Pteridine a Colored Heterocycle and its Anticancer Activity: An Overview
1Department Of Pharmacy, Sumandeep Vidyapeeth Deemed to be University, Piparia, Waghodia, Vadodara, Gujarat, India.
2Department Of Pharmaceutical Chemistry, A. R. college of pharmacy and G. H. Patel Institute of Pharmacy, Vallabh Vidyanagar, Anand, Gujarat, India.
Corresponding Author E-mail: neil.dop@sumandeepvidyapeethdu.edu.in
DOI : http://dx.doi.org/10.13005/ojc/380402
Article Received on : 04 May 2022
Article Accepted on :
Article Published : 24 Aug 2022
Reviewed by: Dr. S. J. Takate
Second Review by: Dr. Parthiban D
Final Approval by: Dr. Ioana Stanciu
The objective of this work is to provide an overview of the numerous pharmacological features that are associated with the pteridine molecule. Pteridines are nitrogen-containing heterocyclic compounds that are well-known and noteworthy. Their chemical formula is C6H4N4.In recent years, pteridine's various potential uses in the field of medicinal chemistry research have garnered significant attention. In the expanding field of intensive study, Pteridine is regarded as a privileged scaffold, and the alteration created with diverse substituents around the centroid opened the way for researchers to deal with it at ease. The heterocycle, which is a fused ring, has a high pharmacological quality. A pteridine is one of the heterocycles that has attracted a lot of interest in terms of biological uses. The pteridine nucleus serves as the quintessential framework in a range of physiologically energetic chemicals and pharmacological molecules. This evaluation is necessary in order to bring to light the remarkable potential that this ring device possesses as a result of the wide variety of pharmacological effects it may perform. This research might unquestionably hasten the graph and synthesis procedures, which would ultimately yield in a wide array of therapeutically feasible medicinal options.
KEYWORDS:Anti-Tumour; Cancer Cell-lines; Pteridine; Tumourgrowh
Download this article as:| Copy the following to cite this article: Panchal N. B, Vaghela V. M. Pteridine a Colored Heterocycle and its Anticancer Activity: An Overview. Orient J Chem 2022;38(4). |
| Copy the following to cite this URL: Panchal N. B, Vaghela V. M. Pteridine a Colored Heterocycle and its Anticancer Activity: An Overview. Orient J Chem 2022;38(4). Available from: https://bit.ly/3TpKGwL |
Introduction
Chemists in medicinal chemistry strive to create, investigate, and manufacture medications or drugs that will help humanity. The chemistry of heterocyclic molecules is critical in the development of novel medications. A pteridine is an aromatic heterocycle that results from the reaction of pyrimidine with pyrazine. Pteridine derivatives are mostly obtained from insects wings,(Pterones means wings in Greek).1 Pteridine,basic nucleus are yellow in colour and its derivatives are mostly coloured compounds. Compounds made up of fused pyrimidine and pyrazine rings. Pteridine research has long been recognized as critical to many biological processes,thatincludes amino acid metabolism, synthesis of various nucleic acid, neurotransmitter production, chemotherapy of cancer, cardiovascular function, and the overall necessity of practically all living species.1 Analgesic, anti-inflammatory, anticonvulsant, sedative-hypnotic, anti-histaminic, anti-hypertensive, anti-tumor, anti-microbial, ischemia or reperfusion surgery, and anti-parasite activity are some of the pharmacological responses ascribed to these systems.2 This diversity in the pharmacological action contours of pteridine has piqued the interest of medicinal chemists in exploring this fused Heterocycle’s potential against a wide range of activities.
Pteridine
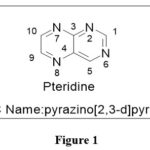 |
Figure 1 Click here to View figure |
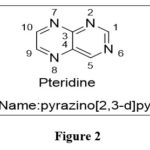 |
Figure 2 |
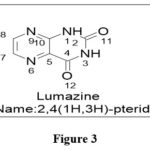 |
Figure 3 Click here to View figure |
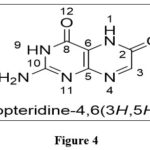 |
Figure 4 Click here to View figure |
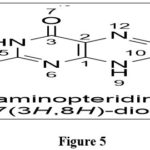 |
Figure 5 Click here to View figure |
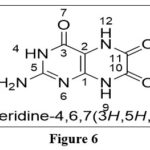 |
Figure 6 Click here to View figure |
The name pteridine refers to the pyrazino[2,3-d]pyrimidine nucleus suggested by scientist Wieland in 1941.The IUPAC regulations govern the nomenclature of this fused-ring system; however, the central CTC bond may be replaced. Because the pyrimidine moiety of the nucleus of pteridines found naturally has the same pattern of substituents, it has been widely accepted to call 2-amino-4(3H)-pteridine ‘pterin.’3As a result, this phrase is frequently employed as a distinguishing feature of the common name of naturally occurring pteridine compounds. Similarly, the 2,4(1H,3H)-pteridinedione derivatives 3 are referred to as ‘lumazines.’ Prefixes such as “dihydro-,” “tetrahydro-,” and so on are used to indicate reduced compounds. The numbers following the prefixes represent the locations of the additional hydrogen atoms in nucleus. In pteridine derivatives, tautomerism is prevalent, and the structural formulae should represent the preferred tautomers in them. Amino groups do not exist in the iminodihydro form, but hydroxy and thiol functionalities 9 near or at the para position to a ring nitrogen atom favoramide (lactam) and thioamide (thioxo) configurations, which are thermodynamically more stable.1,2
 |
Figure 7 Click here to View figure |
Physical Properties4
Pteridine yellow-colored is a solid material.
Melting Point: 139.5⁰C.
