Mineral Oil Shale Part Based-Support as Heterogeneous Catalyst for Organophosphorus Synthesis Assisted by Ultrasounds
Elmustapha Ennesyry , Bahija Mounir
, Bahija Mounir , M’hammed Elkouali, Mohammed Hamza
, M’hammed Elkouali, Mohammed Hamza and Fathallaah Bazi*
and Fathallaah Bazi*
Laboratoire de chimie analytique et moléculaire (LCAM), Faculty of science Ben M’sick, University Hassan II of Casablanca, Boulevard Cdt Driss Harti, BP.7955, Ben M'sick, Casablanca, Morocco.
Corresponding Author E-mail: fathallaah.bazi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380408
Article Received on : 08 Apr 2022
Article Accepted on :
Article Published : 08 Jul 2022
Reviewed by: Dr. Pawan Kumar
Second Review by: Dr. Sanonka Tchegueni
Final Approval by: Dr. Bal krishan
A new heterogeneous catalyst has been developed based on moroccan oil shale raw matter. This new support was used in the α-hydroxyphosphonates synthesis by Pudovik pathway using dialkylphosphites and carbonyls compounds. The transformation was performed by using oil shale-based catalyst under room temperature and by ultrasound-assisted synthetic approach. Both approaches have been found to be efficient in this organophosphorus synthesis. The reaction was carried out with a high yield in dry media, the catalyst is separated easily and reused several times without losing its activity.
KEYWORDS:α-hydroxyphosphonate; Oil shale; Heterogeneous catalysis; Ultrasound
Download this article as:| Copy the following to cite this article: Ennesyry E, Mounir B, Elkouali M, Hamza M, Bazi F. Mineral Oil Shale Part Based-Support as Heterogeneous Catalyst for Organophosphorus Synthesis Assisted by Ultrasounds. Orient J Chem 2022;38(4). |
| Copy the following to cite this URL: Ennesyry E, Mounir B, Elkouali M, Hamza M, Bazi F. Mineral Oil Shale Part Based-Support as Heterogeneous Catalyst for Organophosphorus Synthesis Assisted by Ultrasounds. Orient J Chem 2022;38(4). Available from: https://bit.ly/3bZJTkM |
Introduction
One of the important classes of organophosphorus there is α-hydroxyphosphonates which have attracted an immense attention due to their biological activities such as enzymes inhibitors 1, anti-bacterial and anti-fungal 2,3, anti-cancer 4,5, and anti-HIV activities 6. Adding to that best-known example of phosphonates activity is their potent insecticidal activities 7. Moreover, α-hydroxyphosphonates are synthetic intermediates to achieve a wide important phosphorus compounds of alpha and beta functionalized phosphonates such α-keto 8,9, α-acetoxy 10,11 β-malono 12,13 and α-aminophosphonates 14-16. Many synthetic pathways are used in α-hydroxyphosphonates synthesis, the two main routes are the addition of dialkyl or trialklphosphite to an oxo compound, these methods were described for the first time by Pudovik and Abramov 17. Since then, this reaction got the attention of chemists, a numerous of different catalysts were used to obtain the α-hydroxyphosphonates such as guanidine hydrochloride 18, Amberlyst-15 19, natural phosphate 20, KH2PO4 21, oxalic acid 22 MoO2Cl2 23, MgCl2/Et3N 24, K3PO4 25, Na-FAP 26, MNPs-Guanidine 27, and CeCl3⋅7H2O 28.
On the other hand, Oil shale raw matter is considered as an energy source by conversion this matter to energy and chemical products through the thermochemical conversion technology 29. A number of environmental alternatives have also been developed using Moroccan oil shale as adsorbent material to remove industrial dyes, heavy metals, and radioactive elements 29-36.In this paper, we present the use of this raw matter as heterogeneous catalyst. We present here a simple, rapid, environmentally friendly and high yielding protocol for the synthesis of α-hydroxyphosphonates in in dry media at room temperature or under ultrasonic irradiations (scheme 1), this elaborated support has been used successfully in Knoevenagel reaction 37.
 |
Scheme 1: Synthesis of -hydroxyphosphonates in the presence of the oil shale catalyst. |
Experimental
Generalities
The raw matter comes from the Tarfaya region, Morocco, the catalyst has been prepared as described previously 37. The structural and textural proprieties of the obtained catalyst have been studied using X-ray diffraction, FX, MEB-EDS and BET-BJH analysis.
