Kinetics of Biogas Production from Goat Dung and Pawpaw Seed
Charles Otobrise* , Chidi Wisdom Udubor and Emmanuel Osabohien
, Chidi Wisdom Udubor and Emmanuel Osabohien
Department of Chemistry, Delta State University, P.M.B. 1, Abraka, Nigeria.
Corresponding Author E-mail: otobrisec@delsu.edu.ng
DOI : http://dx.doi.org/10.13005/ojc/380411
Article Received on : 04-May-2022
Article Accepted on :
Article Published : 02 Aug 2022
Reviewed by: Dr. Sadeem
Second Review by: Dr. Nyoni, Bothwell
Final Approval by: Dr.Kallesha N
Anaerobic digestion of goat dung and pawpaw seed and the fitness of some kinetic models in predicting the rate and cumulative production of biogas were investigated in this study, to compare biogas potential of plant and animal based wastes as well as evaluate the effect of co-digestion on biogas production. The results revealed that the goat dung produced higher volume of biogas (4943 mL) than the pawpaw seed (4329 mL). The mixture of both produced the highest volume (5871 mL) of biogas in comparison with the mono-substrates. Polynomial regression model gave the best correlation with R2 value ranging from 0.9650 - 0.9810 for the three experiments when compared with linear regression model for the ascending limb with R2 values ranging from 0.9210 – 0.9500. For descending limb, polynomial regression also gave a better fit with R2 value in the range of 0.9690 – 0.9770 than the linear regression (R2: 0.9560 – 0.9700).
Keywords: Biogas, kinetics; models, goat dung, pawpaw seed, co-digestion
Biogas; Co-digestion; Goat dung; Kinetics; Models; Pawpaw seed
Download this article as:| Copy the following to cite this article: Otobrise C, Udubor C. W, Osabohien E. Kinetics of Biogas Production from Goat Dung and Pawpaw Seed. Orient J Chem 2022;38(4). |
| Copy the following to cite this URL: Otobrise C, Udubor C. W, Osabohien E. Kinetics of Biogas Production from Goat Dung and Pawpaw Seed. Orient J Chem 2022;38(4). Available from: https://bit.ly/3QdB8SV |
Introduction
Energy is an essential commodity in the world. In the past and present times, Nigeria has relied on the hydrostatic power plants for electricity generation1 and fossil fuel for transportation. However, Nigeria’s energy grid is experiencing some crises due to lack of development. This problem is coupled with the fact that burning fossil fuel emits a lot of greenhouse gas (e.g. Carbon dioxide- CO2), which causes environmental degradation and global warming. With such prevalent problems, there is need to consider alternative sources of energy as we seek to improve our energy supply. The key to making a more reliable energy sector is to find and use a renewable energy resource, rather than simply relying on the country’s non-renewable resources2.
Biogas is generated when anaerobic microbes feed on carbohydrates and fats (without oxygen), producing CH4 and CO2 as waste products3, 4. These microbes feed off fats, carbohydrates and proteins, and then through a complex chain of reactions generates biogas consisting mainly of methane (55-60%) and CO2 (35-40%) along with traces of other gases such as H2S (0-2%), H2 (0-1%), N2 (0-2%), O2 (0-2%), water vapour, siloxanes, depending on the waste matter decomposed3, 5-8. The constituents other than methane are contaminants and have various effects on the application of biogas or the environment. These negative impacts range from lowering its calorific value, corrosion of equipment and piping system, emitting SO2 and NOx on combustion9.
Biogas can be used in several ways, either as raw or upgraded. It can be an alternative to fossil fuel.10In its raw state, biogas is utilized in the production of heat and electricity, while upgraded biogas is used as vehicle fuel, in which liquid fuels such as petrol and diesel are replaced5.It serves as green gas when introduced into the natural gas grids3, 11. In addition, biogas is used to produce energy in Combined Heat and Power (CHP) units3. Indeed, if these inexpensive but essential wastes from plants and animals are properly harnessed, biogas technology could become a sustainable way to meet the future energy demands of rural households, especially those in developing countries.
For biogas to fulfill its potential as limitless and versatile source of sustainable energy, most of the contaminants present in it must be removed; and anaerobic digestion must be optimized with appropriate models that can be used in control theory12. The process of removing CO2 from the biogas to improve its energy content with the end product being bio-methane is known as biogas upgrading13, 14.