It is soluble in water
Log log-logp: -0.58
Method of Preparation
Gabriel-Isay (Gabriel-Colman) synthesis:Pteridines are synthesized via cyclo-condensation of 5,6-diaminopyrimidine withbenzil or glyoxal to get 4-methyl-6,7-diphenylpteridine.This reaction is also known as the Gabriel-Colman synthesis.5
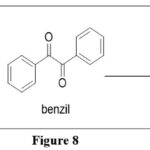 |
Figure 8 Click here to View figure |
On the other hand, when diaminopyrimidine was reacted with diketones, keto aldehydes, keto acids, which are unsymmetrical in nature or their esters, a number of different pteridine derivatives were produced.5
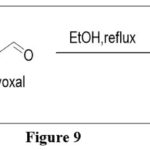 |
Figure 9 Click here to View figure |
Polonovski-Boon Reaction
In synthetic procedure,when 6-chloro-5-nitropyrimidine reacted with an α -aminocarbonyl molecule yields an intermediate, resultant product 2-amino-6-methyl-7,8-dihydropteridin-4(3H)-one is obtained after reduction and cyclization. 6
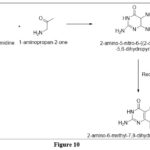 |
Figure 10 Click here to View figure |
Timmis synthesis
Dehydration synthesis of 4-amino-5-nitrosopyrimidines via Base-catalyzed process with molecules such as ketones, aldehydes, nitriles, and cyanoacetic acid,(Which have an activated methylene group,) results in condensed pteridines derivatives. 7
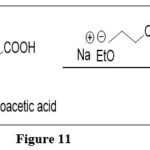 |
Figure 11 Click here to View figure |
Condensation of α-methylenenitrile with 5-nitropyrimidine-2,4,6-triamine resulted in triamterene, a potassium-sparingdiuretic.
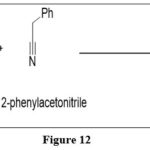 |
Figure 12 Click here to View figure |
Pachter synthesis
The reaction is modified by reacting 4,6-diamino-5-nitroso-2-phenyl pyrimidine with, benzyl methyl ketone, in the presence of potassium acetate to create 4-amino-7-methyl-2,6-diphenylpteridine derivative.8
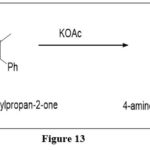 |
Figure 13 Click here to View figure |
Blicke synthesis
When amino pyrimidines react with various aldehydes and hydrogen cyanide by dehydration reaction followed by cyclization using Sodium methoxide as a base, leads pteridine derivatives via cyanoamines.6
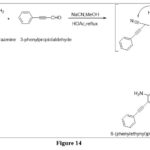 |
Figure 14 Click here to View figure |
From pyrazines
The oxidative aromatization of 3-(aminomethyl)pyrazin-2-amine with orthoformates results in 6-substituted pteridine.6
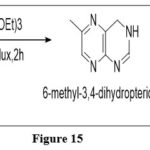 |
Figure 15 Click here to View figure |
Viscontini reaction
Sugar phenylhydrazones Condensed with 5,6-diaminopyrimidines under mild acidic conditions, yielded 6-substituted pterins in this effective synthesis process which is regioselective in nature. 6
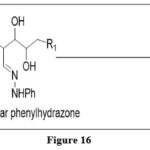 |
Figure 16 Click here to View figure |
Chemical Properties
The pyrimidine ring of pteridineis sensitive enough and degrades with acid and alkali under the reaction circumstances. Heating 2,6-dihydroxypteridine with 100 percent sulfuric acid at 240°C yielded 2-aminopyrazine; the same when undergoes heating (2,6-dihydroxypteridine) with 2–3 equivalents of 12 percent alkali for 2 hours at 170°C yielded 2-amino-3-carboxylic acid. Both these acquired in substantial quantities.9
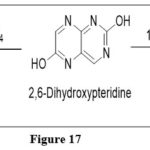 |
Figure 17 Click here to View figure |
Nucleophilic addition
The fused Pteridine nucleus rapidly forms nucleophilic adducts with water, amines and alcohol, primarily at the C-4 and C-7 sites. Covalent hydrates are formed via addition of water molecule to pteridine. Under acidic reaction environment, 3,4-hydrated cation forms first and gradually equilibrate with dehydrated 5,6,7,8-cation.10
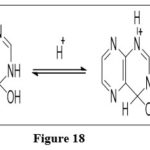 |
Figure 18 Click here to View figure |
An equilibrium mixture of 3,4-dihydro-4-methoxypteridine and 5,6,7,8-tetrahydro-6,7-dimethoxypteridine are obtained when A pteridine is nucleophilically added to methanol in the(1:1 adduct) and(2:1 adduct) respectively .6,10
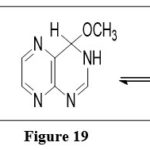 |
Figure 19 Click here to View figure |
Nucleophilic substitution reaction
Using the pteridine in conjunction with the Grignard reagent, followed by oxidation, it is possible to form a derivative with new carbon-carbon bond on the pyrazine ring of the nucleus, at the C-7 location of the ring.6
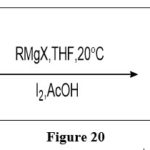 |
Figure 20 Click here to View figure |
Oxidation
Pteridine derivatives, such as xanthopterin, are transformed to leucopterinwhen preferably reacted to hydrogen peroxide or molecular oxygen over platinum. 9
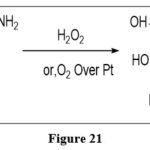 |
Figure 21 Click here to View figure |
Reduction
Tetrahydropteridine derivatives are very reactive to oxidation, making isolation problematic. Avaibility of functional groups which are electron withdrawing in nature on the ring, provide separation easily. As a result of reacting properly substituted pteridine with sodium borohydride, 5,6,7,8-tetrahydro pteridine-4-carboxylate can be extracted. 11
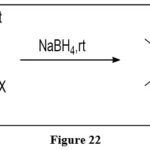 |
Figure 22 Click here to View figure |
Reaction with PCl5 and POCl3
Reactions which involve Leucopterin with very strong chlorinated agents like phosphorous pentachloride and phosphorous oxychloridelead to form a substituted chloro derivative, which via reduction with hydroiodic acid generates 6-deoxyleucopterin.6
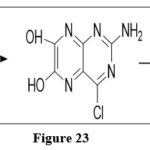 |
Figure 23 Click here to View figure |
Ring fission
Pteridines have high thermal stability; nonetheless, they are unstable in hot solutions. In aqueous acid, they fission the pyrimidine ring to produce 3-aminopyrazine-2-carbaldehyde.6
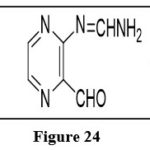 |
Figure 24 Click here to View figure |
Heck reaction
The coupling reaction of 6-chloropteridine with acetylenes, mediated by palladium, produces alkynyl substituted pteridines. 12
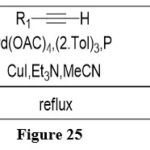 |
Figure 25 Click here to View figure |
Pharmacological scaffold
Pteridine has a significant pharmacological impact because of its varied scaffold. For the previous decade, pteridine has been examined for its many aspects of impact to find the capability to create and isolate a range of pharmacological activity.13
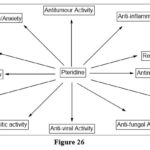 |
Figure 26 |
Anticancer Activity
Despite significant advances in early detection and treatment, cancer remains a primary health and social-economic concern.
