Batch Adsorption Study of Synthetic Dye Mix in Aqueous Solution using Activated Carbon Prepared from Coconut Shell
Department of Chemistry, Seethalakshmi Ramaswami College, Affiliated to Bharathidasan University, Tiruchirappalli, India.
Corresponding Author E-mail: mvasuki63@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380434
Article Received on : 20 Jul 2022
Article Accepted on : 30 Aug 2022
Article Published : 31 Aug 2022
Reviewed by: Dr. Amanullakhan Pathan
Second Review by: Dr. Mapule P Seabelo
Final Approval by: Dr. Charanjeet kaur
This investigation focuses on the effectiveness of coconut shell activated carbon in removing synthetic dye mixture from aqueous solutions. Coconut shell activated carbon, an economical and effective adsorbent, was made from agricultural waste raw material and chemically activated by sulphuric acid treatment. Activated carbon is characterised using FT-IR and SEM analysis. Batch adsorption studies were conducted by adjusting conditions such contact time, adsorbent dosage, initial dye concentration and temperature. The equilibrium of the adsorption process was described through analysis of isothermal models including Freundlich, Langmuir, and Scatchard. Kinetic data followed a pseudo-second order model. Thermodynamic studies showed that the adsorption was endothermic, spontaneous, and feasible. The results of the experiment indicate that coconut shell activated carbon is an effective, environmentally acceptable adsorbent for eliminating synthetic dye mixt from aqueous solution.
KEYWORDS:Adsorption; Adsorption Kinetics; Coconut shell; H2SO4 activation; Thermodynamics; Synthetic dye mix
Download this article as:| Copy the following to cite this article: Vasuki M, Karthika M, Saraswathi G, Akila S. Batch Adsorption Study of Synthetic Dye Mix in Aqueous Solution using Activated Carbon Prepared from Coconut Shell. Orient J Chem 2022;38(4). |
| Copy the following to cite this URL: Vasuki M, Karthika M, Saraswathi G, Akila S. Batch Adsorption Study of Synthetic Dye Mix in Aqueous Solution using Activated Carbon Prepared from Coconut Shell. Orient J Chem 2022;38(4). Available from: https://bit.ly/3Kxbh6K |
Introduction
Synthetic dyes are organic compounds which are frequently employed in the leather, rubber, paper, plastic, cosmetic, and textile industries. Because of their intricate chemical structure, dyestuffs are difficult for living things to degrade. They have teratogenic, carcinogenic, mutagenic, and toxic properties 1, 2. When these dyes are released into water, it frequently leads to water pollution, which usually inhibits sunlight from penetrating the water, which is necessary for aquatic life’s photochemical and biological processes. As a result, in order to lessen environmental pollution, industrial effluents containing dyes must be appropriately treated before being discharged into water bodies.3. Although there are numerous conventional techniques for removing dye from aqueous solutions, including precipitation, ion exchange, solvent extraction, electrochemical treatment, biosorption, and filtration, these techniques are either expensive or insufficient for removing contaminants with high concentrations. Adsorption is a typical, effective, and widely applied realistic method for dyestuff removal from wastewater. Many researchers have studied the development and application of cost effective adsorbents for the efficient elimination of dyestuffs from effluent. 4,5.The carbon derived from biomass mixed with metal oxides and other semiconductors posess good photocatalytic activity.6, 7 Activated carbon is very effective support material in water treatment due to its excellent hydrophobicity, porus structure, stronger adsorption capacity, structural stability low cost and large surface area that facilitates a better adsorption and acts as a promising material that supports photocatalytic process. 8-10 . Activated charcoal made from biomaterials has very low costs when compared to commercial activated carbon 5. Hence coconut shells were best utilized for the adsorption process as adsorbents once they are properly treated and activated in order to increase the pore size and surface morphology 11, 15. Brilliant green, Naphthol Blue Black-B, and Alizarin Red-S are the three dyes utilised to prepare the synthetic dye mix solution for the investigation. These dyes have longer wavelength and are supposed to cause severe discomfort and illness when exposed to human beings. The investigation was conducted to determine the effectiveness of synthetic dye mixture removal by H2SO4 activated carbon produced from coconut shells.