To adequately harness the potentials of biogas technology in meeting present and future energy needs, it may not be enough to just produce biogas. There is need to assess the potentials of different biomasses, operational conditions and models, which could be useful in predictive mode for optimal production for mass utilization. This study is therefore, aimed at evaluating and assessing the quantity and quality of biogas generated from goat dung and pawpaw. The suitability of using selected anaerobic digestion models in predicting biogas production was also evaluated.
Materials and Methods
Material collection and preparation for anaerobic digestion
Pawpaw seeds used for this study were collected from ripe pawpaw (Carica papaya) fruits. The goat dung was collected from various goat sellers, while the cow dung used as inoculums was collected from fresh cow excreta. Both samples used for the mono-digestion were sun dried for several days to lower their moisture contents. After which they were ground into powder in order to increase their surface area and make them have fine particle sizes. Slurries of each of the samples were made by mixing 1000 g of each powdered sample with 3500 mL of water. This constitutes 22% total solid (TS) to maintain satisfactory stability15.One hundred gram (100 g) of fresh moist cow dung was added to each of the slurries as inoculums to boost the microbial counts of the samples. Fresh cow dung was chosen as the inoculums because it has been reported to contain all the vital groups of microbial consortium needed for anaerobic digestion process16.The slurry for co-digestion was also prepared in the same way, except that the co-digested substrate contained 1:1 mole ratio of a mixture of pawpaw seed and goat dung.
Digester set-up
The experiment was done at the Environmental Laboratory of Landmark University, Omu – Aran, Kwara State, Nigeria; using a five-liter automated twin anaerobic digester. The digester is an all-glass apparatus, housed in a glass water bath, whose temperature is automatically regulated by a computer system. The digester is also connected to a water displacement system to determine the volume of gas produced. Figures 1 and 2 show the digestion apparatus and the flow diagram of the digester set-up respectively.
 |
Figure 1: Picture of the twin anaerobic digesters |
 |
Figure 2: Digester Set–up for Production and Measurement of Biogas. |
Anaerobic digestion (AD)
The digestion of the slurry was carried out batch by batch and monitored for 24 days. 4000 mL of each of the slurries was packed into the digester and corked. The digester temperature was set at 35 OC. The slurry was stirred daily by the in-built stirrer of the digester. Stirring prevents caking of the slurry and also aids uniform temperature and bacteria distribution.17.
Measurement of the biogas produced
The biogas produced was measured every 24 hours to determine its production rate as shown in figure 2. This was done by checking the fall in the level of water in the graduated cylinder. The volume of biogas formed for each day is proportional to the amount of water expelled.18, 19
Determination of volatile solid (VS)
Available organic matter for the action of bacteria during digestion constitutes volatile solid20. This solid would burn off when subjected to a furnace temperature of about 550 OC.
The total solid residue was heated in a muffle furnace at 600 OC for 2 hours, after which it was cooled in a desiccator and weighed. The volatile solid was calculated using equation (1).
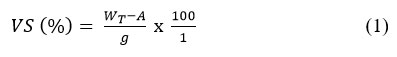
WT = weight of dried residue from total solid
A = weight of residue after further heating at 600OC
g = Initial sample weight
Determination of biochemical oxygen demand (BOD)
The dissolved oxygen meter (Mw 600 by Milwaukee) was first calibrated using a standard solution. Serial dilutions were done on the sample, and then initial dissolved oxygen (DOi) reading was taken. The same sample was kept in the laboratory incubator (DNP-9052 by SANFA) for five days, after which, the final dissolved oxygen (DOf) was measured. The BOD after five days is the difference between DOi and DOf.
Determination of chemical oxygen demand (COD)
The test tube heater was turned on and set to 150 OC, while the safety screen was positioned. The test tube was vigorously agitated to suspend all sediment. 2 mL of the sample slurry was introduced into the test tube with the aid of the pipette. The test tube was then covered and inverted slowly in order to allow mixture of the contents. A blank reagent was prepared similarly using 2 mL of deionized water in another test tube. Both tubes were placed in the heater and digested for 2 hours. The tubes were cooled and their photometric readings taken and recorded in mg/L.
Kinetic models for simulation of daily biogas production rate
Biogas production rates of goat dung, pawpaw seed and the mixture of both substrates were simulated using linear and polynomial plots on Microsoft Excel.
