Electrochemical Performance of Carbon Materials
P. Nandakumar1* , K. N. Amba Sankar1
, K. N. Amba Sankar1 , A. Shankar Ganesh1
, A. Shankar Ganesh1 , BA. Anandh1
, BA. Anandh1 and R. Deepa2
and R. Deepa2
1Department of Electronics, PSG College of Arts and Science, Coimbatore, India.
2Department of Computer Science, PSG College of Arts and Science, Coimbatore, India.
Corresponding Author E-mail: nandacjb@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380308
Article Received on : 04 May 2022
Article Accepted on : 08 Jun 2022
Article Published : 28 Jun 2022
Reviewed by: Dr. Binoj Kunjukunju
Second Review by: Dr. A Ayeshamariam
Final Approval by: Dr.Suja Mathai
The surface modification on electrode materials generally improves the electron mobility and surface interactions at carbon materials. Exfoliate graphite has been prepared by the ball milling technique with three different milling time. The graphene oxide, reduced graphene oxide were prepared modified Hummers method and carbon quantum dots was prepared using chemical synthesis-pyrolysis technique. The synthesized materials were characterized by X-ray diffraction (XRD), Transmission electron microscopy (TEM), and investigated the electrochemical performances of Cyclic Voltammetry (CV) analysis to understand their specific capacitance.
KEYWORDS:Ball Milled graphite; CQD; Electrochemical Properties; RGO
Download this article as:| Copy the following to cite this article: Nandakumar P, Sankar K. N. A, Ganesh A. S, Anandh B. A, Deepa R. Electrochemical Performance of Carbon Materials. Orient J Chem 2022;38(3). |
| Copy the following to cite this URL: Nandakumar P, Sankar K. N. A, Ganesh A. S, Anandh B. A, Deepa R. Electrochemical Performance of Carbon Materials. Orient J Chem 2022;38(3). Available from: https://bit.ly/39TCtPu |
Introduction
In recent day’s sustainable energy storage and consumption are significant challenges in our world due to current lifestyle1,2. The key achievement is not only the creation of renewable and sustainable energy sources, but the main thing is the efficient storage and supply of energy as needed, in particular portable electronic devices, hybrid electric vehicles, mobile applications and transport systems3,4. The energy is stored by batteries and supercapacitors in three ways such as electrically, electrochemically and chemically. Carbon materials have high electrical conductivity, good thermal stability and it is lightest element used for energy storage, configured in different forms to deliver superior surface area and energy efficiency5-10. Carbon materials are broadly used for supercapacitor applications because of their low cost and versatility of 0D, 1D, 2D and 3D forms, such as carbon quantum dots (CQD), carbon nanotube (CNT), graphene and graphite nanomaterials. During the last decade, CNTs have been playing a significant role in the production of energy storage devices due to their lightweight and also CNTs offer many other benefits. CNTs exhibit drawbacks, such as the presence of toxic residual metal impurities, which are more difficult to remove and more expensive to synthesize11. A Graphene nanomaterial has recently developed as an alternative energy storage material with excellent properties such as lightweight, thinnest and low-cost materials. Graphene is a single-layer two-dimensional sheet of sp2-bonded carbon atoms that has unique electrical conductivity, mechanical, electrochemical, thermal stability and optical properties12. The CQD get attention due to its electronic properties, good biocompatibility, and low cost. The effective application area of CQD is a supercapacitor, optoelectronics, polymer solar cell, photocatalysts, and sensors13.
In the present work, we prepare ball-milled exfoliate graphite (MG), graphene oxide (GO) and reduced graphene (RGO) from natural graphite powder. The MG was prepared by the dry ball milling process, GO and RGO were synthesized by chemical process and CQD was prepared by Pyrolysis technique. The electrochemical properties of prepared nanomaterials MG, GO, RGO and CQD had been investigated and the electrochemical performance and compared the specific capacitance values.