The isolated synthesized compounds were identified using IRTF on a Bruker tensor 27 FTIR spectrometer and nuclear magnetic resonance analysis performed on a Bruker ADVANCE II NMR spectrometer, using CDCl3 as a solvent.
General procedure for the synthesis of the α-hydroxyphosphonates in dry media
Equimolar mixture (2.5 mmol) of carbonyl compound and dialkylphosphite, 1 g of the support was added in the absence of solvent at room temperature without stirring or under ultrasound irradiation. After completion of the reaction, the mixture was extracted with 15-20 mL of ethyl acetate to give a residue which is purified by chromatographic column (hexane/ethyl acetate), the support recovered off by simple filtration, and the products were identified by their melting points, IRTF and NMR spectroscopy. The recovered support was washed with water and ethanol, then dried at 150°C for 2 hours before reuse or washed with water and ethanol dried and calcined at 900°C for 30 min to regenerate it.
Results and Discussion
Catalyst characterization
The characterization of the support was carried out by XRD which showed that it formed principally of different oxides such as CaO and MgO, also the presence of alumina and silice were identified. The surface area of the catalyst was determined by BET method from the adsorption-desorption isotherm using nitrogen as adsorbent at 77.373 K, the surface area of 3.3103 m²/g was found. According to the IUPAC classification, the isotherm of catalyst is assigned to type (IV) with a distinct hysteresis loop of type H3 and reversible adsorption–desorption process, which is characteristic of mesoporous materials. Furthermore, the pore size distribution curve determined by (BJH) method shows the pore size distribution is centered approximately at 25.20 Å. In addition, the total pore volume is VT= 0.004440 cm³/g.
 |
Figure 1: Catalyst nitrogen adsorption/desorption isotherms (a) and BJH pore size distribution (b) |
In the regard to broaden the application of this new catalyst, a green protocol was adopted for the synthesis of α-hydroxyphosphonates via Pudovik pathway under solvent-free at room temperature with no agitation.
Further it was studied the influence of the catalyst weight on diethyl (hydroxy(phenyl) methyl)phosphonate synthesis. The obtained results have shown that the optimum catalyst weight should be 1 g (Fig.2).
 |
Figure 2: Catalyst weight influence on diethyl (hydroxy(phenyl) methyl)phosphonate synthesis |
Additionally, it was studied the effect of the reaction time with the purpose to identify the minimum time which could lead to the optimum yield (Fig.3). The results showed that the optimum time to achieve the maximum yield was observed and fixed at 60 min. After this time, the yield presents the same values indicating on the possibility of catalyst poisoning, which could happen when dealing with phosphorous reagents such phosphites. That idea will impose itself when was studied the kinetic of this reaction between dimethylphosphite and acetophenone.
 |
Figure 3: Reaction time influence on the synthesis of diethyl (hydroxy(phenyl) methyl)phosphonates. |
Furthermore, (Fig.4) of the kinetic study of the reaction between dimethylphosphite and acetophenone showed that despite the progressive increase in the reaction time, the yield remains unchanged with no significant improvement, the results achieved after 6 and 18 hours respectively gives a yield of 59% and 71%. To improve the used catalytic system, the model reaction was conducted under ultrasonic irradiations, solvent-free conditions, and 1 g of the catalyst (Fig.5). The reaction proceeds smoothly and the formation of the targeted products was accelerated by ultrasounds within a short reaction time, a yield of 77% achieved after 8 min, the large gap of reaction time between room temperature and ultrasounds conditions showed the positive effect of this last.
 |
Figure 4: Kinetic study on the synthesis of dimethyl (1-hydroxy-1-phenylethyl) phosphonates Click here to View figure |
 |
Figure 5: Kinetic study on the synthesis of diethyl (hydroxy(phenyl) methyl)p hosphonates under ultrasound irradiation |
Studying the recyclability of the catalysts considered as the most important feature of this last. In fact, a catalyst will preferably be chosen if after several re-uses its activity stays unchanged or slightly modified. For this and depending on the encouraging obtained results, the reusability and regeneration of the catalyst have been examined, the synthesis was carried out under similar conditions previously used.
