Extensive Employment of Chemically Treated Elephas maximus Dung in Sequestration of Oxyfluorfen: Batch, Isothermal and Kinetic Modeling
Department of Chemistry, PSGR Krishnammal College for Women, Coimbatore – 641004, TamilNadu, India.
Corresponding Author E-mail: muthulakshmiandal@psgrkcw.ac.in
DOI : http://dx.doi.org/10.13005/ojc/380420
Article Received on : 23 Jun 2022
Article Accepted on :
Article Published : 26 Aug 2022
Reviewed by: Dr. Balachandran S.
Second Review by: Dr. Manoj Kumar
Final Approval by: Dr. Nenad Ignjatovic
Presently, one of the most employed herbicides is Oxyfluorfen, used to control the growth of annual broadleaf and grassy weeds. On the other hand, effective disposal of redundant Elephas maximus dung (EMD) is important for environmental protection and utilization of resource. Aim of the article is focused on sequestration of Oxyfluorfen from aqueous media employing Elephas maximus dung (EMD), a natant biowaste, seldom reported elsewhere. Experimental setup is planned via batch mode under varying operational factors viz., particle size, initial Oxyfluorfen concentration, MEMD dosage, contact time, pH and temperature. The obtained results validated through isothermal and kinetic models imply notable fit in of Langmuir isotherm and Pseudo II Order kinetic models with a maximum of 88.9 % oxyfluorfen removal. Based on the derived observations, supported by theoretical calculations, it is concluded that Elephas maximus dung (EMD) powder, possess maximum potential towards chelation of Oxyfluorfen, thereby succoring an alternate eco-friendly process.
KEYWORDS:Adsorption; Biowaste; Herbicide; Isotherms; Oxyfluorfen; Operational Factors; Redundant
Download this article as:| Copy the following to cite this article: Preethi G, Andal N. M. Extensive Employment of Chemically Treated Elephas maximus Dung in Sequestration of Oxyfluorfen: Batch, Isothermal and Kinetic Modeling. Orient J Chem 2022;38(4). |
| Copy the following to cite this URL: Preethi G, Andal N. M. Extensive Employment of Chemically Treated Elephas maximus Dung in Sequestration of Oxyfluorfen: Batch, Isothermal and Kinetic Modeling. Orient J Chem 2022;38(4). Available from: https://bit.ly/3QUE87m |
Introduction
Pesticides have an outstanding importance in agriculture as to improve productivity of crops1. In their absence, crop yield would drop linearly resulting in the price hike of commodities, a relevant ratio. Typically, herbicides are xenobiotic, toxic and complex to be biodegraded. They are strongly adsorbed by different types of soil, but later, after a certain period they are prone to leaching into surface or groundwater2. Oxyfluorfen, a non-ionic halogenated organic herbicide issued in controlling annual broadleaf and grassy weeds in a variety of tree fruit, blackberries, sunflowers and field crops3,4. Oxyfluorfen is classified as low acute toxicity compound by the World Health Organization (WHO) and by the Environmental Protection Agency (EPA). Limit of oxyfluorfen is 0.01 – 0.27 µg/L in Normal water according to US EPA standards. Oxyfluorfen is a type of contact herbicide5 which requires sufficient light for its activity through the formation of free radical via inhibition of protoporphyrinogen oxidase, in turn curbing necrosis in plants6,7. However, it is considered as lethal to aquatic organisms, further, impose adverse impact on terrestrial plants and ecological systems at all levels8. Thence, few inevitable treatment methods are of prime necessity before discharging the wastewaters into landfills and aquifers9. Several methods reported for removal of pesticides from wastewaters include anaerobic decolourisation, chemical oxidation, reverse osmosis, ion exchange, adsorption, electro-coagulation, sono electrolysis, UV assisted Electrolysis and biological treatment methods, among which adsorption using biomaterials is referred to as an effective method10. This is due to its initial cost, simplicity of design, easy operation, insensitivity to toxic substances, sludge free clean process and high adsorption capacity11.