Cytotoxic drugs achieve their therapeutic effects by preventing the replication of DNA. Because cancer cells proliferate rapidly, they create a large amount of new DNA swiftly, which, if damaged, causes the cell to die. Interfering with DNA replication may be done with three different substances. Antimetabolites are substances that resemble nucleotides. Alkylating agents are substances that attach to DNA and change its structure permanently.14Regrettably, they also bind to a wide range of other substances in cells. Anthracyclines, antitumor antibiotics, platinum analogues, and some alkylating compounds are all examples of different types of DNA-binding drugs. DNA-binding antineoplastics are responsible for tissue damage because they promote deadly DNA crosslinking and strand breaks, both of which are induced by free radicals and ultimately result in cell death. These are often used in combination with an enzyme. Currently, cytotoxic medicines are utilized to treat cancer, either alone or in combination, and numerous of these medications are in various stages of clinical studies. These cytotoxic drugs have several drawbacks, including the inability to discriminate between malignant and normal cell types; a target-specific effect that is necessary for increased treatment success; andas a result, they can cause serious adverse reactions to the recipient, which are usually cumulative and dose-limiting. Because of the emergence and dissemination of multidrug resistance to many conventional medications, as well as the ongoing significance of healthcare spending, a significant number of researchers have concentrated their efforts on the development and testing of novel and efficient anticancer drugs. A pteridine is a scaffold that is commonly utilized in bioactive compounds.
Several pteridine compounds have been licensed for clinical use as anticancer medicines by the Food and Drug Administration (FDA). Methotrexate, Pralatrexate are approoved two examples for the same.15,16
Various cancer treatment targets have been identified to provide more relevant and accurate treatments. A number Several studies have been published in the literature concerning the discovery and development of innovative potential pteridine derivatives for cancer therapy.17,18
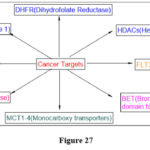 |
Figure 27 Click here to View figure |
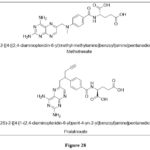 |
Figure 28 Click here to View figure |
Literature Review
Marques SM et al. reported and synthetically prepared a novel Pteridine–sulphonamide conjugates,which act as carbonic anhydrases and dihydrofolate reductase inhibitors. Inhibition investigations were done on a group of CAs and DHFRs. Assays for cell proliferation employing two types of tumor cell lines – 1)A549(adenocarcinomic human alveolar basal epithelial cells), 2) prostate carcinoma, PC-3), Amongsynthesized and analysedcompounds, 1a and 4a were found to be the highest active, with KI of 4.5 and 260 nM,respectively19
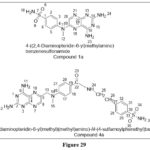 |
Figure 29 Click here to View figure |
Liu KK et al. reported anew sequence of 4-Methylpteridinones Derivatives, whichwere further refined to increase their efficacy against PI3Ka and mTOR targets. The availability of C4 methyl group is necessity for selectivity action because it matches the one-of-a-kind PI3K/mTOR binding pocket, whereas at the C6 position heteroaryl groups, improved PI3K potency, according to SAR analysis.Both of these findings are based on the fact that the C4 methyl group fits the unique binding pocket.In a xenograft mouse model of U87 glioma cells, compound 2 was orally dosed (PI3K Ki = 2.8 nM and mTOR Ki = 6.8 nM), and found most effective one,demonstrating that treated animals’ tumour volume was reduced by 25%.20
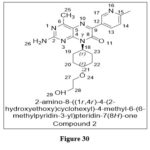 |
Figure 30 Click here to View figure |
Wang H et al.synthesized and analyzed new Pteridine derivatives against the monocarboxylate transporter 1 (MCT1). In the majority of aggressive tumor types, MCT1 and MCT4 are substantially expressed, making them ideal targets for cancer therapy. Compounds 3 and 4ac, respectively, were shown to inhibit the development of Raji Burkitt lymphoma cells expressing MCT1 but noother cell lines likeMCT4 and MCF7 breast cancer cells engineered to overexpress murine MCT1. In these cells, Decrement of of C-lactate transport which correlated with the antiproliferative effects in these cells. According to SAR analysis, Compounds 3 and 4a-c share a 1-naphthyl methyl or isobutyl substituent. In addition, these compounds have distinct hydroxyl groups in alkyl and thioether-containing tethers in their structures, It may have an important role in the action of these substances.21
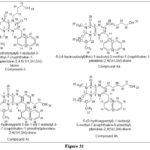 |
Figure 31 Click here to View figure |
Okada Let Al. reported a series of novel Compounds based on pteridine7(8H). Compound 5 is the most structurally favorable of the produced compounds: containing, (1) the presence of a 4-methylpiperazinyl group and a methyl group in the phenyl substituent, and (2) the unavailability of a Michael acceptor in the aminophenyl radical. It was also evaluated on many cancer cell lines, including the AML cell line MV411, which carries an FLT322 activating mutation often identified in AML patients (IC50 = 51 nM) Compound 5 was able to block the activity of FLT3-fms-like tyrosine kinase (IC50 = 1.56 nM, Kd = 0.25 nM) as well as some of the enzyme’s downstream signalling proteins. This reported derivative stopped the G0/G1 phase in the cell cycle and caused cell apoptosis in the MV411 xenograft mice model. It also inhibited tumour development.23
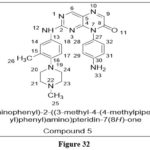 |
Figure 32 Click here to View figure |