Linear regression equation
The linear equation of the biogas production rate for the ascending and descending limb is expressed by equation (2) below21.It is assumed that biogas production rate will increase linearly with increase in time and after reaching a maximum point, it would decrease linearly to zero with increase in time.
Y = a + bT (2)
y = biogas production rate in mL/day.
T = time in days for digestion.
a (mL/day) is a constant obtained from y-intercept of the graph of y vs T.
b (mL/day) is a constant obtained from the slope of the graph of y vs T.
For the ascending limb, the slope, b, is positive and it is negative for the descending limb.
Polynomial Regression Equation
The polynomial plot of the ascending and descending limb is represented by equation (3). Here, it is assumed that biogas production rate shows a polynomial increase in time and after reaching the peak, it will decrease in the same vein to zero with further increase in time.
y = aT2 + bT + c (3)
y is the biogas production rate (mL/day)
T is the retention time in days
a, b and c are regression constants.
Polynomial regression equation seems to be very reliable in predicting biogas production in anaerobic digestion of animal wastes22, 23.
Empirical models for simulation of the cumulative biogas production
The evaluation of anaerobic digestion was carried out by fitting the experimental data into modified Gompertz and modified Logistic models.
Modified Gompertz Model
Gompertz function is presented in equation (4)
Gt = A × exp [ -exp (b – ct)] (4)
Where, Gt is Gompertz cumulative gas yield per time.
A is biogas production potential (mg/TS)
b and c are constants of the Gompertz model
t is cumulative time for biogas production in days24.
The modified Gompertz equation is shown in equation (5)
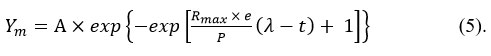
Ym is cummulative biogas yield (mL) attime ( t )
Rmax is maximum biogas production rate (mL/day).
t is retention time (days)
A is biogas production potential (mL)
e is mathematical constant; 24, 25.
Equation (5) can be used to analyze biogas production. The P, Rmax and λ, cannot be used for predictive purposes because they are limited to particular experimental conditions26.
Modified Logistic Model
Equation (6) is an expression of the Logistic model.
Lt = A × [1 + exp (b – ct)] -1 (6)
Where, Lt is Logistic cumulative gas yield per time.
A is biogas production potential (m/g-TS)
b and c are constants of the Logistic model
t is cumulative time for biogas production in days24.
The modified logistic function is shown in equation (7) is modified and re-written thus:
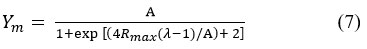
A is maximum production potential of biogas (mL)
Rmax is maximum production rate of biogas(mL/day)
t is retention time in days
λ is lag phase/delay time in days.24
The data from the three experiments were fitted into equations (5) and (7) using non-linear regression analysis with solver tool in Microsoft Excel 2007. The equations were used to determine biogas production potential (A), maximum production rate ( Rmax ) and duration of the lag phase ( λ) . The predicted biogas yields from the non-linear regression analysis were plotted against the retention time and the experimental yields. The correlation coefficient (R2) was calculated using the regression data analysis tool in MS Excel, 2007. R2 was obtained to examine the goodness of fit of the models to the experimental data. A confidence interval of 95% was chosen for the goodness of fit for the predicted data.R2, visual inspection of the curve and experimental values must be taken into account to determine the suitability of the prediction models.
One-way analysis of variance (ANOVA) was performed using Microsoft Excel 2007 to determine whether there is significant difference between the experimental data and the predicted data for the biogas yield each of substrate. A confidence interval of 0.05 was chosen, hence, the difference was considered significant if the probability (p-value) was less than 0.05.
Results and Discussion
The physical and chemical properties of samples in this study are shown on table 1. The pH values of the substrates ranged from 7.12 to 7.46, this is presented in figure 3. This pH range is suitable for optimal performance of anaerobic digestion processes23, 27.