Utilization of biomaterials viz., plant / animal / industrial / marine wastes as pesticides scavengers is accountable in terms of their chemical and thermal stabilities. The current investigation is focused on the employment of a seldom reported material, elephant dung for the removal of Oxyfluorfen from aqueous media. Elephant dung disposal leads to dumped solid waste generation and air pollution once burnt. In view of this, expediate use of elephant dung as a bio-sorbent not only minimizes their litter problem, but also favors solution for trapping excess pesticides leachates. Further, the zero or negligible cost of elephant dung makes this project feasible and cost effective for the removal of Oxyfluorfen, a herbicide from aqueous solution.
The study objective comprises of Determination of EMD sorption capacity in chelating Oxyfluorfen
Best optimization of operating factors viz., particle size, adsorbent dose, initial pesticide concentration, pH, temperature, co-pesticides and contact time on the sorption process
Ascertainment of adsorption / desorption and regeneration parameters
Applicability of isothermal and kinetic data pertaining to Oxyfluorfen– EMD system.
Materials and Methods
Adsorbent – Preparation and Characterization
Elephas maximus dung (EMD) generally available in large quantities throughout the year in the western districts of Tamil Nadu and Kerala12,13 was collected for the present work from the forests of Sathyamangalam, Erode, TamilNadu, India. Later, the dried dung was hammered into smaller pieces, washed off the impurities with doubly distilled water, completely sun dried and arid for 3 hours in hot air oven to ensure moisture elimination. The obtained lumps were crushed, pounded in electrical mixer and assorted into varying mesh sizes (22, 36, 52, 76 & 85 BSS) using molecular sieves which are scientifically tested14. Following, these sized materials were immersed in boiling 0.1N HCl / NaOH, for 3 hours each, rinsed in DDW to neutralize pH, oven dried and labeled as MEMD (Modified Elephas maximus dung) in air locked containers. Images of pulverized EMD and MEMD (85BSS) are depicted in figures 1 a and b15.
 |
Figure 1: (a). Pulverized EMD. |
 |
Figure 1: (b). Modified EMD (MEMD). |
MEMD (raw and granular – 85 BSS) was characterized using Binocular Microscope to categorize the particle size (Ocular micrometer [OLYMPUS make, Model CX21I]), Surface Analyzer to derive Brunauer, Emmett, Teller (BET) / Barrett, Joyner, Halenda (BJH) plots (Micrometrics, BEL, Japan, Inc) for determination of surface area / porosity / pore volume of MEMD. Raw and OX- loaded MEMD was subjected to to analyze the presence of surface functional groups by Fourier Transform Infra-red Spectroscope (Shimadzu Infrared Spectrometer), determination of surface topography / elemental composition through Scanning Electron Microscopy / Energy Dispersive X-Ray Analyzer (Automated JOEL JEM – 6390 Scanning Electron Microscope under a vacuum of 1.33* 10-6m Bar) and to assess the thermal stability of sized material employing Thermogravimetry / Differential Thermal Analyzer (Perkin Elmer, USAA of range 0 – 1000oC). Specific analysis were studied for pre and post experimental run MEMD samples. Physico chemical properties of MEMD were determined by adopting various standard operating procedures, to mention a few are Drift method and Surface acidic group was determined by Boehm titration studies.
Oxyfluorfen – Preparation of Standards
Oxyfluorfen of high technical quality was purchased from Kongu Agro Chemical Pvt Ltd, Sathyamangalam in the marketing name of Marshal. Configuration of Oxyfluorfen is listed in Table 1. A stock solution (1000 mg/L) was prepared by dissolving 1 mL Oxyfluorfen in 1000 mL ethanol. Working standard solutions ranging from 50 – 300 mg/L were diluted appropriately. All solutions were made using DDW only to confirm to non – interference of other ions present in water. Complexing agents for UV- Visible spectrophotometric analysis with respect to chosen pesticide viz.,0.5% -1,2-naphthoquinone-4-sulfonate (NQS) and KCl – NaOH buffer (pH 13) were prepared16.