Experimental
Graphite powder, Hydrogen Peroxide, Sulfuric acid, Acetone, Methanol, and Hydrochloric acid were bought from Loba Chem. Sodium nitrate (NaNO3) was purchased from SDFCL. Potassium permanganate (KMnO4) was obtained from Merck, Chloroform, Poly (sodium 4-styrene sulfonate, 99.8%, M.W. 70,000) (PSS) and CTAB were purchased from Sigma Aldrich. The citric acid (CA) (99.7%) was purchased from Himedia Chemicals. Ethanol was procured from Changshu Longsheng Fine Chemical Co. Ltd., and Milli-Q water was used throughout the experiments.
The synthesis process of GO, RGO and carbon quantum dots had been discussed in our previous publications14-16. In short, Modified Hummer’s method was used to prepare graphene oxide and reduced graphene oxide. The Oxidizing agents H2SO4, KMnO4, and NaNO3were used to exfoliate from graphite stacked form and hydrazine hydrate was used as a reduction agent. In CQD, Citric acid and PSS are dissolved in Milli Q water. The mixture solution was stirred with temperature and continued up to get a gel form. Followed by evaporation then we get powder. Then the powder was pyrolyzed, and finally, we get brownish-black CQD powder.
A planetary ball mill machine (Fritsch ulverisette-5) was used for high-energy ball milling of natural graphite with 250ml stainless steel vials and a 4g weight and10 mm diameter stainless steel ball used. The milling process was carried out with a ball to graphite power ratio of 10:1. As per the ball to powder ratio, 2g of natural graphite powders and 20g of stainless-steel balls were taken per vial17. The milling rotation speed was kept at 200rpm. The milled powders were collected in hours at 2h, 4h and 8-hour time durations. The resultant milled graphite (MG) was washed in a 6 mol of HCL solution at the temperature of 100°C to remove metallic impurities18. Followed by washing with Milli Q water and continuing this process until the pH level reaches a neutral value of 7. The solution was kept in vacuum filtration to separate EG. Finally, the resultant EG was dried for 2 hours in a vacuum oven at 100°C. The X-Ray Diffraction spectrum was recorded using a PANalytical X-ray Diffractometer, in the range of 2q = 10 ° to 80 °. Electrochemical characterizations were carried out using an M/S Biologic Science electrochemical workstation at ambient temperature.
The electrochemical measurements of electrode materials for supercapacitors were tested by cyclic voltammetry (CV) and galvanostatic charge–discharge technique (GCD) were carried out in a typical three-electrode cell, electrochemical setup in ambient conditions. A stranded Ag/AgCl was used as the reference electrode, Pt wire acted as the counter electrode and the GCE was taken as the working electrode. 1 M aqueous solution of Na2So4 was used as the electrolyte for all the measurements.
The specific capacitance was calculated from CV curves using the following equation (1).
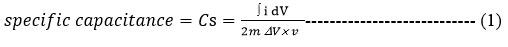
Where C is specific capacitance, ò idV is the integral part that represents the area below the cyclic voltammetry curve, m represents the mass of the active material, v is the scan rate and “ΔV” is the width of the potential window.
Results and Discussion
The characterization of X-ray diffraction is the basic technique to identify the phase purity and crystalline nature of the synthesized materials. XRD patterns in figure 1 show the crystalline nature of ball-milled graphite nanoparticles with different milling times. The ball-milled graphite particles show a sharp and tight peak around 2q = 26° 19-23 which corresponds to (002) the plane of the graphitic framework. The diffraction peaks present at 2h milled sample 2q = 26.59°, 4h milled sample peak value 2q = 26.56°, and 8h milled sample peak present at 2q = 26.51° all the samples attributed to the (002) plane24, 25. The 3D graphite particles are easily destroyed during the ball milling process26. Figure 1 clearly shows that while increasing the milling time of MG the peak intensity value gradually decreases and the diffraction peak intensity was inversely proportional to the milling time. The quality of the MG nanoparticles is based on structural properties and grain size. From this mechanical exfoliation technique we were trying to reduce the grain size of the natural graphite particles, represents by XRD results. The structure and morphological studies of samples were carried out by TEM characterization. Figure 2 (b & c) indicates few-layered graphene sheets present on GO and RGO.
 |
Figure 1: (a) and (b) XRD Patterns of the ball-milled graphite with different time durations. |
 |
Figure 2: TEM images of (a) MG, (b) GO, (c) RGO and (d) CQDs. |
2 (a) But in MG several layers are stacked in bulk form and the edges area is shown thin because of the mechanical exfoliation effect. 2(d) CQD are highly monodisperse with size and shape.
