Sulfur-center Reactivity toward Oxygenation Mediated by Ru : Effective Bioactive Compounds
Sarsuna College, 4/HB/A, Ho-Chi-Minh Sarani, Kolkata-700061, WB, India.
Corresponding Author E-mail: ujjwalsccs@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380305
Article Received on : 23 Apr 2022
Article Accepted on :
Article Published : 25 May 2022
Reviewed by: Dr. M Stella Bharathy
Second Review by: Dr. Neeta Shukla
Final Approval by: Dr. Bal krishan
Transition metal mediated thiolato compounds are highly vulnerable for S-centered oxidation due to its high nucleophilicity and which is immensely important in the point of its bio-activity. It is generally noticeable that a range of chemical changes occurred with molecular O2 and ruthenium thiolato metalloligands in varying conditions. These oxygenations are facile under strictly oxygen environment and produce mono and di sulfenato and/or sulfinato depending on the substrate thiolato. The numerous heteroatomic substituents of thiolato-S ligand have performed a vital task during the course of oxygenation producing oxygenated products as sulfenates, sulfinates and sulfones. There appear to be numerous mechanisms that are involved in the oxygenation process are considerably more complex. Some bizarre photo-induced S-center oxygenation of metal-thiolato to the sulfonated compound is also mentioned. The ruthenium sulfur compounds jointly with the S-oxygenates show remarkable bioactivity as well as enzymatic catalytic activity and interaction with the bio-molecules like DNA that opens a new theme for the researcher for design novel Ru-sulfur-oxygenates compounds as metallodrugs.
KEYWORDS:DNA binding; Dioxirane; Hydrogenase; Metallodrugs; Sulfenato; Sulfinato
Download this article as:| Copy the following to cite this article: Das U. Sulfur-center Reactivity toward Oxygenation Mediated by Ru : Effective Bioactive Compounds. Orient J Chem 2022;38(3). |
| Copy the following to cite this URL: Das U. Sulfur-center Reactivity toward Oxygenation Mediated by Ru : Effective Bioactive Compounds. Orient J Chem 2022;38(3).Available from: https://bit.ly/3lIMHV2 |
Introduction
The sulfur-centered reactivity of transition-metal thiolates generating sulfur oxygenates is well documented, with preliminary efforts focused mainly on FeIII, CoIII and NiII.1-3 One of the most remarkable properties of the metal-bound sulfur atom is their superior nucleophilicity 4-10 which leads to derivatives modified at sulfur center. Transition metal thiolates are prone to S-oxygenation11,12 processes due to high S-centered reactivity. The fact is p-back-bonding from Mn+–t2 to sulfur-3p orbital vis-à-vis drifting of greater electron density to the coordinated thiolato sulfur in {R/Ar-S-M} plays an important role for the enhancement of S-centered reactivity as a nucleophile compared to thiols (R/Ar-S-H).13 Theexternal electrophiles such as molecular oxygen react with nucleophilic sulfur center in these oxygenation processes.Transition-metal complexes with sulfur enclosing functions typically resulted in ligand-based oxidation of the thiolato (RS–) to thiyl radical (RS·), with successive complex deformations can be find in various biological processes.14 Such sulfur functionalised ligands as thiols and thiophenols possessing redox noninnocence nature, play key roles in many biological processes.15-18 In this stand point several thiolato complexes can be converted to different sulfur oxygenates having numerous bioactivity including various enzymatic action with a good number of transition metal complexes.19-21In addition, such type of pro-reactive metal bound sulfur center are more important in the view point of active sites oxygenation of some definite S-rich metalloenzymes22,23 of mostly first row transition metals,for exampledeactivation of metallocysteinate enzymes and to the oxidative metabolism of cysteine.24 The bio-activity of few sulfur populated enzymes [NiFe] hydrogenase and CO-dehydrogenase, is inhibited and irreversibly deactivated by the reaction of a series of metal-thiolates with molecular O2.25,26 Endo and co-workers mentioned that the oxygen affinity of organothiolates appended iron in the biological system. In modern biology, cellular regulatory processes where post-translational oxygenation of the cysteine-derived sulfur donors with a combination of thiolate (RS–), sulfenate (RSO–), and sulfinate (RSO2–) in the active site of NHase to iron(III) was reported.27-30 Again mimicking the active site of such types of valuable enzymes incorporated the oxygenates of sulfur ligands achieved by numerous model complexes synthesis.31-33 In recent time, an array of transition metal mediated sulfur oxygenates like sulfenate, sulfinate and sulfonates have been synthesized mostly by chemical methods and also photochemical and/or electrochemically oxidation processes from the corresponding thiolates. 34,35 Activation of molecular oxygen from air and/or O2 purged into the reaction medium externally, which is believed to oxidize metal-thiolato ligands to corresponding sulfenato and/or sulfinato.Both the triplet [3SO2] and also the singlet [1∆O2] state of molecular oxygen are found to oxidize metal-thiolato to sulfur oxygenated compounds with varying conditions (Scheme 1).36,37 Nonetheless, there are some peroxo and activated oxygen donating sources (H2O2, dimethyldioxirane etc),38-40 can effectively oxygenated the thiolato sulfur center similar to the molecular oxygen. However, analogous scrutiny of S-centered oxidation of aryl thiolates with platinum-group-metals for ruthenium, rhodium, palladium, platinum and gold are less common41-43 and only a few reports for osmium44 and iridium45 have so far been made. It is worth ofmentioning that such type of platinum group metal thiolato is very much prone to alike oxidation producing metallosulfones under aerobic condition and strictly O2 atmosphere is not required.
 |
Scheme 1: Path of Metal-aryl thiolate S-oxygenation. Click here to View scheme |
Here we mostly focused on Ru-mediated organosulfur compounds and their S-oxygenated products reported so far and try to concentrate on the synthesis of this group of compounds utilizing the activation of molecular O2 also paying attention to the underlying mechanism. Most of the metal-thiolato and metal-S-oxygenated compounds were characterized different spectroscopic methods and since the Ru-sulfur compounds are redox active and the electro-active nature of the complexes were analyzed by cyclicvoltametry which reveal many interesting and rich spectral features. The structural behavior including stereochemistry of the representative compounds including bond length bond angle were authenticated very precisely by the crystallographic diffraction techniques using X-ray diffractometer. Finally, unification and correlation of the application in terms of bioactivity and the related field of the reported Ru-S oxygenated compounds are discussed.
