Micellar Effect on Hypersensitive Transitions of Holmium (III) Complexes with Few Indole Derivatives: Doped System Study
GCRC, P.G. Department of Chemistry, Government. Dungar College (A-Grade), M.G. S. University Bikaner, 334003, Rajasthan (India).
Corresponding Author E-mail: hsbhandari78@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380413
Article Received on : 22 Jun 2022
Article Accepted on :
Article Published : 18 Jul 2022
Reviewed by: Dr. Garima Prajapat
Second Review by: Dr. hikmat mohamad
Final Approval by: Dr. Ved Prakash
Optical absorption spectra of Holmium (III)-complexes with Indole derivative [viz:1H-indole -3-carboxylic acid, 2-(1H-indole-3-yl)ethanoic acid and 4-(1H-indole-3-yl)butanoic acid] have been analyzed as a doped system in different medium. Micellar effect is sensational to the transition 5G6← 5I8 and become hypersensitive in UV-Visible absorption spectra. Micellar medium of cationic surfactant found be most effective to enhance the intensity of hypersensitive transition. Intensity parameters, Oscillator Strength (P), magnitude was observed somewhat different in the micellar medium as compared to the non-micellar medium. Applicability of Judd Ofelt theory indulged in spectral analysis for various parameters. Judd Ofelt parameters and other covalency parameters are in favour for the existence of covalency and the degree has been raised in micellar medium. Nonionic surfactant (BRIJ35), Cationic surfactant (CTAB), and anionic surfactant (SDS) shows significant interaction, reflects in spectral study of Holmium(III)-Indole derivative complexes.
KEYWORDS:Covalency Parameters; Hypersensitive Transition; Judd Ofelt Parameters; Micellar Medium; Oscillator Strength
Download this article as:| Copy the following to cite this article: Regar R, Ghasia S, Bhandari H. S. Micellar Effect on Hypersensitive Transitions of Holmium (III) Complexes with Few Indole Derivatives: Doped System Study. Orient J Chem 2022;38(4). |
| Copy the following to cite this URL: Regar R, Ghasia S, Bhandari H. S. Micellar Effect on Hypersensitive Transitions of Holmium (III) Complexes with Few Indole Derivatives: Doped System Study. Orient J Chem 2022;38(4). Available from: https://bit.ly/3uRr6P6 |
Introduction
Metal ligand interaction is key factor to understand the various physicochemical properties of the complexes. Decades passed to understand the nature of bonding in metal complexes in different solvents, as solvent role is crucial, may it be non-micellar or micellar. The extent and way of interaction is very much important in biological systems. The nature of solvent effects the nature of bonding reflecting the change in physicochemical parameters.1-4 Now greener solvents are replacing the hazardous solvents following the principles of green chemistry. In recent decades, considerable interest in lanthanide metal-ligand interaction has been reported. The Optical absorption studies are concerned with the metal-ligand interaction in different solvent systems.5-6 Lanthanide compounds have their use in luminescent chemosensors, shift reagents, medical diagnosis of disease and optical cell imaging.7-9 Holmium which is a heavier lanthanide has a wide range of utility in medical science for the diagnosis of disease. Holmium lasers are used for the removal of prostate cancer and urinary calculas. Holmium-YAG lasers are used for fragmentation of colonic fecalith and kidney stones material.10-12 In oncology, 166Ho microspheres are used for the radioembolization of liver cancer13, hepatocellular carcinoma of liver cancer and bone metastases.14 Due to good penetration depth, high magnetic moment, emission of β– particles and γ-radiations, 166Ho3+ based compounds are used in computed tomography, diagnosis, therapeutic and Magnetic Resonance Imaging.15 High magnetic moment and electron relaxation time properties make the potential possibility of Ho(III) as a contrasting agent in MRI (Magnetic Resonance Imaging).16 Indole derivatives have vast biological importance for therapeutic use as anticancer, antimalarial, antiviral and antitubercular activity,17 due to the presence of the Indole nucleus as a base structure that provides a surface to attach the desirable drug residue on it. Use of Indole derivatives as an anticonvulsant, antipsychotic, antidepressant, sedative and antianxiety drugs for the treatment of central nervous system disorders.18