Electrochemical Measurements
Figure 3 exhibits the CV curve of different carbon electrode materials to examine the capacitive behavior with a potential window range between -1.0 to 0.0 V. From 3(a) to 3(d) CV has been done in the scan rate of 20mVs-1 and 3 (e) shows CV curves of GO electrode was carried out different scan rates from 20 to 100mVs-1. The curves are rectangular in shape even high scan rate and indicate good capacitive nature.
 |
Figure 3: Cyclic voltammograms of (a) MG, (b) GO, (c) RGO, (d) CQD at a scan rate of 20mV/s and (e) different scan rates of GO. |
 |
Figure 4: (a) Galvanostatic charge-discharge curve of different carbon electrode materials at current density 0.125 A/g, and (b) different current density of GO from 0.125 to 1A/g. |
Galvanostatic charge/discharge curves are illustrated in figure 4(a) & 4(b). The voltage-time curve shows a good triangle symmetry shape in obtained graphene, milled graphite and CQD with a small deviation at lower potentials monitored from graphene oxide.
 |
Figure 5: specific capacitance carbon materials. |
Figure 5 illustrates the specific capacitance of different carbon materials and these values calculated from equation (1) at the scan rate of 20mVs-1. From this graph, sample GO exhibits a high specific capacitance value compared to other samples.
The following reasons are GO exhibits higher specific capacitance28,29 than RGO, MG and CQDs,
The main things functional groups are present in graphene oxide due to strong oxidization agents. These functional groups bind on the surface of graphene sheets and their reflections reduce the electrical conductivity like an insulator.
Oxygen-containing group’s carboxyl, carbonyl, epoxy and carbonaceous materials graphite are redox-active. It may contribute to charge storage through the pseudocapacitance process.
But reduced graphene oxide has high electrical conductivity due to the Sp2 hybridization of carbon atoms in the hexagonal lattice and there are no functional groups due to high reduction agents.
The specific capacitance of CQDs showed the lowest value compared to other samples due to their structural properties and absence of oxygen-containing functional groups.
Conclusion
In this summary, the MG nanoparticles were synthesized using the ball milling technique (top-down approach). From XRD results, the milling time is inversely proportional to the peak intensity and it represents the crystalline size. The electrochemical performance of carbon materials ball-milled nanographite, graphene oxide, reduced graphene oxide and carbon quantum dots were compared as electrode materials for supercapacitors application. The specific capacitance of ball-milled nanographite, graphene oxide, reduced graphene oxide and carbon quantum dots were found as 3.84 Fg-1, 38.75 Fg-1, 27.59 Fg-1 and 0.34 Fg-1 respectively. The results indicate that the GO electrode showed a high specific capacitance value of 38.75 Fg-1. Therefore GO nanoparticles shall be a promising electrode materials for supercapacitor applications.
Acknowledgement
The authors acknowledge PSG institutions for helping in doing this research work.
Conflict of Interest
There is no conflict of Interest.
Funding Sources
There is no funding source.
Reference
- Bigdeloo, M.; Kowsari, E.; Ehsani, A.; Chinnappan, A.; Ramakrishna, S. J. Energy Storage. 2021, 37, 102474.
- Sharma, P.; Kumar, V. J. Electron. Mater.2020, 49, 3520-3532.
- Shaqsi, A.Z.A.L.; Sopian, K.; Hinai. A.A.l. Energy Rep. 2020, 6, 288-306.
- Iro, Z.S.; Subramani, C.; Dash. S.S. Int. J. Electrochem. Sci. 2016, 11, 10628-10643.
- Chen, X.; Paul, R.; Dai, L. Natl. Sci. Rev. 2017, 4, 453-489.