Hao Y et al.reported and synthesized a focused,N5-Substituted 6,7-dioxo-6,7-dihydropteridines,Which are (EGFR) Inhibitors.Compounds were screened on T790M mutant and wild-type (WT) EGFR kinases, Among the synthesized compound, Compounds 6a and 6b were found active, withIC50 values 1.21nM and 3.82nM respectively on EGFR wild type cell line, besides this,they found effective on EGFRL858R/T790M cell line with particular IC50 value 0.68nm and 1.07nM and IC50 value 62.2nM and 59nM obtained for H1975 cell line.24
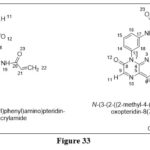 |
Figure 33 Click here to View figure |
Shoji QA et al.Synthesisand analyzedthe Structure-Activity Relationship of 3-Hydroxypyridine-2-thione-base derivatives, synthesized derivatives tested as histone deacetylase (HDAC) inhibitors, The findings revealed that pteroate-based hydroxamates were effective against a wide range of HDAC isoforms, with five (8a) and six (8b) methylene groups providing the optimum linker length (IC50 = 16.1 and 10.2 nM, respectively).Immunoblotting experiments demonstrated that the cytotoxicity was caused by the simultaneous inhibition of both the Histone deacetylase1 and Histone deacetylase6 isoforms.25
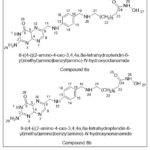 |
Figure 34 Click here to View figure |
Nelson ME et al. disclosed that 2-Amino-O4-benzyl pteridine derivatives are effective inhibitors of the referenced alkyltransferase, however, the availability of DNA, reduced their inhibitory activity (IC50 = 0.01 to 0.4 µM). O4-benzyl folic acid (molecule 9) was the most potent among the derivatives tested. Additionally, the cytotoxic effect of 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) was increased by these compounds when used against A549, KB(human epithelial carcinoma cells), and HT29(adenocarcinomic human alveolar basal epithelial cells) cells. ED90 of compound 9 = 5 µM with BCNU 40 µM were observed, which shows resistance to BCNU alone and more effective in cells that have greater levels of the α-folate receptor expression.26
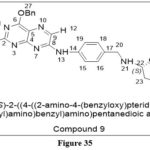 |
Figure 35 Click here to View figure |
Rudolph D et al.reported a of some new sequence of Polo-like kinase 1 (Plk1) inhibitors. Compound 10 inhibits Plk1 (IC50 = 0.87 nmol/L) among the produced compounds. It was effective against number of tumours cell lines in several xenograft models and had significant tissue penetration. At 24 hours, BI6727 caused the cell cycle to remain in the G2/M phase, followed by apoptosis and an increase in sub-G1 peaks.27
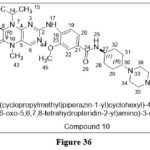 |
Figure 36 Click here to View figure |
Zhan MM et al. reported a novel tetrahydropteridin chemical scaffoldand screened on highly selective polo-like kinase2 inhibitors,Compounds C2 in 3.40 nM concentration against Plk2 and C21 in4.88 nM concentration against Plk2, were found most promising among the produced compounds from the first and second series of selective Plk2 inhibitors, respectively. Furthermore, with selectivity values of 12.57 and 910.06, C21’s selectivity over Plk1/3 was greatly boosted. K562, MCF-7(human breast cancer cell line), HuH-7(hepatocyte derived cellular carcinoma cell line), A549(adenocarcinomichuman alveolar basal epithelial cells), H1975(exhibiting epithelial morphology), and HeLa cell lines were employed.28
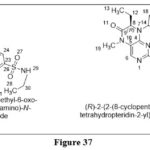 |
Figure 37 Click here to View figure |
Kiryanov A etal. synthesized and reported novel 5,6-Dihydroimidazolo[1,5-f] pteridine derivatives, At 50 nM, bound to Lys82 amino acid and reduced Plk1 activity. They made structural adjustments to the nitrile-containing compounds to enhance the ADME qualities in rats, resulting in compound 13. In xenograft animal models, this drug had a high microsomal stability, favoured the phosphorylation of histone H3, and had a excessive rate of absorption.29
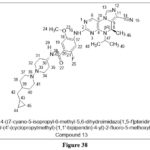 |
Figure 38 Click here to View figure |
Koban LK et al. reported some Tetrahydropteridine derivatives as aBRD4‐selective(Bromodomain-containing protein 4) inhibitor. BRD4, which is responsible in a variety of biological actions including epigenetic control. Compound 14 was the most powerful, inhibiting BRD4 (IC50 = 130nM). When compound 14 was evaluated in MV411(human AML cell line) , showed cytotoxicity (IC50 = 184 to 218 nM).30
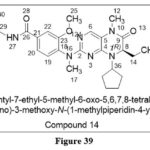 |
Figure 39 Click here to View figure |
Sapkota et al. reportedRibosomal S6 kinases (RSK) inhibitors that have been linked to the development of cancer by being engaged in cell cycle progression and survival, Among the synthesized compound, Compound 17( BI‐D1870) ,By competing with ATP at the N terminal domain, it emerged as a particular selective inhibitor of the four RSK isoforms (about 99 percent inhibition observed at 10 µM concentration), supporting studies on RSK pathways in HEK293 and Rat-2 cells. 31
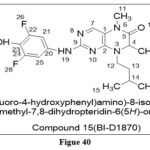 |
Figure 40 Click here to View figure |
Mohammad KS et al. reported and synthesizeda seriesof TGF-b-RI Kinase Inhibitors. Transforming growth factor shows tumor suppressive action on epithelial cells is; yet, when acting on cancer cells, this protein has been linked to tumor development and metastasis. TGF increases the kinase activity of TGF receptors I and II (TGFRI and TGFRII), SD208 (compound 16) was identified from the synthesized compounds as a TGFRI inhibitor (IC50 = 0.048 mol/L). A noticeable tumour growth and metastasis prevention observed when various xenograft mouse models treated with this synthesized derivative along with considerable immune cell infiltration.32
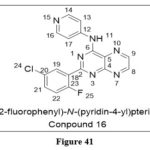 |
Figure 41 Click here to View figure |