Table 1: Physicochemical properties of the slurries before and after digestion.
|
Parameter |
Goat Dung (GTD) |
Pawpaw Seed (PPS) |
Mixture |
|||
|
|
Before |
After |
Before |
After |
Before |
After |
|
Ph |
7.21 |
7.18 |
7.12 |
7.24 |
7.46 |
7.60 |
|
BOD5 (mg/L) |
160 |
146 |
194 |
180 |
306 |
284 |
|
COD (mg/L) |
490 |
312 |
480 |
346 |
512 |
286 |
|
TS (%) |
15.10 |
11.70 |
16.30 |
12.80 |
20.60 |
14.40 |
|
VS (%) |
84 |
66 |
86 |
72 |
82.80 |
64.40 |
|
TN(mg/L) |
3.2 |
2.7 |
2.6 |
2.34 |
2.8 |
2.0 |
|
TC(mg/L) |
28.3 |
22.0 |
32.3 |
28.6 |
43.2 |
38.6 |
 |
Figure 3: Effect of anaerobic digestion on the pH of the slurries. |
The pH of goat dung after digestion dropped from 7.21 to 7.18, while that of pawpaw seed and the mixture increased from 7.12 to 7.24 and 7.46 to 7.60 respectively. The increase in pH for the latter could be attributed to the accumulation of ammonia27, while the slight decrease in pH of goat dung slurry could be attributable to build-up of fatty acids and amino acid within the digester28, 29. The pH values of the slurries after digestion indicate that they can be applied to soil, as fertilizers without adversely affecting the soil pH
There was a decrease in BOD, COD, TS and VS for goat dung, pawpaw seed and the mixture after the 24 days digestion period. Figure 4 shows the percentage decrease in the values of the aforementioned properties. A better degradation efficiency was achieved with the mixture slurries than with goat dung and pawpaw seed substrares. Efficiency in the reduction of TS, VS and COD is important in assessing the performance of an AD process30.
 |
Figure 4:Percntage decrease in BOD, COD, TS and VS values. |
 |
Figure 5: Biogas production rate per day. |
 |
Figure 6: Comparison of maximum biogas yield from the various substrates. |
Biogas production rate per day
The pawpaw seed, goat dung and 1:1 mixture of the two were evaluated for suitability for biogas production at mesophilic temperature range (35 OC) for 24 days. Biogas production for the three substrates started as early as the first day. The effects of time on the daily production rates as well as on the cumulative biogas yield are presented in Figures 5 and 6 respectively. It can be observed from Figure 5 that biogas production rate was slow within the first five days for all the substrates. This slow rate is attributable to the time required for anaerobic microbes to acclimatize to the new environment. Hence, biogas production rate in batch reactor is proportional to specific growth rate of methanogenic bacteria. With increase in retention time, there was gradual increase in the production rate. After attaining the maximum production rate,a decline in biogas production was observed for the three samples. The goat dung had the fastest digestion rate with a maximum production rate of 372 mL occurring on day 11. This was followed by the mixture whose peak production rate of 441 mL/day occurred on day 14. Pawpaw seed had the slowest digestion rate; it had a peak production rate of 338 mL occurring on day 19. One reason for the slow decomposition of pawpaw seed could be as a result of reasonable amount of complex lignocellulose components in it, which could limit anaerobic biodegradability31, 32.
It can be observed on figure 6, that at the end of the 24-day period, the mixture had the highest biogas production of 5,871 mL, followed by goat dung with a total biogas yield of 4,943 mL and pawpaw seed with a total biogas yield of 4329 mL. Essentially, co-digestion or co-substrate is more effective in biogas production than mono-digestion23, 33. Figure 7 shows the effect of retention time on cumulative biogas yield.
 |
Figure 7: Plot showing the effect of retention time on cumulative biogas yield. |
Kinetic Modeling of Biogas Production Rate
Figures 8 and 9 show the linear and polynomial regression plots respectively for the ascending limb of biogas generation rates for the three substrates in this study. The coefficient of determination and model equations derived from the ascending limb plots are presented on table 2. The R2 value generated from the linear plots of goat dung, pawpaw seed and mixture of both are 0.9270, 0.9500 and 0.9290 respectively, while that generated from polynomial plots are 0.9710, 0.9810 and 0.9650 respectively.