Metallo-Sulfur Oxygenated Compounds
The oxygenation of sulfur center of metal-thiolates {M-SR} yielding a number of S-oxygenated products, as metal-sulfenate; [M-S(=O)], metal-sulfinate; [M-S(=O)2] and sometime mixture of the both (Fig 1). Alongside this there are another S-oxygenated compound termed metal-sulfonate [M-SO3–] can be isolable.46 Metal-thiolates to metal-sulfenato, metal-sulfinato conversion by the incorporation of molecular oxygen is two electron and four electron oxidation process respectively.47 In general metal-sulfenates are less stable than the corresponding metal-sulfinates and would disproportionate to metal-thiolate and metal-sulfinates, although a few examples of stable transition metal sulfenates [M-(S(=O)R)] are known.48
 |
Figure 1: Metal aryl sulfur fragments: metal thiolato (left), metal- sulfenate(middle) and metal-sulfinate(right). |
In the oxidation processes, divalent sulfur is oxidized to tetravalent or hexavalent sulfur via oxidative addition in presence of molecular oxygen. Molecular O2 is easily accessible low toxic eco-friendly natural species, thence it is applied in many important organic transformations to produce a lot of starting materials; which are very essential, reasonable and important for industries. As per the findings from the literature both the spin state 3SO2 and/or 1∆O2 of molecular oxygen is believed to oxidize thiolato ligands to sulfenato mostly {(R/Ar)S=O} or sulfinato {(R/Ar)S(=O)2} shown in Scheme 1,36,37 although 3SO2 reacts more slowly than 1∆O2.Conversion of molecular oxygen to singlet oxygen [1∆O2] by utilising energy or to superoxide [O2·] by exchange of electron is the most indispensable and necessary step for the sulfur oxygenation processes and its detail mechanism is discussed afterwards.
Material and Methods
In this present study of synthesis of an array of metal-bound sulfur oxygenates where the main primary targeted compounds are Ru-thiolates having a diverse ligand environment; in which RuCl3. 3H2O and its various derivatives were used as starting metal precursors, which provide the active site of the metal in the metal-thiolato functionality. Principally both aromatic and aliphatic thiols were used as sulfur source during the synthesis of chelate ligands. A several number of coligands having hetero-donor part like bpy, phen, PX3 (X= H, Me, Et, Pr, Ph etc.) Py, halides are linked to the metal-thiolate active zone playing essential task in the course of Ru-sulfur-oxygenates synthesis and the second vital component is oxygen, which is coming from molecular oxygen or any other oxygen supplying resource; like peroxides which are discussed anon in details.
Mechanism of Thiolato Sulfur Oxygenation
Numerous studies have been made en route for the mechanism for ‘metalothioether’ S-oxygenation and it has been documented that the initial drifting of nonbonding electron density from thiolato-S to molecular oxygen occurs during the course of oxygenation. The literature survey reveal that the transition metal thiolato including few soft heavier metals like Ru, Pd and Pt having one or multi S-center are known to form a variety of sulfur oxygenates with both ground state triplet (3SO2) and excited state singlet (1∆O2) with appreciable yield.49,50 The rigorous study in this field explored that the anticipated mechanism shown in Scheme 2 invoke a persulfoxidic species, after the first attachment of molecular oxygen with metal bound thiolato S as has been proposed in the reaction of 1∆O2with R2S,51,52 as the common and active precursor of both the mono and bis sulfinato products. Single-site collapse would yield the thiadioxirane,36,52-54 en routeto the stable metal sulfinate either by involving scission of O-O bond and followed by rearrangement of oxygen atom through intermolecular pathway. Again it may follow the intramolecular orientation of oxygen atom to the sulfinate component after the reactive intermediate collapsed.
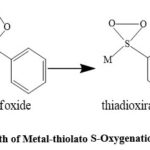 |
Scheme 2: Probable Mechanistic Path of Metal-thiolato S-Oxygenation and the intermediates generated. |
Jensen and co-workers55 first suggested from their computational studies that the persulfoxide and thiadioxirane are the important and effective intermediate in the route of sulfenato and sulfinato synthesis.
Literature analysis explore that though triplet (3SO2) as well as singlet spin state (1∆O2) of oxygen molecules are known to generate metal sulfones/sulfoxides, but their rate of formation is almost 9 to 10 time enhances50,56 with 1∆O2 than the former, though the 3SO2 ®1∆O2 excitation hurdle is only 22.4 kj/mol; which is easily overcome and compensated by the other activation parameters – like formation energy and entropy(Fig 2).56 Such an observation of the slow rate of metal-bound thiolato sulfur oxygenation due to the spin forbidden reaction with ground state triplet oxygen (3SO2) and the rate of reaction enhance for spin allowed reaction with 1∆O2, shown in the simplified cartoon diagram in Fig 2. The formation of sulfinato {S(=O)2}, from the corresponding metal-thiolato by intramolecular addition of dioxygen as in other cases.36 Expectedly, 3rd row transition metals (5d) acts as a better aspirant for back-donation M(t2) ® SO2(π*) as compared to 2nd row (4d) or 1st row (3d) transition metals attributable to its more dilated d orbitals due to relativistic effects.57
 |
Figure 2: Diagram exhibiting spin-forbidden and spin-allowed transformation of metal-thiolato to metal-S-oxygenates with 3SO2 and 1∆O2 |
Sulfur center oxygenation having two or more thiolato groups incorporated metal ion is further interesting and producing a variety of S-oxygenated products. There are many reports in the literature of oxygenation of adjacent sulfur site in cis-position for especially d8metals possessing generally square planar geometry.44,58 In this geometry two S atoms of the thiolato ligand adopted generally in the cis position of the centered metal ion and facilitate the S-centered oxygenation. The dioxygen insertion across the neighbouring sulfur of metal-bisthiolato complexes followed by O-O bond scissions both intramolecular and intermolecular pathway, generating metal-bissulfenato complexes either passing through M-monosulfenato complex following Path A, or via a five membered cyclic form M(SR)2O2 following intramolecular path (Path B) which isshown in Scheme 3, where it is supposed that in both the route passing through a common metal-persulfoxidic intermediate.