Song et al. recently studied and reported a new and potent Transient receptor potential melastatin 7 kinase inhibitor, which has the ability to suppress breast cancer cell migration and invasion.Among the potential compounds, Inhibitory action of migration and invasion in breast cancer cells were found in prepared Compound 17, with IC50=1.07µM.Wound healing and transwell invasion studies using MDAB468 breast cancer cells and TRex293 cells, screened to this synthesized derivative revealed suppression of relocation and metastasis with minimal cytotoxicity.33
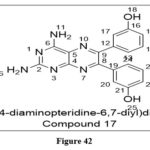 |
Figure 42 Click here to View figure |
Xiaoyuan Liet al. reported and synthesizedderivatives of 5-substituted 2-amino-4-chloro-8-((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)-7,8-dihydropteridin-6(5H)-ones derivatives as Hsp 90 inhibitor,The prepared Compound 20 was found as the most active and had the best selectivity for cancer cells among the preoared compounds.Results were observed: MCF-7, IC50 = 50 nM, HT29 IC50 = 60 nM, SKBR-3, IC50 = 20 nM, and BT474, IC50 = 30 nM. 34
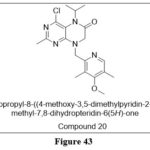 |
Figure 43 Click here to View figure |
Su et alsynthesized derivatives, which focused onkinases (Jak) are a family of nonreceptor tyrosine kinases that have been produced and tested.AutophosphorylatedJaks , activates signal pathways, including STAT (STATs), resulting in cell proliferation and apoptotic arrest. This scaffold has derivatives, including synthesized compound 22, with substituted pteridine ring.It inhibited Jak1 and Jak2 in vitro with IC50 values of 1.5µM and 0.8µM, respectively, along with the growth of BaF3 TEL- Jak2 cells with IC50=0.53µM.35
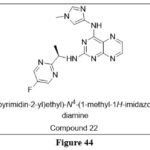 |
Figure 44 Click here to View figure |
Murgueitio MS et al. focused and synthesized Toll-like receptor 2 (TLR2) agonistsand used them as dendritic cell (DC) vaccination adjuvants. DCs from the patient are extracted, activated ex vivo using TLR agonists and tumor antigens, and then reintroduced into the patient. Among the compounds in the series, Compound 23 was discovered to be an allosteric agonist and to be bound to the TLR1-TLR2 complex at distinct locations ,with an IC50 of 5.26 nM36
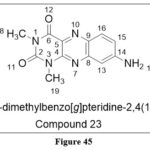 |
Figure 45 Click here to View figure |
Chauhan PM et al. reported a dedicated 6,7‐dimethylpteridine derivatives, with various heterocycles substituted at position 2 that were coupled to thioether or alkylamino in position 4. Some of these synthesized and analyzed derivatives, including compound 24, have demonstrated cytotoxicity against cancer cell lines MCF7 (breast), NCIH460 (lung), and SF268 (center-neuronal system) of 0.1 µM, however the largest inhibitory impact on cell development was not less than 57 percent.37
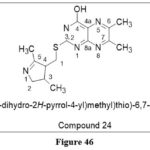 |
Figure 46 Click here to View figure |
Geng PF et al. reported A newclassof 5,8‐dihydropteridine‐6,7‐diones tested on number of cancer cell lines, From the derivatives analysed, those with a piperazine substitution produced the best outcomes, including cell lines like, MGC-803 and SGC7901 (gastric cancer), A549 (lung), PC3 (prostate cancer) (prostate) cell lines. Compound 25 proved to be the most potent anticancer drug, with IC50 values of less than 20 µM for each cell line examined. In a wound-healing experiment, this medication also decreased MGC-803 (indirect neoplastic transformation measurement) cell production and migration. Furthermore, compound 25 emerged with an attractive result with an SI of 4.36 between MGC803 and normal gastric epithelial cells.38
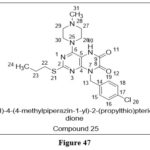 |
Figure 47 Click here to View figure |
Li ZH et al. reportedpteridine‐7(8H)‐one derivative,MKN45 and MGC803 (gastric cancer), H1650 (lung cancer), and EC109 cell lines were tested (esophagus cancer).Compound 26 has IC50 values of less than 10 μM in each case within the tested series. Further research showed that apoptosis was inducted ,a s evidenced by a propidium iodide (annexin V) experiment and validation is observed by increment in Bax expression and lower levels of Bcl-2, caspase-3, and 9 cleavages.39
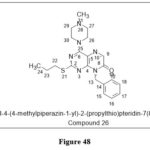 |
Figure 48 Click here to View figure |
Novosad D et al.reported a novel 6‐azapteridines via a cyclocondensation reactionfrom the corresponding aryliminohydrazones.Compounds 27a and 27b were identified as the potent derivatives,In particular, when examined in several cancer cells like MCF7 and K562, 27a had IC50 values of 8.3 and 12.9 µmol/L, respectively, whereas 27b had IC50 values of 7.2 and 14.6 µmol/L.40
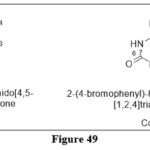 |
Figure 49 Click here to View figure |
MiyoshiT et al. synthesized derivatives from which, two M2‐(N, N‐dimethyl‐aminomethyleneamino)‐3‐pivaloylpteridin‐4‐ones derivatives , From the synthesized compounds , Compounds 28 a and 28 b, screened with Panic-1 cancer cell line, The dosages of prepared derivatives were adjusted from 2mM (28 a) to 400 µM (28 b) reduced cell viability and exacerbated cell apoptosis caused by UVA irradiation. The findings also demonstrated that 28 a induced G2/Marrest.41
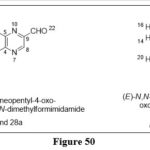 |
Figure 50 Click here to View figure |
Conclusion
By evaluating the distinctive pteridine derivatives, it’s evident that this heterocycle incorporates a big selection of potential as a growth agent. Pteridine, with its effective action and skill to act with different heterocycles, might without delay be utilized to create a range of neoplastic derivatives. Pteridine derivatives have additionally been shown to be effective malignant neoplasm agents, and these promising synthesized derivatives of the heterocycle have the power to influence a good range of cell lines, indicating that theymay be helpful and versatile anticancer agents. It takes heaps less effort to make a scaffold that meets all of the standards for an ideal malignant neoplasm drug.