In lanthanide, 4f subshell electrons are closer to the nucleus than 5d electrons, and are shielded by 5s and 5p subshell electrons. Shielded 4f orbitals exhibit magnetic and luminescent properties as these have lower energy compared to 5d orbitals.19 4f – 4f transition of 4fN electrons which are Laporte forbidden transitions, is the result of optical absorption.20 Hypersensitive transitions in lanthanide metals which have high oscillator strength, have different surrounding environment from other transitions and follows as |ΔJ|≤ 2, | ΔL|≤ 2, |ΔS|=0 selection rule for f-f transition. Polarization of ligand, mixing of f-d orbitals, charge transfer ligand to metal, molecular vibration and spin-orbital configuration interaction affects the hypersensitive transitions.21 Hypersensitive transitions of trivalent Holmium ion are due to the interaction of coordinating solvent ligands.6 Holmium complexes have a positive value of magnetic susceptibilities. The existence of magnetic dipolar nature is the reason for the interaction of f-f transition.22 Sinha investigates the parameter of covalency in the lanthanides.23 Density functional theory (DFT) and scanning tunneling microscopy show the interaction between the metal Holmium ion and organic ligands and reveal the charge transfer, relative stability of complex and bonding environment among the Holmium and organic ligands.5 Spin multiplicity of f-block metals shows the minimum effect on IR spectra which reflect the marginal crystal field splitting that was confirmed by DFT calculations.24
In the present optical absorption study, oscillator strength, hypersensitive transitions, Judd Ofelt parameters, and covalent bonding parameter of indole derivatives 1H-indole-3-carboxylic acid (I3CA), 2-(1H-indole-3-yl)ethanoic acid (2IEA), 4-(1H-indole-3-yl)butanoic acid (4IBA) with Holmium(III) in non-micellar solvent (alcohol+water) and micellar medium BRIJ35 (Polyethylene glycol monododecyl ether), CTAB (Cetyl trimethyl ammonium bromide), SDS (Sodium dodecyl sulphate) have been investigated using Judd Ofelt method. No earlier spectral studies of Ho (III) cation have been reported with indole derivatives I3CA, 2IEA, and 4IBA in non-micellar as well as in nonionic micellar surfactant BRIJ35, cationic micellar surfactant CTAB and anionic micellar surfactant SDS system. Investigations in present work are aimed to know the metal-ligand interaction of Ho(III) complexes with Indole derivatives under the environment effect of micellar medium.
Materials and Methods
Preparation of Solutions
All the chemical used are of analytical grade. Holmium(III)chloride was studied with three indole derivative ligands viz:1H-indole-3-carboxylic acid (I3CA), 2-(1H-indole-3-yl)ethanoic acid (2IEA), and 4-(1H-indole-3-yl)butanoic acid (4IBA). Standard solution of Holmium(III)chloride and ligands of 0.05 M concentration with metal-ligand ratio (1:3) were prepared in non-micellar (ethanol+water) and micellar medium solutions (BRIJ35, SDS, CTAB). Electronic spectrum of doped systems observed and recorded on double beam UV-Visible Spectrophotometer (UV 5704 SS) having high resolution and spectral range of 190 nm to 1100 nm at Green Chemistry Research Centre (GCRC), Bikaner (India). The study done at room temperature (298K).
Experimentation have been done using doped model technique, where the saturated solution of ligand has been prepared in non-micellar and in micellar medium, during solubilization phenomenon metal ion (Holmium(III) chloride) stoichiometric amount is added. The observations of dopped study have been done as the interaction between metal ion Ho(III) and Indole derivative as a ligand in non-micellar solvent medium [Alcohol + Water] as well as in micellar medium BRIJ35, SDS, and CTAB by electronic absorption spectra.
Calculations
Oscillator Strength
Oscillator strength ‘P’, dimensionless quantity, is a measure of the intensity of an absorption band.25-26 The area under the peak of an absorption spectrum represents oscillator strength ‘P’ can also expressed in term of half bandwidth (Δv1/2).
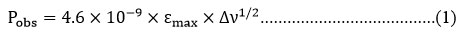
Δv1/2 half bandwidth of an absorption band
εmax can be expressed as

OD = Optical density, C = Concentration (mol/L), L = Path length (cm)
Judd Ofelt Parameters
Energy of a transition in cm-1 and square of matrix element are related with the oscillator strength which is due to an induced electric dipole transition.27-28
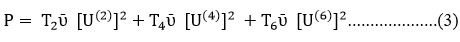
ῡ = energy of transition (cm-1) , and U(2) , U(4) , U(6) = Matrix elements
T2, T4, T6 are Judd Ofelt parameters represented in ratioT4 / T2 as coordination parameter and ratio T4 / T6 as symmetry parameters. Judd Ofelt parameter T2, T4, and T6 are computed by Partial and multiple regression method.29
Covalency/Bonding Parameters ( β , b1/2, δ and η )
β : -Nephelauxetic effect which is used to estimate the ”degree of covalency” is given as.
