Facially Selective Oxo-Diels-Alder Cycloadditions of α-Dienyl-β-Lactam: An Entry to Pyrano Tethered β-Lactams Bifunctional Hybrids
Department of Chemical Sciences, I. K. Gujral Punjab Technical University, Kapurthala, Punjab 144 603, India.
Corresponding Author E-mail: rupesh.manak@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380334
Article Received on : 04 May 2022
Article Accepted on : 21 Jun 2022
Article Published : 27 Jun 2022
Reviewed by: Dr. Rama Sharma
Second Review by: Dr. Murtuja Ali
Final Approval by: Dr. Shamo Tapdiqov
The functionalization of -lactams at C-3 position are useful for the strategic improvement in both the dimensions, namely synthetic utility, as versatile intermediate in organic synthesis and biological potential of these heterocyclic systems. The present manuscript involved the -facial selective synthesis -lactam hybrids employing highly regioselective and diastereoselective oxo-Diels–Alder reactions of diethyl ketomalonate with -dienyl--lactam with stereocentres at its - and - positions. This protocol provided the cycloaddition of - and - stereocentric diene with symmetrical heterodienophiles forming biologically potent regioselective and diastereoselective -lactams substituted pyrano bifunctional hybrids in good yields and -facially selectivity.
KEYWORDS:Diastereoselective synthesis; β-lactams; Dienyl-β-lactams; π-facial selective synthesis; Regioselective synthesis
Download this article as:| Copy the following to cite this article: Mann M. K, Kumar R, Bhargava G. Facially Selective Oxo-Diels-Alder Cycloadditions of α-Dienyl-β-Lactam: An Entry to Pyrano Tethered β-Lactams Bifunctional Hybrids. Orient J Chem 2022;38(3). |
| Copy the following to cite this URL: Mann M. K, Kumar R, Bhargava G. Facially Selective Oxo-Diels-Alder Cycloadditions of α-Dienyl-β-Lactam: An Entry to Pyrano Tethered β-Lactams Bifunctional Hybrids. Orient J Chem 2022;38(3). Available from: https://bit.ly/3bwUROr |
Introduction
The hetero-Diels–Alder (HDA) reactions1-5 are significant tools in establishing 6-membered heterocyclic scaffolds having immense biological relevance. A variety of hetero-Diels-Alder reactions ensured an opening for the development of diverse heterocyclic systems. HDA reactions drew a lot of attention because of their extensive industrial and other important applications.6-8 Different variants of HDA reactions have been explored for a highly efficient stereoselective9-13 synthesis of six-membered ring compounds. Of these, oxo-Diels-Alder (ODA)14-18 variant has considerable potential because of the tactical formation of a variety of six membered derivatives such as dihydropyrans, dihydropyrones etc.
On the other hand, the b–lactams C-3 functionalization19 has continual significant concern of chemists because of its use as important core in the fabrication of organic compounds and their therapeutic biological uses. These 3-substituted prototypes are an important building blocks for development of conformationally constrained and medicinally potent products or for library generation of highly functionalized b-lactams. These C-3 functionalized lactam can efficiently prepared by employing variety of synthetic transformations at its C-3 position.
On the other hand, Diels Alder cycloaddition of functionalized dienes having stereocenter at its α-position have earlier been explored for the preparation of number of functionalized carbocyclic / heterocyclic compounds using variety of functionalized dienophiles.12, 13, 20-24 However, the Diels-Alder cycloadditions of dienes involving stereocenters at its α-and β- positions are still scare in literature. Earlier, diastereoelective Diels Alder cycloadditions of such functionalized dienes like 3-butadienyl-b-lactam are explored for the preparation of number of heterocycles.25 However, the concerned reports on the HDA reactions of 3-butadienyl-b-lactam with hetero-dienophiles are still need to be explored. The cycloadditions of 3-dienyl-2-azetidinones are an important in term of recent usefulness of 1, 3, 4-tri-substituted-b-lactams for various biological activities.26-31
We report herein an earlier unexplored, useful uncatalyzed strategy for the synthesis of functionalized b-lactam pyrano bifunctional hybrids bearing different substituents at 1, 3 & 4-positions of lactam ring (Scheme 1). The methodology involved the synthesis of 3-butadienyl- b-lactams and their previously unexplored regio-, diastereo- and p-facially selective oxo-Diels–Alder reactions with symmetrical oxo-dienophile such as diethyl ketomalonate in absence of any catalyst to afford b -lactam pyrano bifunctional hybrids in excellent yields. 32
Results and discussion
The initial trans and cis-3-butadienyl-2-azetidinones 1a-c & 4a-b needed in this work were obtained through the reaction of in situ formed butadienylketene which is obtained in dry chloromethane from sorbyl chloride and triethylamine with N-aryl and N-aliphatic imines respectively.