 |
Scheme 3: Probable mechanistic paths to cis-metal-bissulfenate from cis-metal-dithiolate via persulfoxidic species. |
This is worth to mention that in the course of metal-bissulfinate formation, initially the monosulfinato generated from thiadioxyrane,which take up the second oxygen from the same O2 molecule for a particular monosulfur center (Path D) and hence required overall two equivalents O2 to reach bissulfinato. While the SO2 fragments of metal-bissulfinate from bissulfenateutilised oxygen atoms from same O2 molecules (Path C) passing through a five membered cyclic form M(SR)2O2 species as shown in Scheme 4, which can be identified from the isotopic labelling studies.59
 |
Scheme 4: Proposed mechanistic paths to cis-metal-bissulfinate from persulfoxidic species via bissulfenate or monosulfinate |
Discussion
Metal-Thiolato Oxygenation and Characterisation
The thiolato S-center oxygenation and the chemistry of the corresponding oxygenated compounds are mainly explored for the Ni group having square planar site; exacting attention has been specified on the NiII then PdII and a few PtII, due to their potential interaction with the biological systems including enzymatic functions. A variety of mono and bisthiolato sulfur oxygenated compounds have been reported in the literature in which mono-sulfenates [M(RS)(RSO)], di-sulfenates [M–(RSO)2], mono-sulfinates [M–(RS)(RSO2)], di-sulfinates [M–(RSO2)2], and mixed sulfenate–sulfinate [M–(RSO)(RSO2)] compounds were pointed out.59 In this present study, the interest has been concentrated on the another heavier transition metal ruthenium, to explore its variation of bio-activities as well as the optoelectronic properties whilst the Ru-bound thiolato sulfur get oxygenated. Remarkably, S-oxygenation of the Ru-thiolato center is the most studied S-oxygenation chemistry after the Ni-group and among the heavier transition metal as well. Syntheses of the sulfur oxygenated complexes from the thiolato is most interesting since the activation of molecular O2 and its incorporation to the sulfur center involved in normal aerobic condition under reflux and/or stirring, most of the cases no externally O2 purging is required. There are some reports in the literature where O2 purging to the reaction medium necessary for the production of metal-sulfur oxygenates. Characterization and identification of these oxygenates done by different spectroscopic methods. Redox nature were analysed by electrochemical study (CV) and information regarding structural detailing and the mass of the molecular or ionic fragments revealed from X-ray diffraction investigation and Mass spectroscopy. Infrared spectra is most informative for the identification of S-oxygenated complexes for the MS(=O)and MS(=O)2 fragments since it exhibit sharp and strong vibrations (symmetric and asymmetric) in the range 900 cm-1 to 1200 cm-1.22,59,60 Electrochemical scrutiny revealed that the accumulation of O atom to the nucleophilic M-thiolato group generating a new S-oxide donor motif , which bring slight stability to the metal center, while the incorporation of the second oxygen atom producing sulfone functionality by which metal oxidation state get more stabilised. This change can be located in the transformed pattern of the cyclicvoltagrams.
Grapperhaus et. al. reported the stepwise conversion of ruthenium(II)-thiolato (1) ([RuII(DPPBT)3]– where DPPBT = 2-diphenylphosphinobenzene thiolato) to ruthenium(II)-sulfinate (2) under stirring condition (Fig 3).34 Interestingly metal center get oxidised from RuII-thiolato (1) to RuIII-thiolato (1a), occurred firstly under purging oxygen gas through RuII-thiolato solution externally and subsequently ligand-centered oxygenation generating RuIII-sulfinate (2a) by further oxygen exposure to (1a) and followed by in situ slow reduction of (2a) yielded target compound RuII-sulfinate (2) in the absence of any reducing agent.
 |
Figure 3: Diagram exhibiting stepwise Ru-centered oxidation and ligand –S-centered oxygenation of ruthenium(III) thiolato compound. (Reproduced with permission from Reference No. 34. Copyright 2005 American Chemical Society.) |
The oxygenation of metallo-thiolates ligand facilitate O↔M interaction,59 and it is believed that the donor property of metallo-S group decreases on S-oxygenation, which bring stability to the metal center.The production of RuII-sulfinato was authenticated by FTIR analysis, in which two strong signals near 1017 cm-1 and 1115 cm-1 were exhibited as conferred previously, which is shown in Fig 4.59
 |
Figure 4: FTIR spectrum of Ru−Ssulfinato (2) as KBr pellet, (Reproduced with permission from Reference No. 34. Copyright 2005 American Chemical Society.). |
Also a slightly decrease in Ru–S bond length of ~ 0.09 Å for Ru−Ssulfinato than that of Ru−Sthiolato confirmed the conversion and which is the case of RuII ® SO2-p* back-donation due to involvement of oxygen discussed afterward. Synthesis of a less common monosulfinates (3) L(S·SO2) of Ru(II) is described in another study by A. B. P. Lever et.al.,61 where the metal-sulfur bound sulfydryl monosulfinates complex, which is slowly but spontaneously oxidised to the RuII-disulfinates (4) L(SO2·SO2) in solution under aerobic condition shown in Fig 5.
 |
Figure 5: Formation of metal-bissulfinates(4) through metal- monosulfinates(3). |
This transformation took place more rapidly upon irradiation with white light62 or applied hydrogen peroxide as external oxidant. Interestingly although the group were able to synthesized the RuII-(bpy)2dithiolato L(S·S) [L =1,2-benzenedithiolate)], starting from the cis-isomer Ru(bpy)2Cl2 and benzene-1,2-dithiol in inert argon medium. But they were unsuccessful to purify or isolate the RuII-(bpy)2dithiolato L(S·S) compound, since it was prone to oxidation in the presence of air to the rare RuII-monosulfinates L(S·SO2).62
These Ru−S oxygenated complexes were characterised using NMR, mass spectrum, CV, UV-vis absorption spectra, infrared spectra and compared the results with the DFT calculation. The newly appeared dominant vibrations of S=O symmetric stretch at 989 cm-1 (cal. 983 cm-1); antisymmetric S=O stretch at 1119 cm-1 (cal.1105 cm-1) in the infrared spectra, which are in the expected ranges.63,64 Masitasand co-workers reported a unique “family” of S-oxygenated complexes by the restricted oxygenation of RuII-Ldithiolato (5)22 [L = bmmp-TASN] with distinct time frames by limiting the amount of dioxygen and the products, which varies on the extent of oxygenation of thiolato sulfur (Fig 6). It is important to note that with time they were succeeded to synthesize and isolate the mono-sulfinate [(bmmp-O2-TASN)-Ru(PPh3)](6)[~5min], oxygenates having both sulfenate/sulfinate functionality [(bmmp-O3-TASN)Ru(PPh3)] (7)11 [~15min to12h] with bis-sulfinate [(bmmp-O4-TASN)Ru(PPh3)] (8)[~120h] starting from RuII-dithiolato (5) and also the oxygenated products can be interchanged to one another with varying conditions.11,22 The S-oxygenetion process occurred is stepwise fashion depending on the limiting concentration of O2 and the other reaction conditions. Rapid oxygenation was noted down in Ru-Sthiolato (5) yielded the partial oxygenated Ru-Ssulfinato (6) [S·SO2] and the covalent nature enhanced for the sulfur- metal group due to the interactions of π-π* orbitals among t2g-rich Ru ions and the thiolate sulfur atoms, which promoted the said oxygenation.65
 |
Figure 6: Conversion of Ru-monosulfinates(6), Ru-bissulfinates(8) and Ru-sulfenate-sulfinates(7) from Ru-thiolate(5) |
Interestingly further oxygenation of (6) to metal-sulfenate and sulfinate (7) [SO·SO2] and metal-bissulfinate (8) [SO2·SO2] proceeds drastically more slowly than the previous steps, due to steric interactions between the presence of bulky coligands (PPh3)around the active site of oxygenation.11 The IR sulfinate stretching bands near 1139 and 1020 cm-1 for (6), 1137 and 1020 cm-1 for (7) and 1136, 1120, 1029, and 1015 cm-1 for (8) were confirmed by isotopic labelling studies11 and are ascribed to the O=S stretches of both symmetric and asymmetric nature for sulfinate functionality.