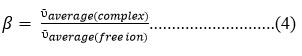
β = Nephelauxetic ratio
ῡaverage(complex) = average bond position of complex in absorption spectra in wavenumber ( cm-1 ) ῡ
ῡaverage(free ion) = average bond position of free ion in absorption spectra in wavenumber ( cm-1 )
b1/2 = Expression of metal wave function when covalent bond formation takes place by f-orbitals cm-1
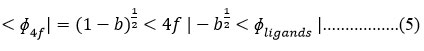
b1/2 = amount of 4f orbital mixing
For electronic transition, required energy depends on inter electronic repulsion and spin orbit coupling constant. Nephelauxetic ratio β and covalency factor correlated as.30
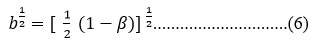
δ = Sinha expressed δ scale to explain the covalency in complexes.23

The value of δ is found positive then it represents covalent bonding in the metal-ligand Complexes while negative value of δ represents the ionic bonding in the metal-ligand complexes.
η = Covalency angular overlap parameter represented as.31

Result and Discussion
Solution behavior of Ho(III) ion with I3CA, 2IEA, and 4IBA doped systems have been analyzed through the study of various parameters exploited from electronic spectrum obtained in the spectral range of 350 nm to 800 nm in non-micellar (alcohol+water) and micellar medium (BRIJ35, SDS, and CTAB). Nine bands for Ho(III) ion with I3CA, 2IEA, and 4IBA doped systems with significant shapes and enhanced intensities have been recorded as multiplet-to-multiplet transitions32 as 3K7, (5G,3G)5, 5G6, 5F2, 5F3, 5F4, 5S2, 5F5, 5I4 ← 5I8 resolved into a Gaussian shaped curve which are represented in figure – 1, 2 and 3.
 |
Figure 1: Absorbance versus Wavelength of Ho(III)-I3CA Complex in non-micellar (alcohol + water) and micellar medium (SDS, CTAB and BRIJ35).Transitions are shown from 5I8 to different energy levels. Hypersensitive transitions are magnified in upper right. |
 |
Figure 2: Absorbance versus Wavelength of Ho(III)-2IEA Complex in non-micellar (alcohol+water) and micellar medium (SDS, CTAB and BRIJ35). Transitions are shown from 5I8 to different energy levels. Hypersensitive transitions are magnified in upper right. |
 |
Figure 3: Absorbance versus Wavelength of Ho(III)-4IBA Complex in non-micellar (alcohol+water) and micellar medium (SDS, CTAB and BRIJ35). Transitions are shown from 5I8 to different energy levels. Hypersensitive transitions are magnified in upper right. |
The present investigation reveal that metal-ligand interaction occurred between the Ho(III) ion and indole derivatives in all 12 metal-ligand doped systems i.e. Ho(III) ion complexes with indole derivatives (I3CA, 2IEA, 4IBA).
Previous literature witnesses Oscillator strength (Pexp), measurement of intensity, of few transitions supposed to be very much sensitive to the environment, named as hypersensitive transitions. A correlation is well established in between increase of intensity of hypersensitive transitions33-35 and increase of coordination number.36 Computed oscillator strength (Pcal) is also obtained for comparative analysis of Ho(III)-with (I3CA, 2IEA, and 4IBA) doped systems, tabulated in Table-1(A). and Table-1(B).Present study reveals hypersensitive transitions and other transitions dramatically found to be of greater oscillator strength in micellar medium rather non micellar medium. The hypersensitive transitions are observed as (5G,3G)5 ← 5I8 and 5G6 ← 5I8, where 5G6 ← 5I8 transitions found to be most intense shown in figure -1,2 and 3. 5G6 ← 5I8, f-f transition has the maximum oscillator strength because the matrix element has a high value, whereas 5I4 ← 5I8, f-f transition has the lowest oscillator strength since the matrix element has a low value except zero.32 The augmentation of εmax and oscillator strength is demonstrated via metal-ligand interaction. In CTAB micellar medium, the highest oscillator strength is found in Ho(III)-I3CA complex. Micellar media produces better outcomes than non-micellar medium, such as an increase in εmax and oscillator strength.
