitro benzene are negative (Fig 3) over the entire range of composition. Toluene + nitrobenzene liquid mixtures have a higher intermolecular interaction than furfural + toluene liquid mixtures, as indicated by the positive value20. The negative value of deviates more from ideality, indicating that there is a dispersion forces between furfural and nitrobenzene. The positive values of binary liquid mixtures are greater than furfural + toluene and lower than toluene + nitro benzene. The graph’s values are in the same order as the VE and ∆KS values.
 |
Figure 5: Deviation in Acoustic Impedance(∆Z) relative to the mole fraction(X1) of furfural at 308.15K & 318.15K for the binary liquid mixtures of furfural (FF) with Toluene (T) and Nitro benzene(NB). |
The observed value of ∆LF, ∆VF, ∆πreflect the same ideal as obtained above. Due to an increase in the thermal movements of interacting molecules, the nature of interaction for the three liquid mixtures changes as the temperature rises. Dispersion forces between the mixed liquids cause negative values, whereas attractive forces like dipole –dipole interaction. ∆Z be haves in a manner opposed to ∆LF, the positive and negative deflection of mixtures indicates the degree of association or dissociation between the mixing components21. The measured values of ∆η and ∆Z are positive and negative across the whole composition range (Fig.3&5), which both strongly and weakly support the aforementioned concept. The systems interact in the following order: furfural + nitro benzene < furfural +toluene < toluene + nitro benzene.
There is excellent agreement between experimental and anticipated results for the degree polynomial solution. Table 5 displays the outcomes in terms of the parameters a, b, c & σ. The degree polynomial solution produced using VE, ∆KS, η, ∆η, ∆Z, ∆LF, ∆VF and ∆πwas found to be in good agreement with the Redlich – Kister parameters.
FT-IR Results
Figures 6 to 8 show FTIR results for toluene and nitro benzene in the binary liquid mixtures with furfural in a molar fraction of 0.5.
 |
Figure 6: FT-IR spectra for the following substances: (a) Pure Furfural liquid, (b) equimolar mixture of Furfural + Nitrobenzene, (c) Pure Nitro benzene liquid. |
 |
Figure 7: FT-IR spectra for the following substances: (a) Pure Furfural liquid, (b) equimolar mixture of Furfural + Toluene, (c) Pure Toluene liquid. |
 |
Figure 8: FT-IR spectra for the following substances: (a) Pure Furfural liquid, (b) equimolar mixture of Furfural + Toluene, (c) Pure Toluene liquid. |
Pure furfural molecule shows a peak at 1686.93cm-1 which is due to the C=O bond, pure nitrobenzene liquid molecule has a peak at 1523.72cm-1 due to the N=O bond, and pure toluene molecule has a peak at 3032.58cm-1 due to the C-H bond, according to FT-IR study.
Figure 6 shows a peak at 1680.18cm-1 in equimolar composition of furfural with toluene. The change in frequency and intensity indicates the existence of an intermolecular partial H-bond between hydrogen atom of the toluene molecule and oxygen atom of the furfural. Figure 7 shows a frequency and intensity of the equimolar combination of furfural + nitrobenzene do not change, indicating that dispersion forces exist between –C=O and –N=O. Figure 8 shows a peak at 3078.38 cm-1 in equimolar composition of toluene with nitro benzene. The alteration in frequency and intensity is evidence that there is a partial H-bond between hydrogen atom of the toluene molecule and oxygen atom of the nitro benzene.
Conclusion
The mixing characteristics of binary liquid mixtures of furfural + toluene, furfural + nitrobenzene, and toluene + nitrobenzene were investigated in the current study. The magnitude of the chemical interactions between the molecules excess volume, deviation in isentropic compressibility, deviation in intermolecular free length, deviation in intermolecular free volume, deviation in internal pressure, deviation in viscosity and deviation in acoustic impedance has been used to interpret their magnitude.