Table 1: Oxyfluorfen – Structure and Physicochemical Properties.
 |
Table 1: Oxyfluorfen – Structure and Physicochemical Properties. |
Batch Adsorption Studies
Oxyfluorfen removal using MEMD was experimentally verified by Batch mode. Varying controlling parameters viz., particle size (0.18mm – 0.71mm) / dose (50 – 250 mg: 50 mg) of MEMD, initial Oxyfluorfen (OX) concentration (50 – 300 mg/ L: 50 mg/L), pH (1– 11), temperature (283 – 333K: 10K) and agitation time frames (10 – 60 mins: 10 mins) were screened to fix the MEMD sorption efficiency. Appropriate volumes of NQS and pH 13 buffer were added to the supernatant solutions, post filtration, allowed to stand for 20 min, followed by the absorbance measurement of complexed samples in UV- visible spectrophotometer at a λmax value of 460nm.Generated MEMD solid residue of batch experiments were dried and agitated with 0.1N HCl as desorbing agent to favor the leaching of loaded OX from the MEMD surface matrices, further stirred with DDW to neutralize the pH condition. Regenerated MEMD was dried and subjected to successive adsorption-desorption cycles, in order to register its regenerating efficacy. Adsorbed Oxyfluorfen concentration was calculated17 as per mass balance equation 1.
qe= C0 – Ce/ W * V (1)
where, C0, Ce , V, W and qe refer to initial and equilibrium OX concentrations (mg/L), volume of solution (L), mass of the adsorbent (g) and amount of OX adsorbed (mg/g). Desorption percentage was calculated from equation 2.
% Desorption = qde/ qad * 100 (2)
where, qad and qde imply the amounts of adsorbed and desorbed OX concentrations. Notable theoretical models (isotherm / kinetic) were applied to validate the experimental data so as to support and predict the studied system parametrics, through coefficient of determination (R2).
Effectual nature of MEMD in trapping OX was encountered in presence of other competing pesticides viz., malathion, carbosulfan and phorate at varying concentration ranges between 50 – 300 mg/L. Experimental set up was planned in such a way that a mixture of the three pesticides and OX at specific concentrations were agitated at pre-set time frames and MEMD dosage, followed by systematic registration of residual OX concentration under the influencing pesticides at UV – visible spectrophotometer.
Results and Discussion
Characterization Studies
Microscopic Analysis
The particle size calculated was 0.18mm against the mesh size 85 BSS for native and modified granular EMD are depicted as microscopic images in figures 2 a & b. The distinct and enlarged open porous nature of MEMD is in favor of extended sorption capacity of the modified material.
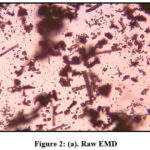 |
Figure 2: (a). Raw EMD. |
 |
Figure 2: (b). Modified EMD. |
Physico – Chemical Analysis
Physio – Chemical parametric values of modified Elephas maximus dung (MEMD – 0.18 mm) are listed in Table 2.