By the incorporation of experimental value of oscillator strength and matrix element value in equation Judd Ofelt parameter Tλ is obtained in three parameters set viz: T2, T4, T6. Computed value of T2, T4, T6 are represented inTable-2. Micellar sensitivity reflects in Judd Ofelt parameter T2 having a positive value establishes the existence of covalency in lanthanides. The T4/T2 ratio, also known as coordination parameters, is essentially constant, indicating that the Ho(III) ion is in the same coordination environment. As a result, the Indole derivatives I3CA, 2IEA, and 4IBA are said to have the same coordination environment around the Ho(III) ion. The high variability in the values of the symmetry parameter T4/T6 ratio indicates that the symmetry of central metal Ho(III) ions has changed.
Covalency parameters, viz: Bonding Parameter (b1/2), Nephelauxetic ratio β, Sinha’s covalency parameter δ and covalency angular overlap parameter (η) are given in Table-3. Due to decrease in inter electronic repulsion parameter, highest nephelauxetic effect β as red shift is observed in Ho(III)-2IEA in cationic surfactant (CTAB) micellar medium. Higher value of red shift shows higher extent of mixing of 4f orbital by b1/2. Sinha’s covalency factor δ and covalency angular overlap parameter η represent covalent bonding between Ho(III) ion and indole derivatives (I3CA, 2IEA, 4IBA). In absorption spectra, the nephelauxetic ratio β is less than one, revealing a red shift in electronic transition spectra. In comparison to the free Ho(III) metal ion, interaction of Ho(III) ions and indole derivative complexes causes absorption bands to shift towards higher wavelengths or lower wave numbers. The value of r.m.s σ is too low, validating the data for Holmium(III) with indole derivative systems doped study.
Table 1: (A). Values of Oscillator Strength (P x 106) of 3K7, (5G,3G)5, 5G6, 5F2, 5F3 ← 5I8 transition Computed for Ho(III)-Complexes with Indole derivative Ligands (I3CA, 2IEA, 4IBA) in non-micellar (alcohol+water) and micellar medium (SDS, CTAB and BRIJ35).
|
S. No. |
LEVEL |
3K7 |
(5G,3G)5 |
5G6 |
5F2 |
5F3 |
|||||
|
Ho(III) with Indole derivatives, doped systems |
Pexp |
Pcal |
Pexp |
Pcal |
Pexp |
Pcal |
Pexp |
Pcal |
Pexp |
Pcal |
|
|
1 |
Ho(III)-I3CA (ALCOHOL+WATER) |
4.35913 |
0.03196 |
6.80725 |
2.02000 |
6.75717 |
3.14556 |
4.03737 |
0.05713 |
4.85608 |
0.100462 |
|
2 |
Ho(III)-I3CA ( BRIJ 35) |
5.32692 |
0.04968 |
10.3726 |
2.54322 |
10.8989 |
5.28824 |
9.61097 |
0.08851 |
3.65567 |
0.15564 |
|
3 |
Ho(III)-I3CA ( SDS) |
5.63254 |
0.04665 |
7.44599 |
2.90968 |
8.14306 |
5.1262 |
4.06796 |
0.07262 |
4.03963 |
0.127553 |
|
4 |
Ho(III)-I3CA ( CTAB) |
8.17122 |
0.05319 |
11.2662 |
3.13037 |
11.60083 |
6.21511 |
4.94374 |
0.07748 |
7.32353 |
0.136385 |
|
5 |
Ho(III)-2IEA (ALCOHOL+WATER) |
5.83981 |
0.0933 |
7.19311 |
2.87017 |
9.132193 |
5.93173 |
2.29482 |
0.27484 |
4.65538 |
0.483271 |
|
6 |
Ho(III)-2IEA ( BRIJ35) |
8.77026 |
0.04665 |
9.56421 |
3.08273 |