The efficiency of the recovered catalyst was checked, the results show that the product yield has dramatically decreased after the 2nd cycle as it shown in Fig.6. These results can be explained in this kind of solid catalysts by the exhaustion of the active sites of the surface by reagents or the products, the thing that prevents contact between the reagents and these active sites. These unsatisfactory lead toward finding the total regeneration of the catalyst, which has been achieved by the thermal treatment of the recoverable catalyst, the results presented in Fig.6 confirm the positive effect of this treatment, and showed that the catalyst can be reused until 4 cycles without any loss of its activity.
 |
Figure 6: Recyclability and the regeneration of the catalyst in the synthesis of diethyl (hydroxy(phenyl) methyl)phosphonates |
To demonstrate the scope of applicability of the catalyst, different derivatives of α-hydroxyphosphonates have been synthesized using the optimized reaction conditions which already in hand. This scope was explored using various aromatic carbonyl compounds bearing substitutions at meta and para positions with a dialkylphosphite compound. As shown in Table 1, when the reaction is carried out at room temperature it affords the corresponding products in good yields (65-86%) within 60 min. The condensation is preceded easily when the aldehydes are used instead of ketones, the thing that has been observed in the case of acetophenone when a yield of 59% and 71% has been achieved respectively after 6 and 18 hours. The efficiency of the catalyst improved when the catalytic system has been assisted by ultrasound irradiations, the formation of the desired compounds was accelerated by ultrasounds irradiation with equal or higher yield compared to the conventional method. These results accentuate the positive effect of ultrasound on the reaction outcome.
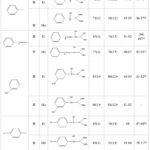 |
Table 1: α-Hydroxyphosphonates synthesis by Pudovik pathway over oil shale catalyst alone and assisted by ultrasounds oil shale catalyst /ultrasounds. |
Finally, the efficiency of the catalyst was compared with that of other catalysts reported previously in the literature, the synthesis of diethyl (hydroxy(phenyl) methyl)phosphonate as a model product compound was chosen. The comparison targeted the catalysts performed in this synthesis under quite similar conditions.
The data summarized in Table 2 clearly shows that this catalyst has an activity similar or high over to other compared catalysts. The method is also effective for a variety of aromatic carbonyls and achieved the products in good yields and in a short time, therefore it’s a novel, environmentally friendly and economically viable method for the synthesis of α-hydroxyphosphonates in dry media. The application of ultrasound and solvent-free methodology makes this protocol a green chemistry approach.
Table 2: Comparison of the efficiency of oil shale catalyst over to other catalysts.
|
Catalyst |
Conditions |
Yield (min) |
References |
|
oil shale catalyst 1g |
2.5 mmol Dry media |
72(60) |
This work |
|
oil shale catalyst 1g,<)))) |
2.5 mmol Dry media |
83(12) |
This work |
|
NP 1g |
2.5 mmol Dry media |
93(15) |
[20] |
|
FAP 1g |
2.5 mmol Dry media |
50(60) |
[26] |
|
HAP 1g |
2.5 mmol Dry media |
59(60) |
[38] |
|
Na2CO3 1g, MW900W |
5mmol Dry media |
75(2) |
[39] |
|
CaO 1g,MW900W |
5mmol Dry media |
70(2) |
[39] |
Conclusion
In this work, it was demonstrated that a solid elaborated depending on the mineral part of oil shale could be considered as an active and promising solid heterogeneous catalyst. XRD and chemical analysis confirmed that the prepared material contains essentially a variety of oxides such as CaO, SiO2, Al2O3 and MgO, which formed due to the thermal treatment of the raw matter (fresh oil shale). Moreover, the textural analysis showed an isotherm of the catalyst which is assigned with characteristics of mesoporous materials.
The obtained catalyst was effectively used in the synthesis of a variety of α-hydroxyphosphonates via Pudovik pathway, easily separated from the reaction mixture, and was reused several times without any loss of activity. In addition, it was demonstrated that the effect of ultrasound led to a decrease in the reaction time and an increase in the yield of the reactions. These results open a way for the use of this inexpensive solid as a catalyst for other transformations.
Physical and Spectral Data of model compound
Diethyl (hydroxy(phenyl) methyl)phosphonates
White solid; Yield : 72% (Method A), 83% (Method B); Melting point : 74-75°C; 1H NMR : (500 MHz, Chloroform-d) δ 7.50 – 7.40 (m, 2H), 7.31 (t, J = 7.4 Hz, 2H), 7.28 – 7.20 (m, 1H), 4.99 (dd, J = 11.1, 7.2 Hz, 1H), 4.07 – 3.89 (m, 4H), 1.18 (dt, J = 21.9, 7.1 Hz, 6H); 13C NMR : (151 MHz, Chloroform-d) δ. 16,48 ; 63.39, 78 ; 127.13 ; 128.05 ; 128.34 ; 136.78; 31P NMR : (202 MHz, Chloroform-d) δ 22.00; IR (KBr) : v cm-1 = 1220 (P=O); 3260 (OH).