Acknowledgement
I am grateful to Dr. A.K Sheth, Principal and Dean, SumandeepVidyapeeth, Department of Pharmacy and Dr. M. K. Mohan Maruga Raja, Associate Professor, Department of Pharmacognosy & Phytochemistry, PIPR, Parul University, for their insightful comments, direction, and help.
Conflicts of Interest
We state unequivocally that we do not have any conflicts of interest.
Funding Sources
There is no funding source.
References
- Theophil, E.; Hauptmann, S. The Chemistry of Heterocycles: Structure, Reactions Synthesis and Applications, 2003rd ed.; Wiley-VCH, Weinheim, 2003.
- Suckling, C.; Gibson, C.; Huggan, J.; Pfleiderer, W. Bicyclic 6-6 Systems : Pteridines. 2008, 915–975.
CrossRef - Pfleiderer, W. Pteridines. Compr. Heterocycl. Chem.1984, 3–7, 263–327. https://doi.org/10.1016/B978-008096519-2.00038-2.
CrossRef - National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 1043, Pteridine. https://pubchem. ncbi.nlm.nih.gov/compound/Pteridine (accessed 2022 -04 -18).
- Brown, T. Deisign Thinking. Harv. Bus. Rev.2008, 86 (6), 84–92. https://doi.org/10.1002/med.
- Ji Ram, V.; Sethi, A.; Nath, M.; Pratap, R. Six-Membered Heterocycles. Chem. Heterocycles2019, 3–391. https://doi.org/10.1016/B978-0-12-819210-8.00002-3.
CrossRef - Synthesis, T.; Activity, P. A. A. SPickett and Timmis . 2887 The Synthesis. 1952, No. 1119541.
- Pachter, I. J. Pteridines. II. Synthesis of 6-Substituted 7-Aminopteridines from Aldehydes. J. Org. Chem.1963, 28 (5), 1191–1196. https://doi.org/10.1021/jo01040a007.
CrossRef - Gates, M. The Chemistry of the Pteridines. Chem. Rev.1947, 41 (1), 63–95. https://doi.org/10.1021/cr60128a002.
CrossRef - Albert, A.; Mizuno, H. Phys. Org. 2423 Pteridine Studies. Part XL1I.l Addition Reactions between Alcohols and Pterid Ine. 1971, 2 (C), 4–8.
CrossRef - Derivatives, S.; Pendergast, W.; Hall, W. R. I- 2’ ’. 1985, No. 8, 388–390.
CrossRef - Taylor, E. C.; Ray, P. S. Pteridines. 51. A New and Unequivocal Route to C-6 Carbon-Substituted Pterins and Pteridines. J. Org. Chem.1987, 52 (18), 3997–4000. https://doi.org/10.1021/jo00227a011.
CrossRef - Martínez, V. C.; Vera, M. Therapeutic Potential of Pteridine Derivatives : A Comprehensive Review. 2018, No. July. https://doi.org/10.1002/med.21529.
CrossRef - Panchal, N. B. QUINAZOLINE HETEROCYCLE AND ITS ANTI-CANCER ACTIVITY: AN OVERVIEW Neil B. Panchal Department of Pharmacy, Sumandeep Vidyapeeth Deemed to Be University, Piparia, Waghodia, Vadodara – 391760, Gujarat, India. 2022, 13 (3), 1065–1078. https://doi.org/10.13040/IJPSR.0975-8232.13(3).1065-78.
- Marchi, E.; Mangone, M.; Zullo, K.; O’Connor, O. A. Pralatrexate Pharmacology and Clinical Development. Clin. Cancer Res.2013, 19 (24), 6657–6661. https://doi.org/10.1158/1078-0432.CCR-12-2251.
CrossRef - O’Connor, O. A.; Amengual, J.; Colbourn, D.; Deng, C.; Sawas, A. Pralatrexate: A Comprehensive Update on Pharmacology, Clinical Activity and Strategies to Optimize Use. Leuk. Lymphoma2017, 58 (11), 2548–2557. https://doi.org/10.1080/10428194.2017.1306642.
CrossRef - Ke, X.; Shen, L. Molecular Targeted Therapy of Cancer: The Progress and Future Prospect. Front. Lab. Med.2017, 1 (2), 69–75. https://doi.org/10.1016/j.flm.2017.06.001.
CrossRef - Hahn, W. C.; Bader, J. S.; Braun, T. P.; Califano, A.; Clemons, P. A.; Druker, B. J.; Ewald, A. J.; Fu, H.; Jagu, S.; Kemp, C. J.; Kim, W.; Kuo, C. J.; McManus, M.; B. Mills, G.; Mo, X.; Sahni, N.; Schreiber, S. L.; Talamas, J. A.; Tamayo, P.; Tyner, J. W.; Wagner, B. K.; Weiss, W. A.; Gerhard, D. S.; Dancik, V.; Gill, S.; Hua, B.; Sharifnia, T.; Viswanathan, V.; Zou, Y.; Dela Cruz, F.; Kung, A.; Stockwell, B.; Boehm, J.; Dempster, J.; Manguso, R.; Vazquez, F.; Cooper, L. A. D.; Du, Y.; Ivanov, A.; Lonial, S.; Moreno, C. S.; Niu, Q.; Owonikoko, T.; Ramalingam, S.; Reyna, M.; Zhou, W.; Grandori, C.; Shmulevich, I.; Swisher, E.; Cai, J.; Chan, I. S.; Dunworth, M.; Ge, Y.; Georgess, D.; Grasset, E. M.; Henriet, E.; Knútsdóttir, H.; Lerner, M. G.; Padmanaban, V.; Perrone, M. C.; Suhail, Y.; Tsehay, Y.; Warrier, M.; Morrow, Q.; Nechiporuk, T.; Long, N.; Saultz, J.; Kaempf, A.; Minnier, J.; Tognon, C. E.; Kurtz, S. E.; Agarwal, A.; Brown, J.; Watanabe-Smith, K.; Vu, T. Q.; Jacob, T.; Yan, Y.; Robinson, B.; Lind, E. F.; Kosaka, Y.; Demir, E.; Estabrook, J.; Grzadkowski, M.; Nikolova, O.; Chen, K.; Deneen, B.; Liang, H.; Bassik, M. C.; Bhattacharya, A.; Brennan, K.; Curtis, C.; Gevaert, O.; Ji, H. P.; Karlsson, K. A. J.; Karagyozova, K.; Lo, Y. H.; Liu, K.; Nakano, M.; Sathe, A.; Smith, A. R.; Spees, K.; Wong, W. H.; Yuki, K.; Hangauer, M.; Kaufman, D. S.; Balmain, A.; Bollam, S. R.; Chen, W. C.; Fan, Q. W.; Kersten, K.; Krummel, M.; Li, Y. R.; Menard, M.; Nasholm, N.; Schmidt, C.; Serwas, N. K.; Yoda, H. An Expanded Universe of Cancer Targets. Cell2021, 184 (5), 1142–1155. https://doi.org/10.1016/j.cell.2021.02.020.