Table 2: Physio- Chemical Characteristics
|
Properties |
MEMD (0.18 mm) |
|
pH of 1 % solution |
6.52 |
|
Moisture (%) |
1.64 |
|
Bulk density (g/L) |
0.66 |
|
Specific gravity |
1.39 |
|
Porosity |
54.59 |
|
Ash content (%) |
3.29 |
|
Acid Soluble Matter (%) |
2.16 |
|
Water Soluble Matter (%) |
1.02 |
|
Ion Exchange Capacity (meq /g) |
0.67 |
|
pHzpc |
4.13 |
|
Surface area (m2/g) |
35.31 |
|
Mean Pore volume (nm) |
2.4 |
|
Surface Acidic groups (m mol g-1) |
|
|
Phenolic |
0.63 |
|
Carboxylic |
1.58 |
|
Lactonic |
0.14 |
Neutral pH and lower moisture content values exhibited by MEMD are indicate by its stable nature. Calculated bulk density being less than 1 g/L implies the presence of porous particles. This statement is supported by the standard porosity value and appreciable surface area / mean pore diameter values evidenced from BET / BJH plots (figs 3 a & b) 18. Internal pore structure mostly decides the extent of adsorption for any material. BET analysis provided a precise surface area value 35.31 m2/g, done by nitrogen multilayer adsorption method, wherein, the measurement had been carried out as a function of relative pressure using an automated analyzer. Pore size distribution and specific pore volume which is independent of external area due to sample’s particle size were determined by BJH analysis19. The centered BJH peak display the sample pore diameter as 2.4 nm, favoring mesoporous nature of the sample.
 |
Figure 3: (a). BET Plot. |
 |
Figure 3: (b). BJH Plot. |
FT – IR
FT – IR spectra of raw, modified and OX – loaded EMD is shown in figure 4. Peaks at 3,289 and 2,959 cm−1 could be assigned to –OH and –NH2 groups and –CH stretching vibrations, respectively. Other bands on the ED surface refer to: 1,687 cm−1(–C=O stretching, –C–N (amide), 1,519cm−1 (amine groups), 1,424cm−1 (–N–H bending), 1,352 cm−1 (–S=O stretching vibrations), 1,161 cm−1 (–C–O–C stretching), 1,031 cm−1 (–C–N, –C–O–C, and –C–C stretching vibrations), and 912cm−1 (–S=O stretching. Overall peak shifts, confirm the participation of functional groups present in the biomass surface during pesticide binding process. A peak at 1038 cm−1 in OX loaded spectrum, referring N-O stretching, register the sequestration of nitrogen ions by the modified material.
 |
Figure 4: FT-IR Spectra. |
SEM / EDAX Analyses
The appearance of heterogeneous surface with aggregate particles was found to undergo surface morphological changes with opened pores due to chemical modification (fig 5 a). Smoothening of unoccupied pores after the experimental run with oxyfluorfen moieties reflect homogenous structures instead of porous nature (fig 6 a) facilitating the adsorption of oxyfluorfen.
Presence of Chlorine, Nitrogen and Fluorine peaks at 2.6, 0.39, 0.67 keV respectively substantiate the adsorption of oxyfluorfen onto MEMD surface (fig 6 b) against their absence in the unloaded spectra (fig 5 b).
 |
Figure 5: (a). SEM – MEMD. |
 |
Figure 5: (b). SEM – OX – MEMD. |
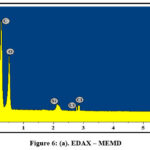 |
Figure 6: (a). EDAX – MEMD. |
 |
Figure 6: (b). EDAX – OX – MEMD. |
TG – DTA Analysis
Thermo Gravimetric analysis of MEMD is shown in figure 7. MEMD is thermally stable up to 230oC. At higher temperatures, the material underwent decomposition leaving behind ash residue. The initial weight loss at 100o C is attributed to 5.6 % moisture loss20. However, decomposition of MEMD had occurred between 230oC -570o C. 14% of MEMD residue constituted ash content. Thermo gravimetric data obtained are complementary with proximate analysis results.
 |
Figure 7: Thermogram of EMD. |
Adsorption Studies 17
Impact of Particle Size
Sequestration of chosen pesticide using different particle sizes viz., 0.18 mm, 0.24 mm, 0.30 mm, 0.42 mm and 0.71 mm of MEMD are shown in figure 8. Smooth gradient decline of the curves indicate that a maximum of 89% pesticide chelation had occurred at a smaller particle size (0.18 mm), which may be due to extended surface area, promoting greater toxicant trapping. Further, lower gradation in the sorption rate at increasing particle size shall be contributed by the higher diffusional resistance to mass transport property, thereby ensuring least utilization of utmost internal surface for sorption. From the above observations, 0.18mm particle size of MEMD had been fixed for the verification of other factors.