Acknowledgement
This research has not received specific funding from any funding agencies in the public, commercial or non-profit sectors.
Conflicts of interest
The authors declares no conflict of Interest
Funding Sources
There is no funding Source.
References
- Ganzhorn, A.J.; Hoflack, J.; Pelton, P.D.; Strasser, F.; Chanal, M.C.; Piettre, S.R. Inhibition of myo-inositol monophosphatase isoforms by aromatic phosphonates. Bioorgan Med Chem. 1998, 6 (10), 1865-1874.
CrossRef - Kategaonkar, A.H.; Pokalwar, R.U.; Sonar, S.S.; Gawali, V.U.; Shingate, B.B.; Shingare, M.S. Synthesis, in vitro antibacterial and antifungal evaluations of new α-hydroxyphosphonate and new α-acetoxyphosphonate derivatives of tetrazolo [1,5-a] quinoline. European. J Med Chem. 2010, 45 (3), 1128-1132.
CrossRef - Pokalwar, R.U.; Hangarge, R.V.; Maske, P.V.; Shingare, M.S. Synthesis and antibacterial activities of α-hydroxyphosphonates and α-acetyloxyphosphonates derived from 2-chloroquinoline-3-carbaldehyde. Arkivoc. 2006, 2006, 196-204.
CrossRef - Kalla, R.M.N.; Lee, H.R.; Cao, J.; Yoo, J.W.; Kim, I. Phospho sulfonic acid: an efficient and recyclable solid acid catalyst for the solvent-free synthesis of α-hydroxyphosphonates and their anticancer properties. New J Chem. 2015, 39 (5), 3916-3922.
CrossRef - Babazadeh, S.; Kazemi Miraki, M.; Pazoki, F.; Heydari, A. Tandem oxidative pudovik reaction Using Fe3O4@ SiO2‐Metformin‐Cu (II) as an efficient and recoverable catalyst. ChemistrySelect. 2020,5 (14), 4263-4266.
CrossRef - Zheng, X.; Nair, V. Synthesis of isomeric nucleoside phosphonates: Cyclic analogs of the anti-HIV active compound, PMEA. Tetrahedron. 1999, 55 (40), 11803-11818.
CrossRef - Kafarski, P.; Lejczak, B. Application of bacteria and fungi as biocatalysts for the preparation of optically active hydroxyphosphonates. J Mol Catal B-Enzym. 2004, 29(1-6), 99-104.
CrossRef - Firouzabadi, H.; Iranpoor, N.; Sobhani, S. Preparation of α-ketophosphonates by oxidation of α-hydroxyphosphonates with neutral alumina supported potassium permanganate (NASPP) under solvent-free conditions and potassium permanganate in dry benzene. Tetrahedron Lett. 2002, 43 (3), 477-480.
CrossRef - Kaboudin, B.; Nazari, R.A. Convenient and mild procedure for the preparation of α-keto phosphonates of α -hydroxyphosphonates under solvent-free conditions using microwave. Synthetic Commun. 2001, 31 (15), 2245-2250.
CrossRef - Rostami, A.; Atashkar, B.; Moradi, D. Synthesis, characterization and catalytic properties of magnetic nanoparticle supported guanidine in base catalyzed synthesis of α-hydroxyphosphonates and α-acetoxyphosphonates. Appl Catal A-Gen. 2013, 467, 7-16.
CrossRef - Baccari, Z.; Sanhoury, M.A.K.; Crousse, B.; Barhoumi-Slimi, T. Synthesis of new α-hydroxyphosphonates and α-acetoxyphosphonates. Synthetic Commun. 2018, 48 (10), 1199-1205.
CrossRef - Sobhani, S.; Parizi, Z.P.; Razavi, N. Nano n-propylsulfonated γ-Fe2O3 as magnetically recyclable heterogeneous catalyst for the efficient synthesis of β-phosphonomalonates. Appl Catal A-Gen. 2011, 409, 162-166.
CrossRef - Yu, Y.Q.; Xu, D.Z. Polystyrene-supported DABCO as a highly efficient and recyclable heterogeneous catalyst for the one-pot synthesis of β-phosphonomalonates. Tetrahedron. 2015, 71 (19), 2853-2857.
CrossRef - Kiss, N.Z.; Kaszás, A.; Drahos, L.; Mucsi, Z.; Keglevich, G.A. neighbouring group effect leading to enhanced nucleophilic substitution of amines at the hindered α-carbon atom of an α-hydroxyphosphonate. Tetrahedron lett. 2012, 53 (2), 207-209.