11.15765 |
6.72263 |
3.96911 |
0.03705 |
6.02175 |
0.065143 |
|
7 |
Ho(III)-2IEA ( SDS) |
7.05907 |
0.04383 |
3.55647 |
1.13004 |
5.30711 |
2.35585 |
1.77672 |
0.14134 |
2.74707 |
0.248265 |
|
8 |
Ho(III)-2IEA ( CTAB) |
6.94468 |
0.03212 |
10.871 |
2.92467 |
10.33983 |
5.07726 |
6.37673 |
0.00559 |
8.36378 |
0.009814 |
|
9 |
Ho(III)-4IBA (ALCOHOL+WATER) |
4.82125 |
0.05201 |
8.4193 |
3.40263 |
10.3168 |
6.87332 |
2.35328 |
0.05491 |
4.6782 |
0.096454 |
|
10 |
Ho(III)-4IBA ( BRIJ35) |
7.18005 |
0.03858 |
8.65789 |
2.85919 |
9.10633 |
4.80502 |
3.5458 |
0.04221 |
3.95804 |
0.074142 |
|
11 |
Ho(III)-4IBA ( SDS) |
8.66276 |
0.0649 |
9.98007 |
3.90646 |
10.23389 |
5.82242 |
3.20659 |
0.13005 |
4.79172 |
0.228429 |
|
12 |
Ho(III)-4IBA ( CTAB) |
8.36809 |
0.01931 |
8.16865 |
1.76261 |
7.613884 |
2.61955 |
3.2255 |
0.0121 |
4.17244 |
0.021254 |
Table 1: (B). Values of Oscillator Strength (P x 106) of 5F4, 5S2, 5F5, 5I4 ← 5I8 transition Computed for Ho(III)-Complexes with Indole derivative Ligands (I3CA, 2IEA, 4IBA) in non-micellar (alcohol+water) and micellar medium (SDS, CTAB and BRIJ35).
|
S. No. |
LEVEL |
5F4 |
5S2 |
5F5 |
5I4 |
r.m.s dev. ±σ x 106 |
||||||
|
Ho(III) with Indole derivatives, doped systems |
Pexp |
Pcal |
Pexp |
Pcal |
Pexp |
Pcal |
Pexp |
Pcal |
||||
|
1 |
Ho(III)-I3CA (ALCOHOL+WATER) |
3.67884 |
0.88211 |
3.25314 |
0.058267 |
3.08792 |
1.16805 |
0.88923 |
0.00143 |
3.584645 |
||
|
2 |
Ho(III)-I3CA ( BRIJ 35) |
8.59699 |
1.16407 |
3.69773 |
0.090188 |
2.70347 |
1.50839 |
2.58153 |
0.00221 |
5.772538 |
||
|
3 |
Ho(III)-I3CA ( SDS) |
5.06718 |
1.24266 |
0.99138 |
0.07398 |
2.13826 |
1.66301 |
0.28576 |
0.00181 |
3.462881 |
||
|
4 |
Ho(III)-I3CA ( CTAB) |
8.10677 |
1.3338 |
3.37756 |
0.079021 |
2.62922 |
1.78598 |
1.52674 |
0.00176 |
5.737065 |
||
|
5 |
Ho(III)-2IEA (ALCOHOL+WATER) |
5.86172 |
1.88288 |
1.77109 |
0.280295 |
3.02396 |
2.08427 |
1.34366 |
0.00686 |
3.402019 |
||
|
6 |
Ho(III)-2IEA ( BRIJ35) |
6.89992 |
1.18661 |
1.8367 |
0.037783 |
2.48818 |
1.67178 |
0.85567 |
0.00093 |
5.011074 |
||
|
7 |
Ho(III)-2IEA ( SDS) |
3.30604 |
0.84793 |
1.62337 |
0.144142 |
3.61102 |
0.89154 |
1.46933 |
0.00353 |
3.171122 |
||
|
8 |
Ho(III)-2IEA ( CTAB) |
6.30009 |
1.03536 |
2.69986 |
0.005709 |
2.93883 |
1.53067 |
0.89595 |
0.00014 |
5.644707 |
||
|
9 |
Ho(III)-4IBA (ALCOHOL+WATER) |
6.13282 |
1.35488 |
1.57604 |
0.055943 |
2.35378 |
1.87719 |
1.95476 |
0.00137 |
3.578133 |
||
|
10 |
Ho(III)-4IBA ( BRIJ35) |
6.86606 |
1.12579 |
3.14635 |
0.043002 |
2.65821 |
1.56881 |
1.778 |
0.00096 |
4.43988 |
||
|
11 |
Ho(III)-4IBA ( SDS) |
7.97941 |
1.76365 |
2.50491 |
0.132488 |
3.1523 |
2.30128 |
1.64716 |
0.00295 |
4.810333 |
||
|
12 |
Ho(III)-4IBA ( CTAB) |
6.51729 |
0.64907 |
5.28223 |
0.012316 |
2.84504 |
0.93676 |
1.79749 |
0.00027 |
5.080689 |
||
Table 2: Values of Tλ Parameters Computed for Ho(III)-Complexes with Indole derivative Ligands (I3CA, 2IEA, 4IBA) in non-micellar (alcohol+water) and micellar medium (SDS, CTAB and BRIJ35).