Materials and Methods
Materials
Naphthol blue black-B (NBB-B) was bought from Loba Chemie Pvt. Ltd. Mumbai. Brilliant Green (BG) and Alizarin Red-S (AR-S) were obtained from Hi Media Laboratory Pvt. Ltd. Mumbai . 0.05g of each dye (Brilliant Green, Naphthol Blue Black-B, and Alizarin Red-S) was dissolved in 100ml of distilled water in a standard flask to prepare stock solution. Synthetic dye mix was standardised by measuring the optical densities of various concentrations of the dye solution at 619 nm by using MAPADA spectrophotometer.
Preparation of Coconut Shell Activated Carbon (CSAC)
The coconut shells were collected, cleaned, dried in the sun, and then treated with sulphuric acid at a 2:1 ratio. The samples that had been impregnated were dried for four hours at 200°C. To remove the free acid, the carbonised material was washed several times with a solution of 1 % sodium bicarbonate, and then rinsed with distilled water. It was then dried at 105°C. As a result, the activated carbon from the coconut shell (CSAC) was ground into a fine powder in a mortar and employed as an adsorbent in all of the studies.
Instrumentation
The optical density of dye solution were determined using MAPADA V-100 D Spectrophotometer. The IR absorption bands were taken by FT-IR (SHIMADZU) spectrometer in the wave number range of 400 – 4000cm-1 . The surface morphological structure of activated carbon was observed using SEM technique (VEGA 3 TESCAN).
Adsorption Studies
The batch adsorption tests were conducted in a 250 ml Pyrex bottle containing 100 ml of a synthetic dye solution with 1.0 g of coconut shell-derived activated carbon (CSAC). The bottle was then shaken at a speed of 250 rpm in an orbital shaker to evaluate the influence of contact period, CSAC dosage, initial dye concentration, and temperature. The optical density was measured using a MAPADA spectrophotometer to study the percentage adsorption. Eqn. 1 is used to calculate percentage decolourisation of synthetic dye mix. 16.
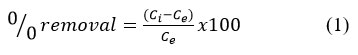
where Ci is initial and Ce is equilibrium synthetic dye mixture concentrations in (mg/L)
Results and Discussion
Characterisation
FT-IR Analysis17 permits spectrophotometric observation of the adsorbent surface in the range 400-4000 cm -1 and predicts a direct means for the identification of the organic functional groups on the surface of activated carbon.IR spectrum (Fig.1) shows a peak at 1143.79 cm-1 and 1203.58cm-1 which is attributed to C-O-C and C-OH bond.
 |
Figure 1: IR Spectrum of Activated Carbon. |
SEM18 analysis is widely used to study the morphological features and surface characteristics of adsorbent materials. SEM image in Fig. 2 shows flaky structure and irregular forms with different sizes on the surface of the activated carbon.
 |
Figure 2: SEM Image of Activated Carbon. |
Adsorption Studies
Effect of contact time
The dye solution (100ml, 4 mg/L) was shaken in an orbital shaker with 1.0g of activated carbon at varied time intervals. After 50 minutes of agitation, the percentage sorption attained equilibrium and showed an increasing trend as the agitation period increased. According to Fig. 3, the percentage of decolorization rises as contact time increases 19.
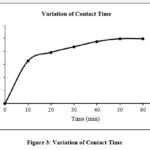 |
Figure 3: Variation of Contact Time. |
Effect of initial dye concentration
Synthetic dye solutions were stirred with 1.0g/L of activated carbon at 34°C for 50 minutes at each of the concentrations of 3.0, 3.5, 4.0, 4.5, and 5.0mg/L in 100ml. As can be seen from Fig. 4, the percentage of decolorization was observed to decrease as initial concentration of the dye is increased. This might be related to the dye monolayer that has developed on the surface of activated carbon (CSAC), which inhibits the formation of subsequent dye layers.