This study clearly indicate that the oxygen atom in Ru-sulfinate is coming from the aerial dioxygen and can be authenticated by the mass spectra where the m/z 731.1138 obtained as parent peak with 16O2 and with 18O2 , the signal is shifted near m/z 737.1267 for (7). Again Ru−Ssulfinato bond length in partially oxygenated compounds 6 and 7 were reported as 2.2473(6) and 2.2548(9) Å respectively, which are reduced than the corresponding Ru−Sthiolato (5) distance 2.3754(10) Å, found from X-ray crystallographic data and the ORTEP representation of the Ru-sulfenate-sulfinates (7) shown in Fig 7., for it’s superior crystal refinement parameters and the crystallographic information for other compounds in this series was reported by Masitas. Another example of partial oxidation of the Ru-dithiolato [(P-P)Ru(pyS)2], in which bis-diphenylphosphine derivative of ethylene (dppe) and butane (dppb) were designated.
 |
Figure 7: ORTEP representation of Ru-sulfenate-sulfinates (7), ellipsoids probability showing 40%. H-atoms are omitted to clearly. (Reproduced with permission from Reference No. 11. Copyright 2010 American Chemical Society.) |
as P-P; reported by Poelhsitz et. al., where mono-sulfinate compound was produced and another thiolato sulfur remain intact.66 An important dimeric chelate of RuIIthiolato, (9) [Ag{(bpy)2Ru(aet)}2]3+, (aet = 2-aminoethanthiolate; and bpy = 2,2′-bipyridine) where two thiolato-bridged of [(bpy)2Ru(aet)]+ fragments were linked with a Ag+ generating RuIIAgIRuII trinuclear complex was reported by Konno and co-worker,60 which can be converted to monomeric RuII-sulfinato [(bpy)2Ru(aesi-N,S)]+ (aesi = o-aminoethanesulfinate) (10) after Ag+ was eliminated as AgI by the application of NaI in air, shown in Fig 8. This is clearly indicates that in [(bpy)2Ru(aet)]+, such type of metal-thiolate functions is extremely nucleophilic and susceptible toward the attack of aerial molecular oxygen to be activated and transformed into a metal-sulfinate functions in which RuII –S coordination was retained. Similar type of optically active isomeric ∆ and Λ- monomeric RuII-sulfinate compounds [(bpy)2Ru(D-Hpsi-O,S)]PF6 (12) were produced from their corresponding isomeric ∆∆ and ΛΛ-dimeric Ru-thiolate-bridged attached with Ag+, containing a RuIIAgIRuII trinuclear motif. The mentioned complexes are the unique example of S^N donor linked to an aliphatic aminothiolate and aminosulfinate ligand to a bis(diimine)-type ruthenium(II) core.67
 |
Figure 8: Ru-thiolates [S and Ag bridged] 9 and 11 to Ru-sulfinates 10 and 12 formation by the removal of Ag+ (Reproduced with permission from Reference No. 60. Copyright 2011 American Chemical Society.) |
The IR stretching bands near 1110 cm-1 and 1010 cm-1 for (10), 1116 and 1010 cm-1 for (∆∆, 12a) and 1119 and 1011 cm-1 for (ΛΛ, 12b) are attributed to the O=S stretches of both symmetric and asymmetric nature for metal-sulfinate functionality. 11,33,61, 68 The electronic absorption spectrum is very informative for the generation of Ru-sulfinato is categorized by an higher energy intense band at 453 nm, generated from metal(Ru) → (bpy)ligand due to MLCT transition, compare to lower energy band at 501 nm for Ru-thiolato (9); this is due to the greater stabilisation of dπ orbitals of sulfinate group produced from corresponding thiolato function. Some bizarre photo-induced S-center oxygenation of metal-thiolato to the sulfonated compound is also present in the literature.34 Photooxidation reactions of sulfur compounds like thioanisoles, thiophene and many other diverse nonaromatic organosulfur compounds, were published,42,69 in which, most of the photooxidation involving 1∆O2 mediated by H2O2 70 or by irradiation of solution of organosulfur compounds saturated by air in presence of a sensitizer. 71 Toma and Hanan reported a photooxidation of a Ru-thiolate, [Ru(Hmctpy)(dmbpy)(κSSpyH)]2+ (13) (Hmctpy = carboxy-substituted terpyridine, dmbpy = methyl-substituted bipyridine) to the corresponding partial S-oxygenated compound, [Ru(mctpy)(dmbpy)(κS-SO2py)] (14) involving 1∆O2 under exposure of UV-vis light in acetonitrile.72 The emission spectra of sulfinate complex (14) in acetonitrile is shown in the Fig 9., exhibits a strong emission band near ~ 766 nm, the sample solution was excited at 424 nm; also a monoexponential nature of decay was observed having lifetime of 98 ns, where as the analogous thiolate compound (13) is nonemissive in nature. It was reported that the photochemical formation of sulfinate (14, Ru-SO2) from corresponding thiolate (13) involving oxygen in acetonitrile solvent.54 In this regard a very agreeable mechanism of self-sensitized photooxidation process was proposed, where the final product sulfinate is obtained starting from thiolato complex passing through terminal-peroxide (13a) or endo-peroxide (13b) intermediates as shown in the Fig 10.36,73
 |
Figure 9: Emission spectra of thiolate compound 13 (blue) and sulfinate compound 14 (green). |
From the UV−vis spectral analysis it was revealed that the photooxidation of metal-sulfides by singlet oxygen (1∆O2) to corresponding sulfinate rather than sulfenate, is facilitated in aprotic solvents like acetonitrile and DMF,72 but with protic solvent as water or alcohols, such type of S-oxygenation is inhibited due to hydrogen-bonding interactions with the intermediate (13a or 13b), also the life time of singlet oxygen (1∆O2) get reduced in presence of protic solvent.74 Interestingly the photooxidation of the thiolate group boost the previously mentioned back-bonding interactions in the strongly acceptor Ru-sulfinate complex, and hence the redox potentials of the RuIII/RuII couple increases from 1.23 to 1.62 V.