|
S.NO. |
Ho(III) with Indole derivatives, doped systems |
T2x1010 |
T4x1010 |
T6x1010 |
T4/T2 |
T4/T6 |
|
1 |
Ho(III)-I3CA (ALCOHOL+WATER) |
0.047242453 |
1.572276994 |
0.14053031 |
33.28102 |
11.1881698 |
|
2 |
Ho(III)-I3CA ( BRIJ 35) |
0.448794228 |
1.981896251 |
0.21771634 |
4.4160467 |
9.10311224 |
|
3 |
Ho(III)-I3CA ( SDS) |
0.246459484 |
2.267501547 |
0.17842732 |
9.2003014 |
12.7082647 |
|
4 |
Ho(III)-I3CA ( CTAB) |
0.474160883 |
2.436548116 |
0.19058478 |
5.1386527 |
12.7845895 |
|
5 |
Ho(III)-2IEA (ALCOHOL+WATER) |
0.455877328 |
2.236519412 |
0.67602029 |
4.9059676 |
3.30836134 |
|
6 |
Ho(III)-2IEA ( BRIJ35) |
0.654131542 |
2.399502715 |
0.09112458 |
3.6682266 |
26.3321116 |
|
7 |
Ho(III)-2IEA ( SDS) |
0.178570872 |
0.879470449 |
0.34764309 |
4.9250499 |
2.5298085 |
|
8 |
Ho(III)-2IEA ( CTAB) |
0.233030114 |
2.290199792 |
0.01375646 |
9.8279135 |
166.481803 |
|
9 |
Ho(III)-4IBA (ALCOHOL+WATER) |
0.55692065 |
2.648491518 |
0.13492468 |
4.7555994 |
19.6294076 |
|
10 |
Ho(III)-4IBA ( BRIJ35) |
0.181554163 |
2.225501467 |
0.10371299 |
12.258058 |
21.4582719 |
|
11 |
Ho(III)-4IBA ( SDS) |
0.011750152 |
3.040593915 |
0.31953604 |
258.7706 |
9.51565241 |
|
12 |
Ho(III)-4IBA ( CTAB) |
0.013665303 |
1.371972909 |
0.02973116 |
100.39828 |
46.1459569 |
Table 3: Values of Bonding parameters [ β, b1/2, δ% and η] Computed for Ho(III)complexes with Indole derivative Ligands in non-micellar (alcohol+water) and micellar medium (SDS, CTAB and BRIJ35).
|
S.No. |
Ho(III) with Indole derivatives, doped systems |
β |
b1/2 |
δ |
η |
|
1 |
Ho(III)-I3CA (ALCOHOL+WATER) |
0.996530905 |
0.041647902 |
0.348117194 |
0.001739074 |
|
2 |
Ho(III)-I3CA ( BRIJ 35) |
0.995886643 |
0.045350616 |
0.413034625 |
0.002063045 |
|
3 |
Ho(III)-I3CA ( SDS) |
0.996147767 |
0.043887545 |
0.386713027 |
0.001931699 |
|
4 |
Ho(III)-I3CA ( CTAB) |
0.996617205 |
0.041126602 |
0.339427691 |
0.001695701 |
|
5 |
Ho(III)-2IEA (ALCOHOL+WATER) |
0.996709687 |
0.040560526 |
0.330117452 |
0.001649227 |
|
6 |
Ho(III)-2IEA ( BRIJ35) |
0.997288392 |
0.036821241 |
0.271898033 |
0.001358567 |
|
7 |
Ho(III)-2IEA ( SDS) |
0.996695431 |
0.040648301 |
0.33155251 |
0.001656391 |
|
8 |
Ho(III)-2IEA ( CTAB) |
0.995427289 |
0.047815849 |
0.459371665 |
0.002294227 |
|
9 |
Ho(III)-4IBA (ALCOHOL+WATER) |
0.99698468 |
0.038828596 |
0.302443945 |
0.001511078 |
|
10 |
Ho(III)-4IBA ( BRIJ35) |
0.997184188 |
0.037522073 |
0.282376304 |
0.001410886 |
|
11 |
Ho(III)-4IBA ( SDS) |
0.996947392 |
0.039067943 |
0.306195526 |
0.001529807 |
|
12 |
Ho(III)-4IBA ( CTAB) |
0.997081453 |
0.03820044 |
0.292709008 |
0.001462476 |
Conclusion
The interaction between metal and ligand in various solvent systems is the principal consequence of the optical absorption investigation. The compartmental behavior of micellar systems provides better ways to species for interaction thus a greater degree of interaction between the Holmium ion and indole derivatives ligands as doped systems is seen in the presence of micellar medium. The lanthanide metal-ligand interaction was described by the optical absorption investigation in terms of increased Oscillator strength, substantial positive Judd Ofelt parameters, and significant bonding parameters.