This result, judging from the R2 value indicates that both linear and polynomial regression models are good fits for simulating the ascending limb of rate of biogas production from goat dung, pawpaw seed and their co-substrate. Hence, progressive increase in biogas production rate can be adequately approximated by linear or polynomial equations (8) to (13) as presented on table 2. However, the polynomial model with higher R2 values in all cases proved to a better fit when compared with the linear model.
 |
Figure 8: Linear Regression Plot for the Ascending Limb. |
 |
Figure 9: Polynomial Regression Plot for the Ascending Limb. |
Table 2: Coefficient of determination (R2) and model equations for ascending limb.
|
SUBSTRATE |
MODEL |
R2 VALUE |
REGRESSION EQUATION |
|
Goat dung |
Linear Polynomial |
0.9210 0.9760 |
y = 33.95 T – 23.93 (8) y = 2.578T2+5.595T+23.33 (9) |
|
Pawpaw seed |
Linear Polynomial |
0.9500 0.9810 |
y = 16.51 T – 8.957 (10) y= 0.575 T2 + 5.584 T + 23.83 (11) |
|
Mixture |
Linear Polynomial |
0.9290 0.9650 |
y = 30.46 T – 27.07 (12) y = 1.572 T2 + 8.459 T +18.60 (13) |
Linear and polynomial regressions for the descending limb of biogas generation rate are displayed in Figures 10 and 11. Table 3 shows the goodness of fit and model equations obtained from figures 8 and 9. Equations (14) – (19) presented on table 3 can be used to predict the decrease in biogas production rate from the substrates in this study. Comparing the R2 values however, it can be observed that the polynomial regression plot gives the best option for simulating biogas production rates.
 |
Figure 10: Linear regression plot for descending limb. |
 |
Figure 11: Polynomial regression plot for descending limb. |
Table 3: Coefficient of determination (R2) and model equations for descending limb.
|
SUBSTRATE |
MODEL |
R2 VALUE |
REGRESSION EQUATION |
|
Goat dung |
Linear Polynomial |
0.968 0.969 |
y = – 21.58 T + 618.5 (14) Y = 0.248T2 -30.52 T + 695.4 (15) |
|
Pawpaw seed |
Linear Polynomial |
0.956 0.977 |
y = -18.28 T + 677.8 (16) y= 1.857 T2 -98.14 T + 1530 (17) |
|
Mixture |
Linear Polynomial |
0.970 0.974 |
y = -24.80 T + 794.0 (18) y = -0.562T2– 3.417 T +596.4 (19) |
Models for fitting cumulative biogas yield
The modified Gompertz model and the Logistic model were used to fit the cumulative biogas production data obtained from the experiment. The predicted cumulative biogas yields by the models were plotted against the experimental data as shown in Figures 12 – 14. One-way analysis of variance (ANOVA) was performed to ascertain whether or not there was a significant difference between experimental data and the results obtained from modified Gompertz and the modified Logistics models. The ANOVA results are presented on tables 4-6.
 |
Figure 12: Simulation of cumulative biogas yield from goat dung. |
Table 4: ANOVA result for Goat dung.
|
Source of Variation |
SS |
df |
MS |
F |
P-value |
F crit |
|
Between Groups |
7468.627 |
1 |
7468.626573 |
0.00232 |
0.961785 |
4.042652 |
|
Within Groups |
1.55E+08 |
48 |
3219501.939 |
|||
|
Total |
1.55E+08 |
49 |
|
|
|
|
 |
Figure 13: Simulation of cumulative biogas yield from pawpaw seed. |
Table 5: ANOVA result for pawpaw seed.
|
Source of Variation |
SS |
Df |
MS |
F |
P-value |
F crit |
|
Between Groups |
730.6097006 |
1 |
730.6097 |
0.000365 |
0.984831 |
4.042652 |
|
Within Groups |
96006345.32 |
48 |
2000132 |
|||
|
Total |
96007075.93 |
49 |
|
 |
Figure 14: Simulation of cumulative biogas yield from the goat dung-pawpaw seed mix. |
Table 6: ANOVA result for the mixture.
|
Source of Variation |
SS |
Df |
MS |
F |
P-value |
F crit |
|
Between Groups |
7096.658229 |
1 |
7096.658 |
0.001636 |
0.967904 |
4.042652 |
|
Within Groups |
208212377.6 |
48 |
4337758 |
|||
|
Total |
208219474.3 |
49 |
|
|
|
|
To compare the performance of the models, the correlation coefficient (R2) between the experimental and estimated data was determined. The R2 values obtained from the two models are presented on table 7. The modified Gompertz model proved to be a better fit for goat dung and the mixture samples, with higher R2 values of 0.9996and 0.9995 respectively. This result concurs with the findings of Dinh and his co-workers as well as the other researchers in the reference24, 34. It has been observed that modified Gompertz model has a better fit when compared with First Order kinetic model and Logistic model in simulating the anaerobic digestion of food waste30.