 |
Figure 10: Proposed mechanism of the formation of Ru-sulfinate (14) from Ru-thiolate (13). (Reproduced with permission from Reference No. 73. Copyright 2017 American Chemical Society.) |
Nature of M−S and M−SO2 bond
From the crystallographic analysis a few characteristic feature concerning the underlying chemistry of Ru−S bonding of both in thiolato and S-oxygenated species are revealed. The decrease in Ru−S bond distance in Ru-sulfinato than Ru-thiolato analogue are very significant and can be ascribed to the following effects: (a) reduction of electron–electron repulsion among the Ru-t2 and the nonbonding π-donor S3p orbital of thiolato function upon oxidation, (b) the slender radius of the oxidized sulfur (Ru−SO2) as compared to the thiolato sulfur (Ru−S) vis-à-vis increased ionic interaction between ruthenium and sulfinato-S, (c) Ru(t2) ® SO2(π*) back donation; where elimination of electrons are involved in the interaction of pπ-dπ (antibonding nature) orbitals between the S3p and Ru4d than Ru-Sthiolato.45 This same changes in M-L bond properties is conjugated with the incident that the sulfur oxygenation to Metal-thiolato center enhances the substrate/new precursor lability and also the hydrolytic behaviour at the NHase and SCNase active sites get increased.75
Bioactivity and Bio-Application of Ru-S-oxygenates
As mentioned earlier that a large number of the metal sulfur compounds are found to be biologically active and most of them show catalytic activity of many active enzymes. It is very interesting that the oxygenation of o-dithiolates bound to FeII, NiII, PdII, PtII, and RuII metal ions producing analogous mono-sulfinates having [S·SO2] and di-sulfinates having [SO2·SO2]donor groups 59,64,66 are well-known unlike the mono-sulfenates [S·SO] or di-sulfenates [SO·SO],76,77 in fact the mono-oxygenation of a metal-bound single thiolates to corresponding metal-sulfenate products are to some extent rare as well, compared to metal-sulfinate. However these compounds containing the M-S, M-SO (mono and di) and M-SO2(mono and di), are extremely much effective to adopt the abnormal active site in the metalloenzyme like nitrile hydratase,78 in addition to the study of their models for themetalloenzyme79 during last few decades. Although some popular biological ligand functionality like amide bound metal-sulfur oxygenated compounds are known to perform the enzymatic activity. NHase-inspired catalytic nitrile hydration of benzonitrile to analogous amide by a series of mixed biological N/S donor with non-biological RuII-sulfur compounds including dithiolato, asymmetric sulfinato-thiolato, sulfenate-sulfenic acid and sulfenato-sulfinato complexes were reported.21 Particularly, some bulky p-acid coligands having extensive p-current like PPh3 coordinated to the metal having higher lability due to sulfur center oxygenation and thus increasing the susceptibility for nitrile exchange80 and also it facilitated the catalytically progressed hydration process. Sriskandakumar et.al. investigated the S-centered chemistry and reactivity of RuII-thiolato, sulfenato and sulfinato compounds as well as the interaction with biomolecules like DNA for RuII-arene compounds.33 Activation of O2 molecule by RuII-arene thiolato and attached co-ligands alter the metal-sulfur
 |
Scheme 5: Mechanism of DNA binding of Ru-arene sulfenates. (Reproduced with permission from Reference No. 33. Copyright 2009 American Chemical Society.) |
bond lability as per their trans effect order and which persuade the H-bonding interaction with the polar biomolecules like DNA.81,82 Ru-incorporated sulfur centered oxygenation and its underlying mechanism as discuss all over the study are very much imperative in view for the design of new anticancer drugs.83
An appreciable biochemical case analysis suggest that such type of Ru(II) mediated ligand S- centered oxidation allowing the binding with DNA in more facile way (Scheme 5),mainly enhancing the lability of the sulfur group ligand by weakening the metal-sulfur bond. Whereas the S-oxygenation of RuII-arene thiolato family to corresponding sulfones of type [Ru(η6-ar)(SOnR)(en)]+ compounds (ar = arene or its derivatives, R= Ph/iPr, n = 1,2) is not amply biologically active and show just different result that shortening and strengthening of the bond (S−Ru) after oxygenation of the S-center of Ru-thiolato,84 due to effective interaction rather charge donation of filled SOπ* orbital to the empty Ru4d orbitals which is confirmed from crystallographic data. Also this electronic involvement concurrently strengthens the S−O bond33,84 owing to removal of electron density from SOπ* orbital, and this will supportive the H-bonding between Ru−SO with amine group of protein like DNA. Although this H-bond interaction is affected rather deteriorate in lower pH. In different proteins and enzymes such thiolate redox processes are occasionally controlled by metal involvement to the S atom.85 Almost no change in oxidation state of Ru upon ligand S-oxygenation is observed, which suggests that the metal center is not directly affected by such oxygenation. There are significant amount of interest among the researchers that the exploitation of biological fate of Ru-based metallodrugs.86-88 Recent research on the coordination polymers of RuII-organosulfur compounds having benzoate group combine with sulfinate functions; their synthesis utilising RuCl3·3H2O and dtdb [2,2′-dithiodibenzoic acid], and arrangements of atoms and groups inside the polymer and chemical behaviour were described.89 Such ruthenium sulfur compounds and their derivatives being the member of heavier platinum group metals, are found to be active component to show the in vitro cytotoxicity 90-92 and can become active precursor for the anticancer drug design93-95 in the field of biological applications because of these components are lesser toxic for remaining healthy part of the body.96,97