CrossRef - Rádai, Z.; Kiss, N.Z.; Mucsi, Z.; Keglevich, G. Synthesis of α-hydroxyphosphonates and α-aminophosphonates. Phosphorus, Sulfur. 2016, 191(11-12), 1564-1565.
CrossRef - Keglevich, G.; Rádai, Z. α-Hydroxyphosphonates as intermediates in the Kabachnik–Fields reaction: New proof of their reversible formation. Tetrahedron Lett. 2020, 61 (23), 151961.
CrossRef - Rádai, Z., Keglevich G. Synthesis and reactions of α-hydroxyphosphonates. Molecules. 2018, 23 (6), 1493.
CrossRef - Heydari, A.; Arefi, A.; Khaksar, S.; Tajbakhsh, M. Hydrophosphonylation of aldehydes catalyzed by guanidine hydrochloride in water. Catalysis Commun. 2006, 7 (12), 982-984.
CrossRef - Tajbakhsh, M.; Heydari, A.; Khalilzadeh, M.A.; Lakouraj, M.M.; Zamenian, B.; Khaksar, S. Amberlyst-15 as a heterogeneous reusable catalyst for the synthesis of α-hydroxy phosphonates in water. Synlett. 2007, 2007(15), 2347-2350.
CrossRef - Smahi, A.; Solhy, A.; Tahir, R.; Sebti, S.; Mayoral, J.A.; García, J.I. Zahouily M. Preparation of α-hydroxyphosphonates over phosphate catalysts. Catalysis Commun. 2008, 9 (15), 2503-2508.
CrossRef - Mandhane, P.G.; Joshi, R.S.; Nagargoje, D.R.; Gill, C.H. Ultrasound-promoted greener approach to synthesize α-hydroxy phosphonates catalyzed by potassium dihydrogen phosphate under solvent-free condition. Tetrahedron Lett. 2010,51(11), 1490-1492.
CrossRef - Vahdat, S.M.; Baharfar, R.; Tajbakhsh, M.; Heydari, A.; Baghbanian, S.M.; Khaksar, S. Organocatalytic synthesis of α-hydroxy and α-aminophosphonates. Tetrahedron Lett. 2008, 49(46) 6501-6504.
CrossRef - De noronha, R.G.; Costa, P.J.; Romao, C.C.; Calhorda, M.J.; Fernandes, A.C. MoO2Cl2 as a novel catalyst for C− P bond formation and for hydrophosphonylation of aldehydes. Organometallics. 2009 28 (21), 6206-6212.
CrossRef - Tajbakhsh, M.; Khaksar, S.; Tafazoli, Z.; Bekhradnia A. MgCl2/Et3N Base System as a New Catalyst for the Synthesis of α‐Hydroxyphosphonate. Chinese J Chem. 2012, 30 (4), 827-829.
CrossRef - Kulkarni, M.A.; Lad, U.P.; Desai, U.V.; Mitragotri, S.D.; Wadgaonkar, P.P. Mechanistic approach for expeditious and solvent-free synthesis of α-hydroxy phosphonates using potassium phosphate as catalyst. C. R. Chim. 2013, 16 (2), 148-152.
CrossRef - Ramananarivo, H.R.; Solhy, A.; Sebti, J.; Smahi, A.; Zahouily, M.; Clark, J.; Sebti S. An eco-friendly paradigm for the synthesis of α-hydroxyphosphonates using sodium-modified fluorapatite under solventless conditions. ACS Sustain; Chem; Eng. 2013, 1 (4), 403-409.
CrossRef - Rostami, A.; Atashkar, B.; Moradi, D. Synthesis, characterization and catalytic properties of magnetic nanoparticle supported guanidine in base catalyzed synthesis of α-hydroxyphosphonates and α-acetoxyphosphonates. Appl Catal A-General. 2013, 467, 7-16.
CrossRef - Mahesh R., Sharma R., Kour P., Kumar A. CeCl3⋅7H2O-catalysed hydrophosphonylation of aldehydes and ketones: An expeditious route to α-hydroxyphosphonates under solvent-free conditions. Phosphorus, Sulfur. 2019, 194 (12), 1091-1097.
CrossRef - Cheikh Moine, E.; Groune, K.; El Hamidi, A.; Khachani, M.; Halim, M.; Arsalane, S. Multistep process kinetics of the non-isothermal pyrolysis of Moroccan Rif oil shale. Energy. 2016, 115, 931-941.