Table 7: Comparison of the goodness of fit of the predictive models.
|
Sample |
Model |
A (mL) |
(mL/day) |
λ (day) |
R2 |
|
Goat Dung |
Gompertz model |
5574.26 |
341.98 |
5.3 |
0.9996 |
|
Logisticmodel |
4973.17 |
373.40 |
6.1 |
0.9985 |
|
|
Pawpaw Seed |
Gompertz model |
11121.09 |
306.58 |
9.6 |
0.9992 |
|
Logisticmodel |
5730.80 |
307.04 |
9.4 |
0.9994 |
|
|
Mixture |
Gompertz model |
7732.29 |
385.43 |
6.8 |
0.9995 |
|
Logisticmodel |
6257.12 |
431.94 |
7.8 |
0.9993 |
Logistic model gave a better fit for pawpaw seed experimental data, with R2 value of0.9994 as against 0.9992 obtained from modified Gompertz model. However, the ANOVA results on tables 4 – 6indicate that with p-values ranging from 0.96-0.98 (P > 0.05); there was no significant difference between the estimated cumulative biogas yields from both models. From table 7, the biogas yield potential (A) estimated by the modified Gompertz model was found to be higher than the values obtained from the modified Logistic model for the three substrates. In all cases, the estimated lag phase time (λ), which is considered as the minimum time in days taken to produce biogas, lies between 5 days and 10 days. This result is not coherent with experimental data, which showed that biogas was actually produced as early as the first day. Other researchers who used modified Gompertz and modified Logistic models also reported similar difference between experimental and estimated lag time24, 34, 35, 36
Conclusions
This study has shown that goat dung can be used as a source of renewable energy and its potency can be optimized by co-digesting it with pawpaw seed. Co-digestion of goat dung with pawpaw seed using cow dung inoculums enhanced biogas production. Polynomial regression performed better than linear regressions for predicting progressive increase and decrease in biogas production. Modified Gompertz and Logistic models were efficient in the estimation of cumulative biogas production. The findings of this study provides stakeholders in the energy sector essential data to develop affordable biogas systems that require little maintenance to produce renewable energy, which will reduce the use of fossil fuel and consequently minimize environmental pollution and degradation.
Acknowledgement
The authors wish to acknowledge the staff of the Environmental Laboratory of Landmark University, Omu-Aran, Kwara State, Nigeria, for the digester set-up to produce and measure biogas production rates. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that there are no conflicts of interests in the publication of this article.
Funding Sources
There is no funding source.
References
- Voice of Nigeria, 2015, Retrieved 29/3/16 from http://voiceofnigeria.org.ng/%E2%80%8Bbio-gas-as-a-viable-alternative-for-electricity-generation-in-nigeria
- Wikipedia, 2015 Retrieved 29/3/16 from http://en.m.wikipedia.org/wiki/ Nigerian_energy_supply_crisis
- Gerlach, F.; Grieb B.; Zerger U. Sustainable biogas production. A handbook for organic farmers, 2013, Available on www.sustaingas.eu).
- Clarke Energy, Biogas|CHP|Cogeneration|Combined heat and power, 2016, Retrieved 29/3/16 fromhttp://www.clarke energy.com/biogas/
- Jorgensen, P.J.. Biogas-Green energy, 2009, Retrieved form http://lemvigbiogas.com/BiogasPjjuk.pdf
- Sharma, B. K. Industrial Chemistry (Including Chemical Engineering). 16th Ed. India: GOEL Publishing House, 2011.
- Van Loon, R. Agilent Technologies, Inc. Middelburg, the Netherlands, 2011, Retrieved 28/4/16 from www.agilent.com/../5990.9508EN.pdf
- Vij, S. Biogas production from kitchen waste. A seminar report submitted in Partial Fulfilment of the Requirement for Bachelor of Technology. Department of Biotechnology and Medical Engineering, National Institute of Technology, Rourkela, 2011.
- Wawrzyniak, R.; Wasiak, W. Ecological Chemistry and Engineering, 2011, 18(4), 537-544.