 |
Figure 8: Impact of Particle Size. |
Impact of MEMD Dosage
A smooth rise in the dosage patterns is evident from figure 9 at a time frame of 40 mins, beyond which a decline was observed for further agitation periods except for 250mg, where an equilibrium was attained. This suffice the statement that higher sorption (89%) had occurred at 250 mg dose.
 |
Figure 9: Impact of MEMD Dosage. |
Impact of Oxyfluorfen Concentration and Agitation Time 21
Initial concentration of any sorbate species plays a key role in an agitation experiment in the process of determining sorbents’ sorption capacity. A trend of wave patterns (figure 10) similar to that envisaged in figure 9 was obtained while studying the influence of sorbate concentration and agitating time intervals. Maximum oxyfluorfen removal at 40 mins was observed for 200 mg/L concentration. This may be due to the attainment of dynamic equilibrium on the biomass surface leading to increased number of unadsorbed pesticide moieties from the aqueous solution.
 |
Figure 10: Impact of Oxyfluorfen Concentration and Agitation Time. |
Impact of pH
pH variations play a pivotal role in the adsorption process through dissociation of functional groups on the surface-active sites. This leads to a change in reaction kinetics and equilibrium characteristics. A maximum percentage removal of oxyfluorfen was registered at pH 5 (figure 11), later a minimal dip was encountered in the curve at higher pH ranges, indicating the attainment of saturation. The reason for the decreased sorption at acidic pH values shall be favored by protonation of surface groups, thus restricting removal of the selected pesticide.
 |
Figure 11: Impact of pH. |
Impact of Temperature
Figure 12 shows the adsorption profiles at different temperature environs (283 – 333 K) for OX – MEMD system. The first half of the curve registered an inclined paradigm upto 303K, however, further temperature rise, triggered an uneven uptake by MEMD, recommending the follow up of an exothermic process.
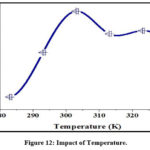 |
Figure 12: Impact of Temperature. |
Impact of Co- pesticides 22
Influence of co – pesticides on OX- MEMD system at varied initial pesticide concentrations (50 – 300 mg/L) showed 23% decrease in pesticide removal due to the inhibition by malathion at a concentration of 150 mg/L, in preference to carbosulfan and Phorate under similar conditions (Table 3). This is due to the binding capability of phosphate and sulphate ions present in malathion with the surface acidic groups of MEMD. Influence of co – pesticides on OX removal shall also be attributed to the varying molecular weights, complexity and functional groups of the former.
Table 3: Impact of Co – pesticides.
|
S.No |
Pesticides |
% Removal |
|||||
|
Concentrations (mg/L) |
|||||||
|
50 |
100 |
150 |
200 |
250 |
300 |
||
|
1. |
Oxyfluorfen (in absence) |
50.9 |
61.6 |
77.6 |
88.9 |
83.5 |
65.5 |
|
2. |
Malathion |
41.7 |
60.3 |
74.3 |
65.8 |
60.6 |
50.5 |
|
3. |
Carbosulfan |
47.2 |
55.2 |
70.8 |
82.1 |
80.3 |
60.4 |
|
4. |
Phorate |
42.4 |
50.9 |
60.9 |
84.2 |
70.1 |
53.5 |
Desorption / Regeneration Studies
Desorbing ability of MEMD loaded with oxyfluorfen and its regenerating capacity studied consecutively for four cycles, are represented in the bar chart (fig 13). It is evident from the diagram, that a maximum of 79.35, 77.86, 75.76and 70.65 mg/g adsorption capacities for the first, second, third and fourth cycles with corresponding desorbed amounts as 33.12, 35.76, 32.96 and 30.65 mg/g had been registered. This observation suggests the marked regenerating capability and reusable nature of MEMD.
 |
Figure 13: Regeneration Studies. |
Theoretical validations of the selected sorption system based on the experimental results were studied. The obtained data were validated using isothermal and kinetic equations.