These 3-butadienyl-2-azetidinones 1a-c & 4a-b were investigated for oxo-DA cycloaddition reactions with symmetrical oxo-dienophile viz. diethyl ketomalonate 2. Our studies were initiated with the treatment of 3-butadienyl-2-azetidinones 1a and symmetrical diethylketomalonate 2 using different set of reaction conditions to get these conditions optimized for cycloadducts (Table 1).
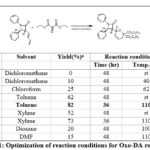 |
Table 1: Optimization of reaction conditions for Oxo-DA reaction. |
The oxo-DA cycloaddition delivered the diastereo- and p-facially selective 2-azetidinone substituted pyrano-lactam bifunctional hybrid in good yields. Very low yields were detected in the reactions of 1 with 2 in solvent such as dichloromethane and chloroform even upon refluxing (Table 1, entries 1-3). Gratifyingly, toluene was found to be the appropriate solvent to give the optomized yield of diastereoselective and regioselective cycloadduct 3 at refluxing temperature (110oC, Table 1, entries 4-5) in comparison to the other solvents with the target product in 82% yield. Xylene also provided the same product in 73% yield (110oC; time 36 h; Table 1, entries 6-7). The oxo-DA cycloaddition of 1 and 2 in dioxane and DMF also proved ineffective and afforded poor yields (Table 1, entries 8-9).
Treatment of 3-butadienyl-2-azetidinones 1a & 1b and 4 with symmetrical oxo-dienophiles 2 without using any Lewis acid give rise to the selective construction of exo- adducts 3a & 3b and 5 respectively in good yields (Table 2). Diastereomerically pure, functionalized 6-(2-oxo-1,4-diphenyl-azetidin-3-yl)-3,6-dihydro-pyran-2,2-dicarboxylic acid diethyl ester 3 thus achieved were characterized based on analytical data and spectroscopic studies.
 |
Table 2: Oxo-DA reactions of 3-butadienyl-2-azetidinones. |
Treatment of 1a & 1b with diethylketomalonate 2 produced very good yield of exo adducts 3a & 3b (77-84%, Table 2). The desirable output with respect to yields with good selectivities is obtained in refluxing toluene at 110oC. However, this synthesis was also studied in xylene under similar conditions but poor yield was obtained (Table 2).
 |
Figure 1: b-lactam hybrids 3a and 5. |
The seterochemistry of the cycloadducts were different from the adducts obtained via Lewis acid catalysed cycloadditions at low temperature as reported earlier.25
The compound, 3a (C26H27O6N) was characterized by mass spectrometry that showed a molecular ion peak m/z at 449. A sharp absorption peak at 1727cm-1 is observed in its IR spectrum because of the presence of carbonyl group of β-lactam ring. 1H NMR (400 MHz) spectrum characterization represented a distinctive multiplet at δ 5.04 corresponding is shown due to the presence of H4 of the β-lactam ring. Further, two doublets at d 2.90 (J = 17.44 Hz) and d 2.72 (J = 17.32 Hz) corresponding to 8a & 8b respectively, a doublet at δ 4.96 due to H5 (J = 2.44 Hz), two multiplets at δ 3.55 and δ 6.07 are also shown due to H3 and H6 respectively in the 1H NMR spectrum of 3a. Three carbonyl carbons at δ 164.2, 167.8 and 168.4 ppm have been observed in the 13C NMR spectrum of 3a.
We further, explored the synthesis of trans 3-butadienyl-2-azetidinones 4 using heterodienophile 2. Diastereoselective, regioselective and π-facially selective pure functionalized exo 6-(1-cyclohexyl-2-oxo-4-phenyl-azetidin-3-yl)-3,6-dihydro-pyran-2,2-dicarboxylic acid diethyl ester 5 is yielded in the reaction. The reaction between 4 and diethylketomalonate 2 gave endo adduct in very good yields (81-83%). Reactions in refluxing toluene (1100C) by employing diethyl ketomalonate 2 as a heterodienophile provided the better yields of products as compared to the xylene (69-72%, Table 2).