- Cioabia, A. E.; Ionel, I. Alternative Fuel, 2011,pP 201-226. DOI:10.5772/25028.
CrossRef - Zhang, P. Biogas – its potential as energy source in rural households with particular emphasis on China. Master’s Thesis, Noragric, Norway. Available online:http://www.umb.no/noagric 2012.
- Fedailaine, M.; Moussi, K.; Khitous, M.; Abada, S.; Saber, M.; Tirichine N. Procedia Computer Science, 2015, 52,730-737.
CrossRef - Swedish Gas Center, SGC. Evaluation of upgrading techniques for biogas report. SGC 142. ISSN 1102-7371. 2003.
- Ramaraj, R.; Dussadee, N. International Journal of Sustainable and Green Energy, 20154(1), 20-32.
- Angelonidi, E.; Smith S. Water and Environment Journal, 2015, 29, 549-557.
CrossRef - Das, A.; Mondal, C. International Research Journal of Environmental Sciences, 2016, 5(1), 49-57.
- Ezekoye, V. A.; Onah, D. U.; Offor, P. O.; Ezekoye, B. A. Global Journal of Science Frontier Research, 2014, 14 (1), 1-8.
- Momoh, Y.; Augusto, D.; Diemuodeke, O. Research in Agricultural Engineering, 2011, 57(3),97-104.
- Somashekar, R. K.; Verma, R.; Naik M. A. International Journal of Geology, Agriculture and Environmental Science, 2014, 2 (2), 1-7.
- Ntunde, D. I.; Edeh, H. I.; Ochu, R. C.; Onyia, B. I.; Atuba, T. International Journal of Research in Advanced Engineering and Technology, 2016, 2(3): 157-161.
CrossRef - Kumar, N.; Dureja, G.; Kamboj, S. International Journal of Modern Engineering Research 2014, 4 (3), 1-6.
- Nnabuchi, M. N.; Akubuko, F. O.; Augustine C.; Ugwu, G. Z. Global Journal of Science Frontier Research, Physics and Space Science, 2012, 12 (7), 20-26.
- Stanley, H. O.; Okerentugba, P. O.; Ogbonna, C. B. International Journal of Advanced Biological Research, 2014, 4(4), 405-411.
- Dinh, P. V.; Fujiwara T.; Pham Phu S. T.; Hoang, M. G. IOP Conference Series: Earth Environmental Science, 2018,159, 012041.
CrossRef - Donoso, B. A.; Perez, E. S. I.; Fodz P. F. Chemical Engineering Journal, 2010, 160, 607-614.
CrossRef - Ye, R. Z.; Doane, J. A.; Morris, J.; Horwath, W. R. Soil Biology and Biochemistry, 2015, 81, 98-107.
CrossRef - Dobre, P.; Nicolae, F.; Matei, F. Romanian Biotechnological Letters, 2014, 19(3), 9283-9296.
- Suyog, V. I. J. Biogas production from kitchen waste. Bachelor of Technology Thesis. National Institute of Technology, Rourketa, Orissa 2011.
- Chibueze, U.; Okorie, N.; Oriaku, O.; Isu, J.; Peters, E. International Journal of Materials and Chemstry, 2017, 7(2), 21-24.
- Pramanik, S. K.; Suya, F. B.; Porhemmat, M.; Pramanik, B. K. Processes, 2019, 7, 600.
CrossRef - Ukpai, P. A.; Nnabuchi, M. N. Research Library Advances in Applied Science Research, 2012, 3(3), 1864-1869.
- Ojikutu, A. O.; Olumide, O. O. The International Journal of Engineering and Science, 2014, 3(1), 01-07.
- Alvarez, R.; Liden G. Renewable Energy, 2008, 33, 726-734.
CrossRef - Latinwo, G. K.; Agarry, S. E. International Journal of Renewable Energy Development, 2015, 4(1), 55-63.
CrossRef - Deepanraj, B.; Sivaubramanian, V.; Jayaraj, S. Journal of Renewable and Sustainable Energy, 2015, 7, 063104.
CrossRef - Bong, C. P. C.; Lim, L. Y.; Lee, C. T.; Ho, W. S.; Klemes, J. J. Chemical Engineering Transactions, 2017, 61,1669-1674.

This work is licensed under a Creative Commons Attribution 4.0 International License.