Biosorption isothermal studies 23
Fit of the recorded values pertaining to Langmuir, Freundlich, Temkin and Dubinin – Radushkevich isotherm corresponding to their plots and their derived constant are shown in Figures (14 – 17) and table 4. A better linearity exhibited by Langmuir isotherm is supported the correlation co-efficient value close to unity (R2– 0.9877) and amount of OX adsorbed (qmvalue -70.12 mg/g). Constants of Temkin – BT (Heat of Adsorption < 10 J/ mol) and AT (Equilibrium Binding Constant), Freundlich – 1/n (> 1) and DKR- E (mean free energy < 20 J/ mol) values registered dissimilar results than the obtained batch data, representing a weaker interaction between the sorbate species and sorbent moieties. A comparison of the varied isothermal studies reveal the order to be as Langmuir > Freundlich >Tempkin > DKR, with respect to R2 values. This shows that the studied system follows monolayer adsorption, favoring the fit in of Langmuir model.
 |
Figure 14: Langmuir Plot |
 |
Figure 15: Freundlich Plot. |
 |
Figure 16: Temkin Plot. |
 |
Figure 17: DKR Plot. |
Table 4: Isothermal Constants.
|
Models |
Constants |
||
|
Langmuir
|
qm(mg/g) |
B (L/g) |
R2 |
|
70.12 |
0.13 |
0.9877 |
|
|
Freundlich
|
K (mg/g) |
1/n |
0.8632 |
|
60.92 |
1.23 |
||
|
Temkin |
AT(L/g) |
BT (J/mol) |
0.4293 |
|
58.76 |
143.87 |
||
|
DKR |
qs(mg/g) |
E (KJ/mol) |
0.7783 |
|
56.75 |
2.10 |
||
Biosorption kinetic studies
Pseudo first order and Pseudo second order model equations were solved using the experimental data, wherein the calculated values are plotted as log (qe – qt) and t/qt vs time (t) (figures 18 & 19). Deviations of the marked values is obvious in figure 20, whereas, almost all the plotted points lie in the straight line as far as figure 19 is concerned. This clearly is indicative of best fit of Pseudo first order for the studied system.
 |
Figure 18: Pseudo First Order. |
 |
Figure 19: Pseudo Second Order. |
Conclusion
Modified Elephas maximus dung (MEMD), an animal litter, being seldom reported elsewhere was employed for the confiscation of diphenyl ether pesticide (Oxyfluorfen) from aqueous environs under variable conditions. Optimized states of the batch studies for ≈ 89% Oxyfluorfen removal was established as 0.18mm particle size, 250 mg MEMD dosage, 200 mg/L initial Oxyfluorfen concentration, 40 mins agitation time, pH 5 and temperature 303K. Pesticide laden MEMD and its precursor were characterized viz., Microscopic, BET / BJH, FT-IR, SEM / EDAX and TG – DTA to assess the MEMD particle size, presence of functional groups, surface morphology / elemental composition and thermal stability. Four Isothermal and two Kinetic models were validated where, Langmuir and Pseudo first order plots well described the experimental data, indicating monolayer adsorption. From the results, it is arrived that Elephas maximus dung, serve as an excellent alternate to chelate the chosen toxicant, oxyfluorfen pesticide from aqueous matrices.
Acknowledgement
The authors gratefully acknowledge the grant from Star College Scheme of Department of Biotechnology (DBT) – New Delhi and TamilNadu Directorate of Collegiate Education, Chennai, India for providing the financial support for this research work.