The compound, 5 (Figure 1) upon mass spectrometric characterization indicated a molecular ion peak at m/z 455. IR spectrophotometric analysis presented a peak at 1727 cm-1, because of C=O group of β-lactam ring. Further, the 1H NMR presented a characteristic multiplet at δ 3.33 corresponding to CH of cyclohexyl ring, doublet at δ 4.88 pertaining to β-lactam ring H4 (J = 5.4 Hz), a ddd at d 2.57 (J = 2.2, 4.96 & 17.1 Hz) corresponding to 8a and a dd at d 2.28 (J = 2.72 & 17.24 Hz) corresponding to 8b, dd at δ 3.45 is assigned to H3 proton (J= 5.4, 10.2 Hz). The presence of 10.2 Hz coupling between H3 and H5 confirm the cis stereochemistry. The 13C NMR of 5 also gave the presence of three carbons of C=O group at δ 166.8, 167.9 and 168.0 ppm pertaining to the carbonyl of b-lactam and esters respectively
In accordance with expectations, the DA synthesis of cis-/trans-3-dienyl-azetidin-2-ones 1a & 1b and 4 with oxo-dienophiles 2 led to exo adducts exclusively. The presence of α-and β-stereocentres at the vicinity of the diene of 3-dienyl-b-lactams creates both facial sides of dienyl component distinguishable. Two possible exo adducts are expected, varying in stereo-relationship among the stereo-centres on the lactam and on the cyclohexyl scaffolds.
 |
Figure 2: Plausible ways of approaching the oxo-dienophiles to 3-butadienyl- azetidin-2-ones. |
Due to the endo addition of dienophiles to lower facial side of butadiene, 3a & 3b adducts are formed. Steric hindrance between the approaching dienophiles and substitution at C-4 of lactam ring, upper facial attack of dienophiles is excluded.
Conclusion
In conclusion, oxo-DA cycloadditions of α-and β-stereocentric diene with symmetrical heterodienophiles have been explored for the fabrication of biologically potent 2-azetidinones functionalized pyrano hybrids with diastereoselectivity and π-facially selectivity. The reported protocol is a significant direct approach for the regio-controlled synthesis of diastereo- and facially selective functionalized lactams.
Experimental Section
General information
Anhydrous solvents were obtained from Sigma Aldrich. Thin layer chromatographic technique (TLC) is performed on procured silica plates from Merck (0.2mm F254 Kieselgel). Visualisation of compounds is carried out under UV light. Bruker 400MHz spectrometer and 75MHz were utilized to record 1H NMR spectra and 13C NMR spectra respectively. Chemical shifts (δ) are quoted in ppm (parts per million) in reference to the internal solvent (d-CHCl3 δ=7.26 for 1H and δ=77.2 for 13C NMR. Coupling constants (J) are presented in Hz and chemical shifts values are shown in δ (ppm) values. Characterization data is described as followed: chemical shift, multiplicity (singlet-s, broad singlet-br s, doublet-d, triplet-t, double of doublet-dd, double of triplet-dt, multiplet-m), coupling constant (Hz) and integration. Brucker-micrOTOF-Q II mass spectrometer was utilized to get the high resolution mass spectra. Melting points determined are uncorrected and recorded using open capillary method on Digital Melting Point Apparatus. Perkin Elmer-Spectrum II spectrophotometer was used for recording IR spectra.
General synthetic Procedure for the formation of 3 and 5
Diethyl ketomalonate (2) was added to a well-stirred solution of cis/trans-3-butadienylazetidin-2-one 1a & 1b and 4 (1eq.) in toluene (5 ml) at room temperature. The reaction was allowed to reflux for 24h. The monitoring of the progress was done using TLC considering 3-butadienylazetidin-2-one as a limiting reactant. After the reaction gets completed, removal of the solvent was achieved under reduced pressure. The purification of the initially obtained product was done through column chromatography employing a mixture of hexane-EtOAc (80:20) as an eluent. The recrystallization of the products was performed with a mixture of diethyl ether and hexane which yielded pure products 3a, 3b and 5 (Table 1 & 2).