VE and ΔKS values are negative of toluene + nitrobenzene shows more interaction than furfural + toluene interaction and VE and ΔKS values are positive of furfural + nitrobenzene is the mixing liquids interact less frequently. The –O atom of furfural deviates from the intermolecular H- bond form between the toluene molecules hydrogen atom, the hydrogen atom toluene molecules from the intermolecular H- bond form between the nitrobenzene molecules oxygen atom and the furfural molecules oxygen atom and the nitro benzene molecule’s oxygen atom consequently, Vander Waals forces are involved. To determine the variable coefficients, the corresponding thermodynamic excess parameters were calculated using the techniques previously mentioned and adapted to the Redlich- Kister polynomial equation. On the basis of experimental and calculated results, the behavior of the liquid mixtures and the deviation from ideality has been examined. An analysis of FT-IR spectroscopy showed the establishment of H-bond between unlike molecules.
Acknowledgement
The authors acknowledge the Management and Principal of St. Joseph’s College, (Autonomous), Tiruchirappalli – 02, for providing the necessary facilities to carry out the research work.
References
- Sharma,; Rani M,; Maken S. J. Mol. Liq. 2021, 321, 114366
CrossRef - Sekhar M.C,; Mohan T.M,; Krishna T.V. J. Mol. Liq.2014, 200, 263–272
CrossRef - Sreenivasulu Karlapudi,; Gardas R.L,; Venkateswarlu P,; Sivakumar K. J. Chem. Thermodyn.2013,67, 203–209
CrossRef - Laura Lomba,; Beatriz Giner,; Ma Carmen Lopez. J. Chem. Eng. Data.2014, 59(2),329-338
CrossRef - Muhammad Saad Qureshi,; Pavel Vrbka,; Vladimír Dohnal. Fuel.2017,191, 518-527
CrossRef - Uma Sivakami K,; Vaideeswaran S,; and Rosevenis A. J. Environ. Nanotechnol. 2018, 7( 3), 22
- Bolloju Satheesh,; Jagadeesh Kumar Ega,; Kavitha Siddoju,; Siddhartha Marupati,; Tangeda Savitha Jyostna. chem. data collect.2022,37 , 100812
CrossRef - Felixa S,; Sivakami U.; and R. Venis. World J. Pharm. Pharm. Sci. 2016, 5,1602
CrossRef - Ubagaramary D,; Muthu Vijayan Enoch,; and Kesavaswamy. Orient. J. Chem. 2016 32(1),321-330
CrossRef - Reddicherla Umapathi,; Narasimha Rao C,; Paramespri Naidoo. J.Chem.Thermodyn. 2020,149,106150
CrossRef - Narendra K,; Sudhamsa B,; Sarath Babu M. J. Appl Sol Chem Mod. 2015, 4,119–127
CrossRef - Laura Lomba,; Beatriz Giner,; Isabel Bandres. Green Chem. 2011, 13, 2062-2070
CrossRef - Revathi U,; Rose Venis A. JETIR. 2019, 4, 6
- Umasivakami K,; Vaideeswaran S,; and Rose Venis A. J. Serb. Chem. Soc. 2018, 83(10), 1131–1142
CrossRef - Ramana CH.V.V,; Kiran Kumar A.B.V,; Satya Kumar A. Thermochim. Acta. 2013, 566, 130–136
CrossRef - Follegatti–Romero L.M,; Sosa F.H.B,; Costa M.C. J. Chem. Thermodyn. 2019,134, 20-30
CrossRef - Bendiaf L,; Bahadur I,; Negadi A. Thermochim Acta. 2015, 599, 13-22
CrossRef - Rajalakshmia R,; Ravikumara S,; Sivakumarb K. J. Chem. Data Coll. 2019, 24,100299
CrossRef - Kumar S,; Jeevanandham P. J. Mol. Liq. 2012, 174,34-41
CrossRef - Prabhu P,; Rose Venis A. Phys. Chem. Liq.2020, 58,529-544
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