- Saleem, A.M.; Desmaris, V.; Enoksson, P. J. Nanomater. 2016, 2016, 1537269.
- Chen, T.; Dai, L. J. Mater. Chem. A. 2014, 2, 10756-10775.
- Li, H.; Tao, Y.; Zheng, Z.; Luo, J.; Kang, F.; Cheng, H. M.; Yang, Q.H. Energy Environ. Sci. 2016, 9, 3135-3142.
- Chen, T.; Dai, L. Mater. Today. 2013, 16, 272-280.
- Dou, Q.; Park, H.S. Energy & Environmental Materials, 2020, 3, 286-305.
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. J.Mater. Chem. 2011, 21, 15872-15884.
- Shi, X.; Zheng, S.; Wu, Z.S.; Bao, X. J. Energy Chem. 2018, 1, 25-42.
- Rasal, A.S.; Yadav, S..; Yadav, A.; Kashale, A.A.; Manjunatha, S.T.; Altaee, A.; Chang, J.Y. ACS Appl. Nano Mater. 2021, 4, 6515-6541.
- Amba Sankar, K.N.; Sathish Kumar, C.; Kallol Mohanta. Materials Today: Proceedings of International Conference on Nanotechnology: Ideas, Innovations & Initiatives-2017 (ICN:3i-2017) India, 2019, 18, 759-764.
- Amba Sankar, K.N.; Nandakumar, P.; Sathishkumar, C.; Deepa, R.; Kallol Mohanta. Rasayan J. Chem. 2021, 14, 2196-2201.
- Amba Sankar, K.N.; Bhattacharjee, L.; Jat, S. K.; Bhattacharjee, R.; Kallol Mohanta. ChemistrySelect, 2017, 2, 4241-4247.
- Panjiar, H.; GakkhaR, R.P.; Daniel, B.S.S. Powder Technol. 2015, 275, 25-29.
- Vieira, M.A.; Gonçalves, G.R.; Cipriano, D.F.; Schettino, M.A.; Filho, E.A.S.; Cunha, A.G.; Emmerich, F.G.; Freitas, J.C.C. Carbon, 2016, 98, 496-503.
- Ban, F. Y., Siti Rohana Majid, Nay Ming Huang and H. N. Lim, Int. J. Electrochem. Sci, 2012, 7, 4345-435.
- Kavitha, M. K., Pramod Gopinath and Honey John, Phys. Chem. Chem. Phys., 2015, 17, 14647-14655.
- Jiao, Xuan, Yangshuai Qiu, Lingyan Zhang and Xudong Zhang, RSC advances, 2017, 7, 52337-52344.
- Radoń, Adrian, and Dariusz Łukowiec, Micro & Nano Letters, 2017, 12, 955-959.
- Siburian, Rikson, Hotmaulina Sihotang, S. Lumban Raja, M. Supeno and Crystina Simanjuntak, Orient. J. Chem., 2018, 34, 182.
- Ain, Qura Tul, Samina Hyder Haq, Abeer Alshammari, Moudhi Abdullah Al-Mutlaq and Muhammad Naeem Anjum, Beilstein J. Nanotechnol., 2019, 10, 901-911.
- Dash, P.; Dash, T.; Kumar Rout, T.; Sahu, A.K.; Biswal, S.K.; Mishra, B.K. RSC Adv. 2016, 6, 12657-12668.
- Tang, Q.; Jihuai Wu; Hui Sun; Shijun Fang. J. Alloys Compd. 2009, 475, 429-433.
- Yue, X.; Wang, H.; Wang, S.; Zhang, F.; Zhang, R. J. Alloys Compd. 2010, 505, 286-290.
- Xu, Bin, Shufang Yue, Zhuyin Sui, Xuetong Zhang, Shanshan Hou, Gaoping Cao, and Yusheng Yang, Energy Environ. Sci., 2011, 4,2826-2830.
- Li, Junfu, James O’Shea, Xianghui Hou, and George Z. Chen. Chem Comm., 2017, 53, 10414-10417.

This work is licensed under a Creative Commons Attribution 4.0 International License.