6-(2-Oxo-1,4-diphenyl-azetidin-3-yl]-3,6-dihydro-pyran-2,2-dicarboxylic acid diethyl ester (3a):
Solid; Pale yellow; mp 103-105oC. 1H-NMR (d-CHCl3, 400MHz): δH=7.29 (m, 10 Aromatic H), 6.07 (m, 1 H, H6), 5.76 (d(t), J = 10.48, 1 H, H7), 5.04 (m, 1 H, H4), 4.96 (d, J = 2.44 Hz, 1 H, H5), 4.22 (m, 4 H, OCH2CH3), 3.55 (m, 1 H, H3), 2.90 (d, J = 17.44Hz, 1 H, H8a), 2.72 (d, J = 17.32Hz, 1 H, H8b), 1.27 (t, 3 H, OCH2CH3), 1.19 (t, 3 H, OCH2CH3) ppm. 13C NMR (d-CHCl3, 75MHz): δ=13.9 (OCH2CH3), 14.2 (OCH2CH3), 29.3 (C8), 56.3 (C3), 62.3 (OCH2CH3), 62.4 (OCH2CH3), 62.9 (C4), 70.1 (C5), 80.2 (C9), 117.2, 124.0, 124.7, 126.0, 128.2, 129.0, 129.1, 137.5, 137.7, 164.2 (N-CO-CH2), 167.8 (COOCH2CH3), 168.4. (COOCH2CH3) ppm. MS: m/z = 449 [M+].
6-(2-Oxo-4-phenyl-1-p-tolyl-azetidin-3-yl)-3,6-dihydro-pyran-2,2-dicarboxylic acid diethyl ester (3b):
Solid; Pale yellow; mp 104-106oC. 1H-NMR (d-CHCl3, 400MHz): δH=7.19 (m, 9 ArH), 6.06 (m, 1 H, H6), 5.76 (d(t), J = 10.48 Hz, 1 H, H7), 5.03 (m, 1 H, H4), 4.92 (d, J = 2.4 Hz, 1 H, H5), 4.22 (m, 4 H, OCH2CH3), 3.53 (m, 1 H, H3), 2.89 (d, J = 17.28 Hz, 1 H, H8a), 2.71 (d, J = 16.16 Hz, 1 H, H8b), 2.25 (s, 3H, Ph-CH3), 1.27 (t, 3 H, OCH2CH3), 1.20 (t, 3 H, OCH2CH3) ppm. 13C NMR (d-CHCl3, 75MHz): δ= 13.9 (OCH2CH3), 14.2 (OCH2CH3), 20.9 (Ph-CH3), 29.3 (C8), 56.3 (C3), 62.3 (OCH2CH3), 62.4 (OCH2CH3), 62.8 (C4), 70.1 (C5), 80.3 (C9), 117.2, 124.3, 125.6, 126.1, 128.1, 128.9, 129.6, 133.7, 135.1, 137.8, 163.9 (N-CO-CH2), 167.8 (COOCH2CH3), 168.4 (COOCH2CH3). ppm. MS: m/z = 463 [M+].
6-(1-Cyclohexyl-2-oxo-4-phenyl-azetidin-3-yl]-3,6-dihydro-pyran-2,2-dicarboxylic acid diethyl ester (5):
Solid; Pale yellow; mp 101-103oC.1H-NMR (d-CHCl3, 400MHz): δH=7.32 (m, 5 ArH), 5.95 (d, J = 10.5 Hz, 1 H, H6), 5.76 (unresolved dddd, 3.5, 5.7, 10.4 Hz, 1 H, H7), 4.88 (d, J = 5.4 Hz, 1 H, H4), 4.23 (m, 1 H, H5), 4.13 (q, 2 H, OCH2CH3), 3.97 (dd, J = 7.1, 10.6 Hz, 1 H of OCH2CH3), 3.77 (dd, J = 7.1, 10.6 Hz, 1 H of OCH2CH3), 3.45 (dd, J = 5.4, 10.2 Hz, 1 H, H3), 3.33 (m, 1 H, Cyclohexyl-CH), 2.57 (dddd, J = 2.2, 4.96, 17.1 Hz, 1 H, H8a), 2.28 (dd, J = 2.72, 17.24 Hz, 1 H, H8b), 2.05-1.25 (m, 8 H, Cyclohexyl-CHH), 1.20 (t, 3H, OCH2CH3) 1.16-1.04 (m, 2 H, cyclohexyl-CHH), 1.00 (t, 3 H, OCH2CH3), ppm. 13C NMR (d-CHCl3, 75MHz): δ=14.0 (OCH2CH3), 14.1 (OCH2CH3), 25.0, 25.3, 29.0 (C8), 30.6, 31.4, 53.4, 57.1 (C3), 59.0, 61.5 (OCH2CH3), 61.8 (OCH2CH3), 63.2 (C4), 68.2 (C5), 79.1 (C9), 122.9, 126.6, 127.8, 128.0, 136.3, 166.8 (N-CO-CH2), 167.9 (COOCH2CH3), 168.0 (COOCH2CH3) ppm. MS: m/z = 455 [M+].