CrossRef - Marques, S. M.; Enyedy, É. A.; Supuran, C. T.; Krupenko, N. I.; Krupenko, S. A.; Santos, M. A. Bioorganic & Medicinal Chemistry Pteridine – Sulfonamide Conjugates as Dual Inhibitors of Carbonic Anhydrases and Dihydrofolate Reductase with Potential Antitumor Activity. 2010, 18, 5081–5089. https://doi.org/10.1016/j.bmc.2010.05.072.
CrossRef - Liu, K. K. C.; Bagrodia, S.; Bailey, S.; Cheng, H.; Chen, H.; Gao, L.; Greasley, S.; Hoffman, J. E.; Hu, Q.; Johnson, T. O.; Knighton, D.; Liu, Z.; Marx, M. A.; Nambu, M. D.; Ninkovic, S.; Pascual, B.; Rafidi, K.; Rodgers, C. M. L.; Smith, G. L.; Sun, S.; Wang, H.; Yang, A.; Yuan, J.; Zou, A. 4-Methylpteridinones as Orally Active and Selective PI3K/MTOR Dual Inhibitors. Bioorganic Med. Chem. Lett.2010, 20 (20), 6096–6099. https://doi.org/10.1016/j.bmcl.2010.08.045.
CrossRef - Wang, H.; Yang, C.; Doherty, J. R.; Roush, W. R.; Cleveland, J. L.; Bannister, T. D. Synthesis and Structure-Activity Relationships of Pteridine Dione and Trione Monocarboxylate Transporter 1 Inhibitors. J. Med. Chem.2014, 57 (17), 7317–7324. https://doi.org/10.1021/jm500640x.
CrossRef - Quentmeier, H.; Reinhardt, J.; Zaborski, M.; Drexler, H. G. FLT3 Mutations in Acute Myeloid Leukemia Cell Lines. Leukemia2003, 17 (1), 120–124. https://doi.org/10.1038/sj.leu.2402740.
CrossRef - Okada, K.; Nogami, A.; Ishida, S.; Akiyama, H.; Chen, C.; Umezawa, Y.; Miura, O. FLT3-ITD Induces Expression of Pim Kinases through STAT5 to Confer Resistance to the PI3K/Akt Pathway Inhibitors on Leukemic Cells by Enhancing the MTORC1/Mcl-1 Pathway. Oncotarget2018, 9 (10), 8870–8886. https://doi.org/10.18632/oncotarget.22926.
CrossRef - Hao, Y.; Wang, X.; Zhang, T.; Sun, D.; Tong, Y.; Xu, Y.; Chen, H. Discovery and Structural Optimization of N5-Substituted 6 , 7-Dioxo-6 , 7- Dihydropteridines as Potent and Selective Epidermal Growth Factor Receptor ( EGFR ) Inhibitors Against L858R / T790M Resistance Mutation Discovery and Structural Optimization of N5. 2016.
CrossRef - Sodji, Q. H.; Patil, V.; Kornacki, J. R.; Mrksich, M.; Oyelere, A. K. Synthesis and Structure − Activity Relationship of 3 ‑ Hydroxypyridine- 2-Thione-Based Histone Deacetylase Inhibitors. 2013.
CrossRef - Nelson, M. E.; Loktionova, N. A.; Pegg, A. E.; Moschel, R. C. 2-Amino- O 4 -Benzylpteridine Derivatives : Potent Inactivators of O 6 -Alkylguanine-DNA Alkyltransferase. 2004, 3887–3891.
CrossRef - Rudolph, D.; Steegmaier, M.; Hoffmann, M.; Grauert, M.; Baum, A.; Quant, J.; Haslinger, C.; Garin-chesa, P.; Adolf, R. Cancer Therapy : Preclinical BI 6727 , A Polo-like Kinase Inhibitor with Improved Pharmacokinetic Profile and Broad Antitumor Activity. 2009, 15 (9), 3094–3102. https://doi.org/10.1158/1078-0432.CCR-08-2445.
CrossRef - Zhan, M. M.; Yang, Y.; Luo, J.; Zhang, X. X.; Xiao, X.; Li, S.; Cheng, K.; Xie, Z.; Tu, Z.; Liao, C. Design, Synthesis, and Biological Evaluation of Novel Highly Selective Polo-like Kinase 2 Inhibitors Based on the Tetrahydropteridin Chemical Scaffold. Eur. J. Med. Chem.2018, 143, 724–731. https://doi.org/10.1016/j.ejmech.2017.11.058.