Conclusion
The current survey on the numerous transformations of a class of ruthenium mediated mainly organosulfur compounds to the corresponding oxygenates; and provides concentration on their chemical behavior and potential activities, and the findings are pointed out as follows. The thiolate sulfur atom interacted metal center like Ru is highly susceptible for S-centered oxidation. The oxygenation at the sulfur center is possible in the normal atmospheric condition either stirring or on reflux. It is noticeable that thiolate complexes of ruthenium undergo a diversity of reactions with molecular oxygen. Remarkably, this type of oxygenated product is not obtained if the reaction is carried out under strictly inert medium. It is believe that these S-oxygenation proceeds via intramolecular and/or intermolecular dioxygen addition pathway. Meticulous investigation on the interactions of Ru bound thiolates species with dioxygen mainly 1∆O2 generating S-oxygenates mostly metal-sulfinate products, where the functionality bound to the S atom especially hetero atoms plays a significant role for the above oxygenation. It is to be noted that for multiple thiolato-S centers a variety of sulfur oxygenates for example monosulfinate alone, bissulfinate alone and a mixture of mono and bis sulfinate products may be obtained, where as sulfenate compounds are rare. There come into sight to be numerous mechanistic paths for the oxygenation route of the nucleophilic sulfur center bound to Ru metal are significantly further complex and that would be unlike the oxygenation reaction of organic sulphides including the nature of the reactive intermediates and products. Some photooxygenation of Ru-mediated thiolato functionality to the corresponding s-oxygenates are also discussed. Most of the ruthenium sulfur compounds including the S-oxygenates were identified applying FTIR O=S stretching values and their structural behavior as the shortening of Ru−S on oxygenation were analyzed by X-ray crystallography. The ruthenium sulfur compounds together with the S- oxygenates show impressive bioactivity including enzymatic catalytic activity and interaction with the protein molecule like DNA are pointed out. The above information and findings will be extremely helpful for designing and planning for the synthesis of many analogous organosulfur systems of soft metals. Extensive research effort required for exploring novel methods and schemes of preparation of a new class of organosulfur and their oxygenated derivatives mediated by heavier transition metals and also to investigating their underlying properties and reactivity including bio-activity.
Acknowledgment
The author is grateful to the Sarsuna College, Kolkata for the infrastructural support. The author heartily acknowledges Smt. Sucheta Sarkar for her support and effort and some helpful discussions in this work. The contribution of Miss Titas Das in this work is cordially acknowledged. The author also thanks Miss Sucharita Sarkar for her valuable technical assistance in this work.
Conflict of interest
The author declares that there is no conflict of interest.
Funding Sources
There is no funding Source.
References
- Liu, T.; Li, B.; Singleton, M. L.; Hall, M. B.; Darensbourg, M. Y. J. Am. Chem. Soc., 2009, 131, 8296–8307.
CrossRef - Kovacs, J. A. Chem. Rev., 2004, 104, 825–848.
CrossRef - Mullins, C. S.; Grapperhaus, C. A.; Frye, B. C.; Wood, L. H.; Hay, A. J.; Buchanan R. M.; Mashuta, M. S. Inorg. Chem. 2009, 48, 9974–9976.
CrossRef - Yoshinari, N.; Konno, T. Chem. Eur. J., 2009, 15, 10021–10024.
CrossRef - Green, K. N.; Brothers, S. M.; Jenkins, R. M.; Carson, C. E.; Grapperhaus C. A.; Darensbourg, M. Y. Inorg. Chem., 2007, 46, 7536–7544.
CrossRef - Kinunda,G. A. Tanzania Journal of Science, 2018, 44, 45–63.
- Tang, H.; Brothers, E. N.; Grapperhaus C. A.; Hall, M. B. ACS Catal., 2020, 10, 3778−3789.
CrossRef - LoPachin R. M.; Gavin,T. Free Radic Res., 2016, 50, 195–205.
CrossRef - Gennari, M.; Duboc, C. Accounts of Chemical Research, 2020, 53, 2753–2761.
CrossRef - Yao, Q.; Wu, Z.; Liu, Z.; Lin, Y.; Yuan, X.; Xie, J. Chem. Sci., 2021, 12, 99–127.
CrossRef - Masitas, C. A.; Mashuta M. S.; Grapperhaus C. A. Inorg. Chem., 2010, 49, 5344–5346.
CrossRef - Lee, C.-M.; Hsieh, C.-H.; Dutta, A.; Lee, G.-H.; Liaw, W.-F. J. Am. Chem. Soc., 2003, 125, 11492–11493.
CrossRef - Grapperhaus C. A.; Mullins, C. S.; Kozlowski, P. M.; Mashuta, M. S. Inorg. Chem., 2004, 43, 2859–2866.
CrossRef - Cobley, J. N.; Husi, H. Antioxidants, 2020, 9, 315(1–25).
CrossRef - Arakawa, T.; Kawano, Y.; Kataoka, S.; Katayama, Y.; Kamiya, N.; Yohda, M.; Odaka, M. J. Mol. Biol., 2007, 366, 1497–1509.
CrossRef - Abou-Hussein, A. A.; Linert, W. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2014, 117, 763–771.
CrossRef - Paison, F., Su, B., Pan, D., Yan, T.; Wu, J. Austin Biochem. 2020,5,1025(1–12).
- Selvaganapathy, M.; Raman, N. J Chem Biol Ther, 2016, 1, 1–17.
- Jiang, Y.; Widger, L. R.; Kasper, G. D.; Siegler, M. A.; Goldberg, D. P. J. Am. Chem. Soc., 2010, 132, 12214–12215.
CrossRef - Dey, A.; Jeffrey, S. P.; Darensbourg, M. Y.; Hodgson, K. O.; Hedman B.; Solomon, E. I. Inorg. Chem., 2007, 46, 4989–4996.
CrossRef - Kumar, D.; Nguyen, T. N.; Grapperhaus C. A. Inorg. Chem., 2014, 53, 12372–12377.
CrossRef - Masitas, C. A.; Kumar, M.; Mashuta, M. S.; Kozlowski, P. M.; Grapperhaus, C. A. Inorg. Chem., 2010, 49, 10875–10881.
CrossRef - Tolman, W. B. J. Biol. Inorg. Chem., 2006, 11, 261–271.
CrossRef - Mirza, S. A.; Day, R. O.; Maroney, M. J. Inorg Chem., 1996, 35, 1992–1995.
CrossRef - Shin, W.; Lindahl, P. A. Biochim. Biophys. Acta, 1993,1161, 317–322.
CrossRef - Coremans, J. M. C. C.; Van der Zwaan, J. W.; Albracht, S. P. J. Biochim. Biophys. Acta, 1992, 1119, 157–168.