Acknowledgement
The authors are highly grateful to the IK Gujral Punjab Technical University, Kapurthala, Punjab to provide all the necessary research amenities to carry out this work.
Conflict of Interests
There are no competing interests to declare.
Funding Sources
There is no funding source.
References
- Reymond, S.; Cossy, J., Copper-Catalyzed Diels− Alder Reactions. Chem. Rev. 2008,108 (12), 5359-5406.
CrossRef - Pellissier, H., Asymmetric hetero-Diels-Alder reactions of carbonyl compounds. Tetrahedron (Oxford. Print) 2009,65 (15).
CrossRef - Moyano, A.; Rios, R., Asymmetric organocatalytic cyclization and cycloaddition reactions. Chem. Rev. 2011,111 (8), 4703-4832.
CrossRef - Pellissier, H., Asymmetric organocatalytic cycloadditions. Tetrahedron (Oxford. Print) 2012,68 (10).
CrossRef - Masson, G.; Lalli, C.; Benohoud, M.; Dagousset, G., Catalytic enantioselective [4+ 2]-cycloaddition: a strategy to access aza-hexacycles. Chem. Soc. Rev. 2013,42 (3), 902-923.
CrossRef - Bodnar, B. S.; Miller, M. J., The Nitrosocarbonyl Hetero‐Diels–Alder Reaction as a Useful Tool for Organic Syntheses. Angew. Chem. Int. Ed. 2011,50 (25), 5630-5647.
CrossRef - Funel, J. A.; Abele, S., Industrial applications of the Diels–Alder reaction. Angew. Chem. Int. Ed. 2013,52 (14), 3822-3863.
CrossRef - Gregoritza, M.; Brandl, F. P., The Diels–Alder reaction: a powerful tool for the design of drug delivery systems and biomaterials. European journal of pharmaceutics and biopharmaceutics 2015,97, 438-453.
CrossRef - Kagan, H. B.; Riant, O., Catalytic asymmetric diels alder reactions. Chem. Rev. 1992,92 (5), 1007-1019.
CrossRef - Johnson, J. S.; Evans, D. A., Chiral bis (oxazoline) copper (II) complexes: versatile catalysts for enantioselective cycloaddition, aldol, Michael, and carbonyl ene reactions. Acc. Chem. Res. 2000,33 (6), 325-335.
CrossRef - Jørgensen, K. A., Catalytic asymmetric hetero‐Diels–Alder reactions of carbonyl compounds and imines. Angew. Chem. Int. Ed. 2000,39 (20), 3558-3588.
CrossRef - Bonjouklian, R.; Ruden, R. A., Versatile allene and carbon dioxide equivalents for the Diels-Alder reaction. The Journal of Organic Chemistry 1977,42 (25), 4095-4103.
CrossRef - kal Koshvandi, A. T.; Heravi, M. M., Applications of Danishefsky’s dienes in asymmetric Oxo-Diels-Alder reactions. Tetrahedron: Asymmetry 2017,28 (11), 1506-1556.
CrossRef - Dai, Q. S.; Zhang, X.; Yang, K. C.; Li, M. H.; Yang, J.; Li, Q. Z.; Feng, X.; Han, B.; Li, J. L., Chiral Amine‐Catalyzed Stereoselective [4+ 2] Annulations of Alkenyl Thiazolones and Aliphatic Aldehydes via a Step‐Wise Mechanism. Adv. Synth. Catal. 2018,360 (22), 4435-4440.