 |
Figure 4: Variation of initial dye concentration. |
Effect of dosage of CSAC
The influence of dosage of CSAC on adsorption was examined by increasing the amount of CSAC from 0.50 to 1.50 g/L while maintaining the optimal dye concentration of 4 mg/L, contact period of 50 min, and temperature of 34°C.As the dosage of activated carbon is increased, a rise in the percentage of decolourisation was noticed (Fig. 5) The reason may be the adsorption sites become available at an increasing rate with an increase in the adsorbent’s surface area.
 |
Figure 5: Variation of dosage of adsorbent |
Effect of temperature
The impact of temperature on process of adsorption was investigated at various temperatures (34, 37, and 40°C).When the temperature rises, the percentage of decolorization likewise rises, improving the availability of active adsorption sites and increasing the porosity of the adsorbents’ pore volume. The percentage decolorization increases as temperature rises (Fig. 6) shows the endothermic nature of adsorption 20,21.
 |
Figure 6: Variation of temperature. |
Adsorption Isotherm Studies
Adsorption isotherms are often defined as the ratio of the amount of dye adsorbed to the amount still in solution at equilibrium and at a constant temperature. To learn about the mechanistic details and the adsorbent’s surface properties, the adsorption parameters are correlated using several isotherm models. To find the isotherm that fits the data the best, the linear regression coefficient (R2) is utilised 19.
Freundlich Isotherm
A solute’s concentration on the adsorbent’surface and equilibrium concentration in solution are related empirically by the Freundlich isotherm 22-24. Freundlich’s equation can be expressed linearly as
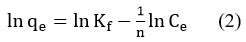
It was observed that the plot of lnCe vs ln qe (Fig. 7) was linear, indicating that the Freundlich isotherm governs the adsorption of synthetic dye mix on activated carbon (CSAC) made from coconut shell.
 |
Figure 7: Freundlich Isotherm. |
Langmuir Isotherm
To calculate the maximal adsorption capacity corresponding to full monolayer coverage on the adsorbent surface, the Langmuir isotherm22-25 is employed, and its expression is
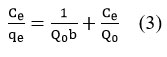
Ce /qe versus Ce plot (Fig.8) was found to be linear. A separation factor termed the equilibrium parameter, abbreviated RL, can be used to represent the Langmuir isotherm. RL which is expressed as
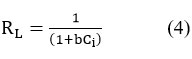
where b is Langmuir constant and Ci is the initial dye concentration.
The RL value reveals both the isotherm’s shape and the nature of the adsorption process. Adsorption is if RL = 0 it is irreversible , unfavourable if RL value > 1, favourable if 0<RL<1 . In the current investigation, RL value was found to be 0.0176. As a result, it can be concluded that the adsorption of synthetic dye mix utilising activated carbon (CSAC) is a favourable process for the concentration range investigated.
 |
Figure 8: Langmuir Isotherm. |
Scatchard Analysis
To further analyse the binding isotherm of dye and adsorbent, Scatchard analysis 26-29 was used. It is possible to express the Scatchard equation as,
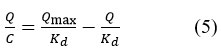
The experimental data for the adsorption of synthetic dye mix was found to be fitted with the Scatchard analysis, which is represented by a single straight line in the Scatchard plots (Fig. 9).
 |
Figure 9: Scatchard Analysis. |
The slope and intercept values for all adsorption isotherm models are listed as follows,
Table 1: Adsorption Isotherms.
|
Name of the isotherm |
Slope |
Intercept |
R2 value |
|
Freundlich |
n = 7.4129 |
Kf = 1029.3677 |
R2 = 0.876 |
|
Langmuir |
Q0 = 26.5957 |
b = 13.9259 |
R2 = 0.990 |
|
Scatchard |
Kd = 0.1 |
Qmax = 0 |
R2 = 1 |
Adsorption Kinetics
Pseudo-first order as well as pseudo-second order kinetic models have been studied for the decolorization of synthetic dye mix solution over coconut shell activated carbon.
Pseudo-first order kinetic model
The pseudo-first order kinetic model [30] can be represented as
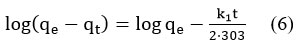
This equation predicts that the log (qe-qt) should be linearly plotted against time. According to the equilibrium data the adsorption of a synthetic dye mix on CSAC, cannot be applied, and the reaction mechanism does not follow first-order kinetics.