Conflict of interest
The authors declare that we have no conflict of interest.
Funding Sources
There is no funding source.
References
- Peshin, S.S.; Srivastava, A.; Halder, N.; Gupta, Y.K. Journal of Forensic and Legal Medicine., 2014, 22, 57 – 61.
CrossRef - Marin-Morales, M.A.; Ventura- Camargo, B.C, Hoshina, M.M. Herbicides – Current Research and Case Studies in Use, 2013, 16, 399 – 443.
- Hussein, A.K.; Balah, M.A. Egyptian Scientific Journal of Pesticides, 2016, 2(4), 8 – 16.
- Wu, C.; Liu, X.; Wu, X.; Dong, F.; Xu, J.; Zheng, Y. Science of the Total Environment, 2019, 658, 87–94.
CrossRef - Sireesha, A.; Chandrasekhar Rao P.; Ramalaxmi, Ch.S.; Swapna, G. Indian Journal of Agricultural Research, 2013, 47, 449 – 452.
- Bharathi, C.; Arthanari, P.M.; Chinnusamy, C. International Journal of Chemical Studies, 2020, 8(6), 1038 – 1041.
CrossRef - Dayan,F.E.; Duke, S.O. Hayes’ Handbook of Pesticide Toxicology, 2010, 81, 1733 – 1751.
CrossRef - Karunarathne, A.; Bhalla, A.; Sethi,A.; Perera,U.; Eddleston, M. BMC Public Health, 2021, 21, 1441.
CrossRef - Naveen, B. P.; Sumalatha, J.; Malik, R.K. International Journal of Geo-Engineering, 2018, 9 – 27
CrossRef - Surra, E.; Correia, M.; Figueiredo, S.; Silva, J.G.; Vieira, J.; Jorge, S.; Pazos, M.; Sanromán, M.A.; Lapa, N.; Matos, C.D. Sustainability, 2021, 13, 3669.
CrossRef - Liua, J.; Wangb, N.; Zhangb, H.; Baeyens, J. Journal of Environmental Management, 2019, 238, 473–483.
CrossRef - Theivarasu, C.; Chandra, S. Oriental Journal of Chemistry, 2011, 2, 573 – 585.
- Çelekli, A.; Bozkurt, H. Environmental Science Pollution Research, 2013, 20, 4647 – 4658.
CrossRef - Preethi, G.; Muthulakshmi Andal, N. International Journal of Environmental Pollution, 2022, 2, 243 – 248.
- Anuradha, J.; Muthulakshmi Andal, N. Environment Asia, 2019, 2, 9 – 13.
- Alrahman, K.F.; Elbashir, A.A.; Ahmed, H.E. Medicinal Chemistry, 2015, 5 -8.
- Foroutan, R.; Peighambardoust, S.J.; Hosseini, S.S.; Akbari, A.; Ramavandi, B. Journal of Hazardous Materials, 2021, 413, 125428.
CrossRef - Khoshbouy, R.; Takahashi, F.; Yoshikawa, K. Environmental Research, 2019, 175, 457 – 467.
CrossRef - Daia, J.; Mengb, X.; Zhanga, Y.; Huang, Y. Bioresource Technology, 2020, 311, 123455.
CrossRef - Rashid, R.; Afroze,F.; Ahmed, S.; Miran, M, S.; Susan, A.B.H. Materials Today: Proceedings, 2019, 15, 546–554.
CrossRef - Kalhor, M. M.; Rafati, A.A.; Rafati, L.; Rafati, A.A. Journal of Molecular Liquids, 2018, 15, 453 – 459.
CrossRef - Theivarasu, C.; Chandra, S. Desalination and Water Treatment, 2013, 51, 7639 –7654.
CrossRef - Gupta, V.K. ; Gupta, B.; Rastogi, A.; Agarwal S.; Nayak, A. Water Research, 2011, 45, 4047 – 4055.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