CrossRef - Kiryanov, A.; Natala, S.; Jones, B.; McBride, C.; Feher, V.; Lam, B.; Liu, Y.; Honda, K.; Uchiyama, N.; Kawamoto, T.; Hikichi, Y.; Zhang, L.; Hosfield, D.; Skene, R.; Zou, H.; Stafford, J.; Cao, X.; Ichikawa, T. Structure-Based Design and SAR Development of 5,6-Dihydroimidazolo[1,5-f]Pteridine Derivatives as Novel Polo-like Kinase-1 Inhibitors. Bioorganic Med. Chem. Lett.2017, 27 (5), 1311–1315. https://doi.org/10.1016/j.bmcl.2016.10.009.
CrossRef - Koblan, L. W.; Buckley, D. L.; Ott, C. J.; Fitzgerald, M. E.; Ember, S. W. J.; Zhu, J. Y.; Liu, S.; Roberts, J. M.; Remillard, D.; Vittori, S.; Zhang, W.; Schonbrunn, E.; Bradner, J. E. Assessment of Bromodomain Target Engagement by a Series of BI2536 Analogues with Miniaturized BET-BRET. ChemMedChem2016, 11 (23), 2575–2581. https://doi.org/10.1002/cmdc.201600502.
CrossRef - Sapkota, G. P.; Cummings, L.; Newell, F. S.; Armstrong, C.; Bain, J.; Frodin, M.; Grauert, M.; Hoffmann, M.; Schnapp, G.; Steegmaier, M.; Cohen, P.; Alessi, D. R. BI-D1870 Is a Specific Inhibitor of the P90 RSK (Ribosomal S6 Kinase) Isoforms in Vitro and in Vivo. Biochem. J.2007, 401 (1), 29–38. https://doi.org/10.1042/BJ20061088.
CrossRef - Mohammad, K. S.; Javelaud, D.; Fournier, P. G. J.; Niewolna, M.; McKenna, C. R.; Peng, X. H.; Duong, V.; Dunn, L. K.; Mauviel, A.; Guise, T. A. TGF-β-RI Kinase Inhibitor SD-208 Reduces the Development and Progression of Melanoma Bone Metastases. Cancer Res.2011, 71 (1), 175–184. https://doi.org/10.1158/0008-5472.CAN-10-2651.
CrossRef - Song, C.; Bae, Y.; Jun, J. J.; Lee, H.; Kim, N. D.; Lee, K. B.; Hur, W.; Park, J. Y.; Sim, T. Identification of TG100-115 as a New and Potent TRPM7 Kinase Inhibitor, Which Suppresses Breast Cancer Cell Migration and Invasion. Biochim. Biophys. Acta – Gen. Subj.2017, 1861 (4), 947–957. https://doi.org/10.1016/j.bbagen.2017.01.034.
CrossRef - Li, X.; Shocron, E.; Song, A.; Patel, N.; Sun, C. L. Discovery of 5-Substituted 2-Amino-4-Chloro-8-((4-Methoxy-3,5-Dimethylpyridin-2-Yl)Methyl)-7,8-Dihydropteridin-6(5H)-Ones as Potent and Selective Hsp90 Inhibitors. Bioorganic Med. Chem. Lett.2009, 19 (10), 2860–2864. https://doi.org/10.1016/j.bmcl.2009.03.074.
CrossRef - Su, Q.; Ioannidis, S.; Chuaqui, C.; Almeida, L.; Alimzhanov, M.; Bebernitz, G.; Bell, K.; Block, M.; Howard, T.; Huang, S.; Huszar, D.; Read, J. A.; Rivard Costa, C.; Shi, J.; Su, M.; Ye, M.; Zinda, M. Discovery of 1-Methyl-1 H-Imidazole Derivatives as Potent Jak2 Inhibitors. J. Med. Chem.2014, 57 (1), 144–158. https://doi.org/10.1021/jm401546n.
CrossRef - Murgueitio, M. S.; Ebner, S.; Hörtnagl, P.; Rakers, C.; Bruckner, R.; Henneke, P.; Wolber, G.; Santos-Sierra, S. Enhanced Immunostimulatory Activity of in Silico Discovered Agonists of Toll-like Receptor 2 (TLR2). Biochim. Biophys. Acta – Gen. Subj.2017, 1861 (11), 2680–2689. https://doi.org/10.1016/j.bbagen.2017.07.011.
CrossRef - Chauhan, P. M. S.; Martins, C. J. A.; Horwell, D. C. Syntheses of Novel Heterocycles as Anticancer Agents. Bioorganic Med. Chem.2005, 13 (10), 3513–3518. https://doi.org/10.1016/j.bmc.2005.02.055.
CrossRef - Geng, P. F.; Wang, C. C.; Li, Z. H.; Hu, X. N.; Zhao, T. Q.; Fu, D. J.; Zhao, B.; Yu, B.; Liu, H. M. Design, Synthesis and Preliminary Biological Evaluation of 5,8-Dihydropteridine-6,7-Diones That Induce Apoptosis and Suppress Cell Migration. Eur. J. Med. Chem.2018, 143, 1959–1967. https://doi.org/10.1016/j.ejmech.2017.11.009.
CrossRef - Li, Z. H.; Zhao, T. Q.; Liu, X. Q.; Zhao, B.; Wang, C.; Geng, P. F.; Cao, Y. Q.; Fu, D. J.; Jiang, L. P.; Yu, B.; Liu, H. M. Synthesis and Preliminary Antiproliferative Activity of New Pteridin-7(8H)-One Derivatives. Eur. J. Med. Chem.2018, 143, 1396–1405. https://doi.org/10.1016/j.ejmech.2017.10.037.
CrossRef - Novosád, D.; Stýskala, J. Analogs of Biologically Active Compounds IX.1 Synthesis of Several New Uracil and Pteridine 6-Azaanalogs Based on Cyclization of Arylhydrazones Derived from Mesoxalic Acid. Arkivoc2017, 2017 (5), 129–140. https://doi.org/10.24820/ark.5550190.p010.123.
CrossRef - Miyoshi, T.; Arai, T.; Nonogawa, M.; Makino, K.; Mori, H.; Yamashita, K.; Sasada, M. Anticancer Photodynamic and Non-Photodynamic Effects of Pterin Derivatives on a Pancreatic Cancer Cell Line. J. Pharmacol. Sci.2008, 107 (2), 221–225. https://doi.org/10.1254/jphs.08002SC.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