CrossRef - Oumam, M.; Abourriche, A.; Adil, A.; Hannache, H.; Pailler, R.; Naslain R., Puillot J.P. Elaboration et caractérisation d’un nouveau matériau adsorbant à partir des schistes bitumineux du Maroc. Ann. Chim.-Sci. Mat. 2003, 28 (4), 59-74.
CrossRef - Miyah, Y.; Idrissi, M.; Lahrichi, A.; Zerrouq, F. Removal of a Cationic Dye–Méthylène Bleu–From Aqueous Solution by Adsorption onto Oil Shale Ash of Timahdit (Morocco). Oil Shale. 2014, 3 (8).
CrossRef - Elhammoudi, N.; Oumam, M.; Mansouri, S. Modeling Using the Response Surface Methodology the Activation Process of Moroccan Oil Shale for Removal of Cd (II). Int. J. Chem. Sci. 2018, 16 (2), 1-10.
CrossRef - Elhammoudi, N.; Oumam, M.; Mansouri, S.; Abourriche, A.; Chham, A.I.; Hannache, H. Modeling and optimization of the activation process of oil shale for removal of cd (II) using the response surface methodology. Int. J. Recent. Sci. Res. 2018, 9 (5A), 26455-26464
- Khouya, E.; Fakhi, S.; Hannache, H.; Abbe, J.C.; Andres, Y.; Naslain, R.; Nourredine A. New adsorbents from oil shales: Preparation, characterization and U, Th isotope adsorption tests. J Radioanal Nucl Ch. 2004, 260 (1), 159-166.
CrossRef - Khouya, E.; Fakhi, S.; Hannache, H.; Ichcho S., Pailler R., Naslain, R.; Abbe, J.C. Production of a new adsorbent from Moroccan oil shale by chemical activation and its adsorption characteristics for U and Th bearing species. J.Phys., IV. 2005, 123, 87-93.
CrossRef - Khouya, E.H.; Legrouri, K.; Fakhi, S.; Hannache, H. Adsorption of uranium and thorium on new adsorbent prepared from Moroccan oil shale impregnated with phosphoric acid. Nat. Preced. 2010 1-1.
CrossRef - Ennesyry, E.; Bazi, F.; Mounir, B.; Elkouali, M.; Hannache, H.; Talbi, M.; Hamza, M. Knoevenagel Condensations Catalyzed by New Oil Shale Recyclable Catalyst at Room Temperature and Assisted by Ultrasounds Irradiations. Orient. J. Chem. 2021, 37 (6).
CrossRef - Solhy, A.; Sebti, S.; Tahir, R.; Sebti, J.; Ould Abba, M.; Bousmina, M.; Zahouily, M. Remarkable catalytic activity of sodium-modified-hydroxyapatite in the synthesis of α-hydroxyphosphonates. Curr Org Chem. 2010, 14 (14), 1517-1522.
CrossRef - Kaboudin B., Nazari R. The synthesis of α-hydroxyphosphonates mediated by microwave irradiation under solvent-free conditions. J Chem Res-s. 2002, 2002 (6), 291-292.
CrossRef - Sardarian, A. R.; Kaboudin B. Surface-mediated solid phase reactions: preparation of diethyl 1- hydroxyarylmethylphosphonates on the surface of magnesia. Synth commun. 1997, 27 (4), 543-551.
CrossRef - Wang, C.; Zhou, J.; Lv, X.; Wen, J.; He, H. Solvent-Free Synthesis of Tertiaryα-Hydroxyphosphates by the Triethylamine-Catalyzed Hydrophosphonylation of Ketones. Phosphorus, Sulfur. 2013, 188 (10), 1334-1339.
CrossRef - Jahani, F.; Zamenian, B.; Khaksar S., Tajbakhsh M. Pyridine 2, 6-dicarboxylic acid as a bifunctional organocatalyst for hydrophosphonylation of aldehydes and ketones in water. Synthesis. 2010, 2010 (19), 3315-3318.
CrossRef - Goldeman, W.; Soroka, M. The preparation of dialkyl 1-hydroxyalkylphosphonates in the reaction of trialkyl phosphites with oxonium salts derived from aldehydes or ketones. Synthesis. 2006, 2006 (18) 3019-3024.
CrossRef - Saito B., Egami H., Katsuki T. Synthesis of an optically active Al (salalen) complex and its application to catalytic hydrophosphonylation of aldehydes and aldimines. J Am Chem So. 2007, 129 (7), 1978-1986.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