Metal-ligand interaction is established as an enhancement of and oscillator strength. Highest oscillator strength is observed in Ho(III)-I3CA in CTAB micellar medium. Better results as enhancement in and oscillator strength are observed in micellar medium than non-micellar medium. Metal-ligand interaction causes increase in oscillator strength and a red shift in absorption spectra. 12 metal-ligand systems, nine absorption bands 3K7, (5G,3G)5, 5G6, 5F2, 5F3, 5F4, 5S2, 5F5, 5I4 ← 5I8 multiplet to multiplet transition are observed among which 5G6 ← 5I8 transition is hypersensitive transition having high oscillator strength than other eight transitions. The electric quadrupolar selection rule governs this transition (5G6 ← 5I8). The f-f transition matrix element has a strong relationship with oscillator strength.
Judd Ofelt parameter T2 is significant as it has positive value in all micellar study mediums. Coordination T4/T2 parameter inferred that Ho(III) metal ion has the same coordination environment around it while change in symmetry confirmed by symmetry T4/T6 parameter around the central metal Ho(III) ion. Covalency parameters, viz: Bonding Parameter ( b1/2 ), Nephelauxetic ratio β, Sinha’s covalency parameter δ and covalency angular overlap parameter (η) shows greater extent of covalency character in metal Ho(III) ion and Indole derivatives in micelllar medium. Application of present work will be helpful in resolution of technologies where the use of lanthanide is incorporated.
Acknowledgement
Authors are thankful to GCRC, P.G. Department of Chemistry, Govt. Dungar College (A-Grade), MGS University Bikaner, Rajasthan (India). for providing better facilities for the experimental research work.
Conflict of Interest
We have no conflicts of interest to disclose.
Funding Sources
There is no funding source.
References
- Pedada, S. R.;Gollapalli, N. R. Ind. J. Chem., 2021, 60A, 236-242.
- Pedada, S. R.; Bathla, S.; Vasa, S. S. R.; Charla, K. S.; Gollapalli, N. R. Bull. Chem. Soc. Ethiop., 2009, 23(3), 347-358.
CrossRef - Xiao, L. V.; Dong, W.; Ti, J. Z. J. Dis. Sc. Tech., 2006, 27, 1073-1077.
CrossRef - Panda, A. K.; Bhattamisra, S. D. Ind. J. chem. Tech., 2015, 22, 253-257.
- Uphoff , M.; Michelitsch, G. S.; Hellwig, R.; Reuter, K.; Brune, H.; Klappenberger, F.; Barth, J.V. ACS Nano., 2018, 12(11), 11552-11560.
CrossRef - Irfanullah, M.; Iftikhar, K. J. Luminesc.,2010, 130(11),1983-1993.
CrossRef - Bunzli, J.C. G.; Piguet, C. Chem. Soc. Rev., 2005, 34(12), 1048-1077.
CrossRef - Hemmila, I.; Laitala,V. J. Fluoresc., 2005, 15(4), 529-542.
CrossRef - Carvan, P. Acc. Chem. Res.,2009, 42(7), 851-862.
CrossRef - Marien, T.; Kadihasanoglu, M.; Miller, N. Res. Rep. Urol.,2016, 8, 181–192.