Pseudo-second order kinetic model
The equation for pseudo-second order reaction kinetics 31 is
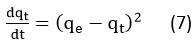
The rearranged equation is obtained by integrating the above equation
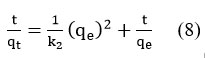
The plot of t/qt against t (Fig.10) was linear. The reaction kinetics is in accordance with a pseudo-second order rate equation, which was supported by the correlation coefficient (R2).
 |
Figure 10: Pseudo-Second order kinetic model. Click here to View figure |
Thermodynamic Studies
The Gibbs free energy change ( ∆G◦) standard entropy change (∆S◦) and standard enthalpy change ( ∆H◦) of the process of adsorption were calculated 31,32 using following equation.,
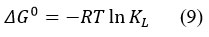
where T-Absolute temperature in K
R- Universal gas constant (R=8.314 J/mol/K)
KL– Equilibrium constant (with respect to Langmuir constant Q0 and b)
The following equation was used to determine the entropy change (∆S◦) and enthalpy change (∆H◦) parameters ,
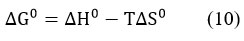
The values were calculated based on the plot of ln KL vs 1/T. (Fig.11).
 |
Figure 11: Adsorption Thermodynamics. |
The process of adsorption is spontaneous in nature, as shown by the negative value of ∆Gօ (-6.7009). The affinity of CSAC for synthetic dye mix is reflected in the positive value of entropy change ∆Sօ (0.0219), and the positive value of ∆Hօ (0.0024) indicates the endothermic character of the adsorption process.
Conclusion
In the current investigation, synthetic dye mix was successfully removed from an aqueous solution using coconut shell carbon that had been subjected to sulphuric acid treatment. According to batch adsorption experiment, the maximum dye removal of 99.1 % was accomplished after 50 minutes. Synthetic dye adsorption increased with contact time, CSAC dosage, and temperature. The results of the experiment are examined using a variety of adsorption isotherm models, including the Freundlich, Langmuir, and Scatchard models. The equilibrium data is best explained by the homogenous, monolayer Langmuir isotherm. Pseudo-second order kinetics is found to govern the adsorption process. The negative value of ∆Gօ indicates the spontaneity and feasibility of the adsorption process. The adsorption process is endothermic as indicated by the positive values of ∆Hօ . In conclusion, coconut shell activated carbon (CSAC) is an efficient adsorbent for removing synthetic colour mix from aqueous solution. Furthermore, coconut shell activated carbon as an adsorbent plays a significant part in waste management by minimising agricultural waste in addition to being used for waste water treatment.
Acknowledgement
We thank Seethalakshmi Ramaswami College, Tiruchirappalli for providing facilities to carry out this research work.
Conflict of Interest
All authors declare that there is no conflict of interest in this paper.
References
- Crini, G. Bioresource technology, 2006, 97(9), 1061-85.
- Aksu, Z. Process biochemistry, 2005, 40(3-4), 997-1026.
- Edokpayi, O.; Osemwenkhae, O.;Ayodele, B.V.;Ossai, J., Fadilat, S.A.; Ogbeide, S.E. Journal of Applied Sciences and Environmental Management, 2018, 22(5), 631-635.
- Meyer, V.; Carlsson, F.H.,;Oellermann, R.A. Water Science and technology, 1992, 26(5-6), 1205-11.
- Srisorrachatr, S ;Kri-arb, P., Sukyang, S.; Jumruen, C. InMATEC Web of conferences, EDP Sciences, 2017, 119, 01019.
- Prasannan, A.Ind.Eng.Chem.Res.2013, 52, 15673-15678
- Hasan, A.K.M.M.; Dey, S.C.; Rahman, M.M.; Zakaria, A.M.; Sarker, M.; Ashaduzzaman, M.; Shamsuddin, S.M. Bull. Mater. Sci. 2020, 43, 1–9
- Amel Taha; Melek Ben Aissa;EnshirahDa’na. Molecules, 2020, 25, 1-18.