CrossRef - Charles, R. L.; Schroder, E.; May, G.; Free, P.; Gaffney, P. R. J.; Wait, R.; Begum, S.; Heads R. J.; Eaton, P. Mol. Cell. Proteomics, 2007, 6, 1473–1484.
CrossRef - Jacob, C.; Knight I.; Winyard, P. G. Biol. Chem., 2006, 387, 1385–1397.
CrossRef - McQuilken, A. C.; Goldberg, D. P. Dalton Trans. 2012, 41, 10883–10899.
CrossRef - Kumar, D. “Sulfur-oxidation enhances nitrile hydration in bioinspired ruthenium complexes: catalytic, kinetic, and DFT investigations.” Electronic Theses and Dissertations. Paper 2098. (2015).
- Amaro-Gahete, J.; Pavliuk, M. V.; Tian, H.; Esquivel, D.; Romero-Salguero, F. J.; Ott, S. Coordination Chemistry Reviews, 2021, 448, 214172 (1–34).
CrossRef - Tietze, D.; Sartorius, J.; Seth, B. K.; Herr, K.; Heimer, P.; Imhof, D.; Mollenhauer, D.; Buntkowsky, G. Scientific Reports, 2017, 7, 17194 (1–9).
CrossRef - Sriskandakumar, T.; Petzold, H.; Bruijnincx, P. C. A.; Habtemariam, A.; Sadler, P. J.; Kennepohl, P. J. Am. Chem. Soc. 2009, 131, 13355–13362.
CrossRef - Grapperhaus C. A.; Poturovic, S.; Mashuta, M. S. Inorg. Chem. 2005, 44, 8185–1887.
CrossRef - Monsour, C. G.; Decosto, C. M.; Tafolla-Aguirre, B. J.; Morales, L. A.; Selke, M. Photochemistry and Photobiology, 2021, 97, 1219–1240.
CrossRef - Zhang, D.; Bin, Y.; Tallorin, L.; Tse, F.; Hernandez, B.; Mathias, E. V.; Stewart, T.; Bau, R.; Selke, M. Inorg. Chem., 2013, 52, 1676–1678.
CrossRef - Chauvin, J.-P. R.; Pratt, D. A. Angew. Chem. Int. Ed., 2017, 56, 6255–6259.
CrossRef - van Bergen, L. A. H.; Roos, G.; De Proft, F. J. Phys. Chem. A, 2014, 118, 6078–6084.
CrossRef - Kassim, R.; Ramseyer, C.; Enescu, M. Inorg. Chem. 2011, 50, 5407–5416.
CrossRef - Song, X.; Fanelli, M. G.; Cook, J. M.; Bai, F.; Parish, C. A. J Phys Chem A., 2012, 116, 4934–4946.
CrossRef - Acharyya, R.; Dutta, S.; Basuli, F.; Peng, S.-M.; Lee, G.-H.; Falvello, L. R.; Bhattacharya, S. Inorg. Chem., 2006, 45, 1252–1259.
CrossRef - Mala, B.; Murtagh, L. E.; Farrow, C. M. A.; Akien, G. R.; Halcovich, N. R.; Allinson, S. L.; Platts, J. A.; Coogan, M. P. Inorg. Chem. 2021, 60(10), 7031–7043.
CrossRef - Römbke, P.; Schier, A.; Schmidbaur. H. Dalton Trans., 2001, 2482–2486.
CrossRef - Tamura, M.; Tsuge, K.; Igashira-Kamiyama, A.; Konno, T. Chem. Commun., 2011, 47, 12464–12466.
CrossRef - Das, U.; Ghorui, T.; Adhikari, B.; Roy, S.; Pramanik S.; Pramanik, K. Dalton Trans., 2015, 44, 8625–8639.
CrossRef - Moneo-Corcuera, A.; Pato-Doldan, B.; Sánchez-Molina, I.; Nieto-Castro, D.; Galán-Mascarós, J. R. Molecules, 2021, 26, 6020(1–13).
CrossRef - Kumar, M.; Colpas, G. J.; Day, R. O.; Maroney, M. J. J. Am. Chem. Soc., 1989, 111, 8323–8325.
CrossRef - Herting, D. L.; Sloan, C. P.; Cabral, A. W.; Krueger, J. H. Inorg. Chem. 1978, 17, 1649–1654.
CrossRef - Kumar, D.; Masitas, C. A.; Nguyen, T. N.; Grapperhaus C. A. Chem. Commun., 2013, 49, 294–296.
CrossRef - Grapperhaus C. A.; Maguire, M. J.; Tuntulani, T.; Darensbourg, M. Y. Inorg. Chem., 1997, 36, 1860–1866.
CrossRef - Farmer, P. J.; Solouki, T.; Soma, T.; Russell, D. H.; Darensbourg, M. Y. Inorg. Chem. 1993, 32, 4171–4172.
CrossRef - Zhang, D.; Hernandez, B.; Selke,M. J Sulphur Chem. 2008, 29, 377–388.
CrossRef - Tuntulani, T.; Musie, G.; Reibenspies, J. H.; Darensbourg, M. Y. Inorg. Chem. 1995, 34, 6279–6286.
CrossRef - Darensbourg, M. Y.; Farmer, P. J.; Soma, T.; Russell, D. H.; Solouki, T.; Reibenspies, J. H. “In The Activation of Dioxygen and Homogeneous Catalytic Oxidation; Barton,” D. H. R., Martell, A. E., Sawyer, D. T., Eds.; Plenum Press: New York, 1993: p 209.
CrossRef - Jensen, F. J. Org. Chem., 1992, 57, 6478–6487.
CrossRef - Selke, M.; Karney, W. L.; Khan S. I.; Foote, C. S. Inorg. Chem., 1995, 34, 5715–5720.
CrossRef - Pyykko, P. Chem. Rev., 1988, 88, 563–594.
CrossRef - Grapperhaus C. A.; Darensbourg, M. Y.; Sumner L. W.; Russell, D. H. J. Am. Chem. Soc., 1996, 118, 1791–1792.
CrossRef - Grapperhaus C. A.; Darensbourg, M. Y. Acc. Chem. Res., 1998, 31, 451–459.
CrossRef - Tamura, M.; Tsuge, K.; Igashira-Kamiyama A.; Konno, T. Inorg. Chem., 2011, 50, 4764–4771.
CrossRef - Begum, R. A.; Farah, A. A.; Hunter, H. N.; Lever, A. B. P. Inorg. Chem., 2009, 48, 2018– 2027.