CrossRef - Daters, A.; Jana, K. K.; Deobald R. G., Int. J. Case Rep. Images., 2016, 7(6), 397-401.
CrossRef - Shalini, S.; Frank, D.S.; Aldoukhi, A.H.; Majdalany, S.E.; Roberts,W.W.; Ghani, K. R. ACS Biomater. Sci. Eng.,2020, 6(9), 5274–5280.
CrossRef - Prince, J. F.; Van den Bosch, M. A. A. J.; Nijsen, J. F. W.; Smits, M. L. J.; Van den Hoven, A. F.; Nikolakopoulos, S. J. Nucl. Med., 2018, 59(4), 582–588.
CrossRef - Klaassen, N. J. L.; Arntz, M. J.; GilArranja, A.; Roosen, J.; Nijsen, J. F. W. Radiopharm. Chem.,2019, 4(1), 19.
CrossRef - Van de Maat, G. H.; Seevinck, P. R.; Elschot, M.; Smits, M. L. J.; Leeuw, H.; van het Schip, A. D. Eur. Radiol., 2013, 23(3), 827–835.
CrossRef - Zhai, T.; Wang, C.; Cui, L.; Du, J.; Zhou, Z.; Yang, H.; Yang, S. ACS Appl. mater. interf.,2020, 12(33), 37470-37476.
CrossRef - Kumar, S.; Ritika, A. Future J. Pharm. Sci., 2020, 6(1), 121.
CrossRef - Kerzare, D. R.; Khedekar, P. B. J Pharm Sci. Bioscient. res., 2016, 6(1),144-156.
- Qin, X.; Liu, X.; Huang, W.; Bettinelli, M.; Liu, X. Chem. Rev., 2017, 117(5), 4488-4527.
CrossRef - Hatanaka, M.; Yabushita, S.; J. Phys. Chem. A., 2009, 113(45),12615–12625.
CrossRef - Jørgensen, C. K.; Judd, B. R. Mol. Phys.,1964, 8(3), 281–290.
CrossRef - Ishikawa, N.; Iino,T.; Kaizu, Y. J. Am. Chem. Soc., 2002, 124(38),11440–11447.
CrossRef - Sinha, S. P. Spectrochim Acta., 1966, 22(1), 57–62.
CrossRef - Houthuijs, K. J.; Martens, J.; Arranja, A.G.; Berden, G.; Nijsen, J. F. W.; Oomens, J. Phys. Chem. Chem. Phys., 2020, 22(27), 15716-15722.
CrossRef - Carnall, W. T.; Fields, P. R.; Wybourne, B. G. J.Chem. Phys.,1965, 42(11), 3797–3806.
CrossRef - Carnall, W. T.; Fields, P. R.; Rajnak, K. J. Chem. Phys., 1968, 49(10), 4412–4423.
CrossRef - Judd, B. R. Phys. Rev., 1962, 127(3), 750.
CrossRef - Ofelt, G. S. J. Chem. Phys., 1962, 37(3), 511–520.
CrossRef - Carnall, W. T.; Beitz, J. V.; Crosswhite, H.; Rajnak, K.; Mann, J.B. Systematics and the properties of the lanthanides. Springer Netherlands:Dordrecht., 1983, 389–450.
CrossRef - Henrie, D. E.; Choppin, G. R. J. Chem. Phys.,1968, 49(2), 477–481.
CrossRef - Goutam, M. P.; Yadav, A.; Limaye, S. N. Asian J. Chem., 1998, 10(3), 415.
- Carnall, W. T.; Fields, P. R.; Rajnak, K. J. Chem. Phys.,1968, 49(10), 4424–4443.
CrossRef - Ansari, A. A.; Hussain, H. A.; Iftikhar, K. Spectrochim. Acta. A. Mol. Biomol. Spectrosc., 2007, 68(5), 1305–1312.
CrossRef - Khan, A. A.; Hussain, H. A.; Iftikhar, K. Spectrochim. Acta., 2003, 60, 2087-2092.
CrossRef - Hussain, H. A.; Ansari, A. A.; Iftikhar, K. Spectrochim. Acta.,2004, 60, 873-884.
CrossRef - Sastri, V.S.; Bunzli, J. C.; Rao, R. R.; Rayudu, G.V.S. Perumareddi, J.R.; Modern Aspects of Rare Earths and Their Complexes, Elsevier Science., 2003, 569-731.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