CrossRef - Yeung, C.-T.; Chan, W. T. K.; Lo, W.-S.; Law, G.-L.; Wong, W.-T., Catalytic asymmetric oxo-Diels–Alder reactions with chiral atropisomeric biphenyl diols. Beilstein journal of organic chemistry 2019,15 (1), 955-962.
CrossRef - Nagpal, R.; Bhalla, J.; Bari, S. S., A Comprehensive Review on C-3 Functionalization of β-Lactams. Current Organic Synthesis 2019,16 (1), 3-16.
CrossRef - Angell, E. C.; Fringuelli, F.; Pizzo, F.; Porter, B.; Taticchi, A.; Wenkert, E., Diels-Alder reactions of cycloalkenones. 9. Diastereofacial selectivity of mono-and dialkylated 2-cyclohexenones. The Journal of Organic Chemistry 1986,51 (14), 2642-2649.
CrossRef - Brown, F. K.; Houk, K.; Burnell, D. J.; Valenta, Z., Modeling of steric control of facial stereoselectivity. Diels-Alder cycloadditions of unsymmetrically substituted cyclopentadienes. The Journal of Organic Chemistry 1987,52 (14), 3050-3059.
CrossRef - Kahn, S.; Hehre, W., Modeling chemical reactivity. 5. Facial selectivity in Diels-Alder cycloadditions. Journal of the American Chemical Society 1987,109 (3), 663-666.
CrossRef - Macaulay, J. B.; Fallis, A. G., Heteroatom-directed. pi.-facial diastereoselection in Diels-Alder cycloadditions of plane-nonsymmetric cyclopentadienes. Journal of the American Chemical Society 1990,112 (3), 1136-1144.
CrossRef - Carruthers, W., Cycloaddition reactions in organic synthesis. Elsevier: 2013.
- Bhargava, G.; Mahajan, M. P.; Saito, T.; Otani, T.; Kurashima, M.; Sakai, K., Highly Diasteroselective and Remarkably π‐Facially Selective Lewis Acid‐Catalysed Diels–Alder Cycloaddition Reactions: Access to Novel 1, 3, 4‐Trisubstituted 2‐Azetidinones. Eur. J. Org. Chem. 2005,2005 (11), 2397-2405.
CrossRef - Adonias, M.; Anayaba, J.; Cámara, J.; Canet, E.; Gateau-Olesker, A.; Gero, S.; Grande, M.; Hernando, J., Enantioselective synthesis and antielastase activity of 1, 3, 4-trisubstituted and 3, 4-disubstituted β-lactam antibiotics. Bioorganic & Medicinal Chemistry Letters 1993,3 (12), 2547-2552.
CrossRef - Edwards, P. D.; Bernstein, P. R., Synthetic inhibitors of elastase. Medicinal research reviews 1994,14 (2), 127.
CrossRef - Mascaretti, O.; Boschetti, C.; Danelon, G.; Mata, E.; Roveri, O., β-Lactam compounds. Inhibitors of transpeptidases, β-lactamases and elastases: a review. Curr. Med. Chem. 1995,1 (6), 441-470.
CrossRef - Vaccaro, W. D.; Sher, R.; Davis Jr, H. R., Sugar-substituted 2-azetidinones as cholesterol absorption inhibitors. Bioorganic & medicinal chemistry letters 1998,8 (1), 35-40.
CrossRef - Kayarmar, R.; Nagaraja, G.; Naik, P.; Manjunatha, H.; Revanasiddappa, B.; Arulmoli, T., Synthesis and characterization of novel imidazoquinoline based 2-azetidinones as potent antimicrobial and anticancer agents. Journal of Saudi Chemical Society 2017,21, S434-S444.
CrossRef - Panditrao, K. S., Synthesis and Biological evaluation of some novel derivatives of 2 Azetidinone. 2018.
- Kumar, Y.; Singh, P.; Bhargava, G., Diastereo-and Facially Selective Imino-Diels–Alder Cycloaddition of 2-Azeditinone-Tethered 1-Azadiene: Synthesis of Functionalized (2-Oxo-4-styrylazetidin-3-yl)–Pyridine Hybrids. Synlett 2015,26 (03), 363-366.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