CrossRef - Connick, W. B.; Gray, H. B. J. Am. Chem. Soc., 1997, 119, 11620–11627.
CrossRef - Keating, C. S.; McClure, B. A.; Rack, J. J.; Rubtsov, I. V. J. Chem. Phys., 2010, 133, 144513 (1–14).
CrossRef - Lauter, M.; Breitinger, D. K.; Breiter, R.; Mink, J.; Bencze. E. J. Mol. Struct., 2001, 563-564, 383–388.
CrossRef - O’Toole, M. G.; Kreso, M.; Kozlowski, P. M.; Mashuta, M. S.; Grapperhaus C. A. J. Biol. Inorg. Chem. 2008, 13, 1219–1230.
CrossRef - Von Poelhsitz, G.; Rodrigues, B. L.; Batista, A. A. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2006, C62, m424–m427.
CrossRef - Tamura, M.; Matsuura, N.; Kawamoto, T.; Konno, T. Inorg. Chem., 2007, 46, 6834–6836.
CrossRef - Sellmann, D.; Hein, K.; Heineman, F. W. Eur. J. Inorg. Chem., 2004, 2004, 3136–3146.
CrossRef - Bonesi, S. M.; Fagnoni, M.; Albini, A. J. Org. Chem., 2004, 69, 928–935.
CrossRef - Ciclosi, M.; Dinoi, C.; Gonsalvi, L.; Peruzzini, M.; Manoury, E.; Poli, R. Organometallics, 2008, 27, 2281–2286.
CrossRef - Bonesi, S. M.; Crespi, S.; Merli, D.; Manet, I.; Albini, A. J. Org. Chem., 2017, 82, 9054–9065.
CrossRef - Rosana, M.; da Silva, E.; Auvray, T.; Laramée-Milette, B.; Franco, M. P.; Braga, A. A. C.; Toma, H. E.; Hanan, G. S. Inorg. Chem., 2018, 57, 4898–4905.
CrossRef - Selke, M. “Reactions of Metal Complexes with Singlet Oxygen. In PATAI’S Chemistry of Functional Groups”; John Wiley & Sons, Ltd.: Chichester, U.K., (2014); pp 1−50.
CrossRef - Bregnhøj, M.; Westberg, M.; Jensen, F.; Ogilby, P. R. Phys. Chem. Chem. Phys., 2016, 18, 22946–22961.
CrossRef - Lugo-Mas, P.; Dey, A.; Xu, L.; Davin, S. D.; Benedict, J.; Kaminsky, W.; Hodgson, K. O.; Hedman, B.; Solomon, E. I.; Kovacs, J. A. J. Am. Chem. Soc., 2006, 128, 11211–12221 .
CrossRef - Buonomo, R. M.; Font, I.; Maguire, M. J.; Reibenspies, J. H.; Tuntulani, T.; Darensbourg, M. Y. J. Am. Chem. Soc. 1995, 117, 963–973.
CrossRef - Schrauzer, G. N.; Zhang, C.; Chadha, R. Inorg. Chem. 1990, 29, 4104–4107.
CrossRef - Takuma, Y.; Yuko, W.-T.; Yuji, K.; Tomonori, S.; Yasuhiro, F.; Tomohiro, M.; Hideki, O. Chemistry Letters, 2008, 37, 66–67.
- Heinrich, L.; Li, Y.; Vaissermann, J.; Chottard, J.-C. Eur. J. Inorg. Chem., 2001, 2001, 1407–1409.
CrossRef - Petzold, H.; Sadler, P. J. Chem. Commun. 2008, 37, 4413–4415.
CrossRef - Petzold, H.; Xu, J.; Sadler, P. J. Angew.Chem., Int. Ed., 2008, 47, 3008–3011.
CrossRef - Wang, F. Y.; Weidt, S.; Xu, J. J.; Mackay, C. L.; Langridge-Smith, P. R. R.; Sadler, P. J. J. Am. Soc. Mass Spectrom., 2008, 19, 544–549.
CrossRef - K.; Goto, M.; Holler, R. Okazaki, J. Am. Chem. Soc., 1997, 119, 1460–1461.
CrossRef - Maret, W. Antioxid. Redox Signaling, 2006, 8, 1419–1441.
CrossRef - Ascone, I.; Messori, L.; Casini, A.; Gabbiani, C.; Balerna, A.; Dell’Unto, F.; Castellano, A. C. Inorg. Chem. 2008, 47, 8629–8632.
CrossRef - Harris, T. V.; Szilagyi, R. K.; McFarlane Holman. K. L. J. Biol. Inorg. Chem. 2009, 14, 891–898.
CrossRef - Mjos, K. D.; Orvig, C. Chem. Rev., 2014, 114, 4540–4563.
CrossRef - Moosun, S. B.; Jhaumeer-Laulloo, S.; Coles, S. J.; Blair, L. H.; Hosten, E. C.; Bhowon, M. G. Inorganic Chemistry Communications, 2015, 62, 71–76.
CrossRef - Ribeiro, G. H.; Guedes, A. P. M.; de Oliveira, T. D.; de Correia, C. R. S. T.; Colina-Vegas, L.; Lima, M. A.; Nóbrega, J. A.; Cominetti, M. R.; Rocha, F. V.; Ferreira, A. G.; Castellano, E. E.; Teixeira, F. R.; Batista, A. A. Inorg. Chem. 2020, 59, 15004–15018.
CrossRef - Xu, L.; Zhong, N.-J.; Xie, Y.-Y.; Huang, H.-L.; Jiang, G.-B.; Liu, Y.-J. PLoS ONE, 2014, 9, e96082 (1–8).
CrossRef - Levina, A.; Mitra, A.; Lay, P. A. Metallomics, 2009, 1, 458–470.
CrossRef - Kanaoujiya, R.; Singh, M.; Singh, J.; Srivastava, S. Journal of Scientific Research, 2020, 64, 364–368.
CrossRef - Adeniyi, A. A.; Ajibade, P. A. Rev Inorg Chem, 2015, 36, 1–23.
CrossRef - Bruijnincx, P. CA.; Sadler, P. J. Curr. Opin. Chem. Biol., 2008, 12, 197–206.
CrossRef - Antonarakis, E. S.; Emadi, A. Cancer Chemother Pharmacol, 2010, 66, 1–9.
CrossRef - Egorova, K. S.; Ananikov, V. P. Organometallics, 2017, 36, 4071−4090.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