- Bappy Mondol; Anupam Sarker ;A. M. Shareque; Shaikat Chandra Dey; Mohammad Tariqul Islam; Ajoy Kumar Das; , Sayed Md. Shamsuddin; Md. Ashraful Islam Molla; Mithun Sarker. Photochem, 2021, 1, 54–66
- Prabhavathy S.;Arivuoli Dakshanamoorthy. Journal of Materials Science and Surface Engineering, 2021, 8(2),1034-1044
- Bhatnagar, A.; Vilar, V.J.;Botelho, C.M.;Boaventura, R.A. Advances in colloid and interface science, 2010, 160(1-2), 1-5.
- Aljeboree, A.M.;Alshirifi, A.N.; Alkaim, A.F. Arabian journal of chemistry, 2017, 10, 3381-93.
- Zhang, L., Tu, L.Y. ;Liang, Y.;Chen, Q.; Li, Z.S.; Li, C.H.; Wang, Z.H. ;Li, W.. RSC advances, 2018, 8(74), 42280-91.
- Kannan, N.; Shakila, O.D. Indian Journal of Environmental protection, 2005, 25(5), 437.
- Begum, S.F.; Uma, C.; Balamurugan, S.; Kalyani, M. Asian Journal of Microbiology Biotechnology and Environmental Sciences, 2005, 7(4), 733.
- Vanderborght, B.M.;Van Grieken,;R.E. Analytical chemistry, 1977, 49(2), 311-6.
- Namasivayam, C.;Kavitha, D. Microchemical Journal, 82, 2006, 43-48.
- Ashish S. Sartape ; Aniruddha M. Mandhare ;Vikas V. Jadhav;Prakash D. Raut ;Mansing A. Anuse; Sanjay S. Kolekar. Arabian Journal of Chemistry, 2017, 10, 3229-3238.
- Karthika, M.; Vasuki, M. International Journal of Applied Engineering Research, 2018, 13(12), 10260-7.
- Baidya, K.S.; Kumar, U. South African Journal of Chemical Engineering, 2021, 35, 33-43.
- Laskar, N.;Kumar, U. International Journal of Environmental Science and Technology, 2019, 16(3), 1649-62
- Rehman, M.S.; Munir, M.; Ashfaq, M.; Rashid, N.;Nazar, M.F.;Danish, M.; Han, J.I. Chemical engineering journal, 2013, 228, 54-62.
- Freundlich, H.M. J. Phys. chem., 1906, 57(385471), 1100-7.
- Adane, B.;Siraj, K.;Meka, N. Green Chemistry Letters and Reviews, 2015, 8(3-4), 1-2.
- Langmuir, I. Journal of the American Chemical society, 1918, 40(9), 1361-403.
- Panahi, H.A.; Shakerin, N.;Zolriasatain, F.;Panahyab, A.; Moniri, E. European Journal Of Experimental Biology, 2013, 3(6), 215-4.
- Foo, K.Y.;Hameed, B.H. Chemical engineering journal, 2010, 156(1), 2-10.
- Shahmohammadi-Kalalagh, S. Caspian Journal of Environmental Sciences, 2011, 9(2), 243-55.
- Shahbeig, H,; Bagheri, N.;Ghorbanian, S.A.; Hallajisani, A.;Poorkarimi, S. World Journal of Modelling and Simulation, 2013, 9(4), 243-54.
- Lagergren, S.K. Sven. Vetenskapsakad. Handingarl., 1898, 24, 1-39.
- Ho, Y.S.; McKay, G.; Wase, D.A.J.; Forster C.F. Adsorption science & technology, 2000, 18(7),639-50.
- Mittal, A.; Mittal, J.;Malviya, A.;Gupta, V.K. Journal of colloid and interface science, 2010, 344(2), 497-507.
- Kul, A.R.;Koyuncu, H. Journal of Hazardous Materials, 2010, 179(1-3), 332-9.

This work is licensed under a Creative Commons Attribution 4.0 International License.










