Effect of Aluminium, Magnesium Doping on Magnetic Nature of Zinc Oxide Nanoparticles Studied by X-Ray Diffraction Method
Department of Chemistry, G.T.N Arts College (Autonomous), Dindigul, Tamil Nadu, India
Corresponding Author E-mail: drshanmugaselvan@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380310
Article Received on : 31 Jan 2022
Article Accepted on : 14 Jun 2022
Article Published : 23 Jun 2022
Reviewed by: Dr. Femina Felix
Second Review by: Dr. T. Raja
Final Approval by: Dr. S.A. Iqbal
Zinc Oxide nanoparticles were prepared by an ultra-sonicated method. It’s characterized with XRD, SEM, and FTIR. X-ray diffraction is utilized to find exact grain size, arrangement of the crystal in the materials and used to prove doping exists. (242), (151) the hkl value proves doping exists in the prepared material. After doping the metal shows the hexagonal structure and size below 100 nm. FTIR finds functional group that shows metal functional group of the nanoparticles. The current study explains the magnetic nature of ZnO by using magnetic (1) symmetry which is identified by XRD and crystal impact Match-2 and Diamond-3 software.
KEYWORDS:Diamond-3; Magnetic symmetry; Match-2; SEM; XRD
Download this article as:| Copy the following to cite this article: Sulochana S, Soundaravalli K, Selvan R. S. Effect of Aluminium, Magnesium Doping on Magnetic Nature of Zinc Oxide Nanoparticles Studied by X-Ray Diffraction Method. Orient J Chem 2022;38(3). |
| Copy the following to cite this URL: Sulochana S, Soundaravalli K, Selvan R. S. Effect of Aluminium, Magnesium Doping on Magnetic Nature of Zinc Oxide Nanoparticles Studied by X-Ray Diffraction Method. Orient J Chem 2022;38(3). Available from: https://bit.ly/3OeeskQ |
Introduction
ZnO is factotum that has good optical, chemical and electrical properties. It’s also found beneficial in constructing piezoelectric dimmers, sensors optical dimmers, surface acoustic dimmers, (3) solar cell, antibacterial activity etc. The reaction of different crystal structured material are used in random access memories. (4, 5) The magnetic and optical properties could be improved by embedding transition metals or rare earth element. First principle calculation is used to stud dopping effect on domin wall. On doping domain wall region the energy is grater compared to bulk region (2).
The synthesis of ZnO nanoparticles numerous methods have been used, such as chemical vapor deposition, gas evaporation sol-gel, spray pyrolysis, precipitation and thermal decomposition but the chemical techniques tend to be preferred as they provide control of nucleation, growth and ageing of particles during synthesis (6,7). Among these, the hydrothermal method is a cost effective, low-temperature, substrate independent and straightforward technique that determines controllable structures (8). Therefore, the purpose of analysis is to characterize ZnO nanoparticles in order to determine its potential use in wastewater treatment.
Preparation of Pure ZnO Nanoparticles
Zinc acetate (Zn C4H6O4) 0.5 moles prepared by using distilled water the same methods are followed to prepare the base solution of Potassium hydroxide (KOH). The sample is prepared by using the Sonication method with frequency at 6000MHz’s. The PEG is added for the extra effective and de-aggregation purposes. The initial pH of the pure sample solution is 6 the base (KOH) is added till the solution reach the base pH 10 to 11 the precipitate is allowed to settle and separated then washed with dill HCL for removing the impurities then dried at 800C for 12 hours then it is calcinated at 3600C for 3 hours. The precipitate is stored and named as (CHEM- 2)
Preparation of Aluminium and Magnesium Doped Materials
Then the pure ZnO nanoparticles where doped with Aluminium and Magnesium under 1200C and 6000 MHz of ultrasound the dopping is made under base PH. The double dopping is made to enhance the magnetic nature of the sample. The sample is dried at 1200C for 3 hours and calcinated under 3600– 4200C for one hour. The doped sample is stored and named as (CHEM- 4)
Table 1: Observation of sample preparation.
|
Sample |
pH |
Color |
Viscosity |
|||
|
|
Initial |
Final |
Initial |
Final |
Initial |
Final |
|
Zinc oxide nanoparticles |
6 |
11 |
White precipitate |
White precipitate |
Normal |
Oily gel |
|
Zinc oxide doped with Al and Mg |
6 |
12 |
White precipitate |
White precipitate |
Normal |
Oily gel |
X-Ray Diffaraction (XRD)
XRD is used as the finger print region for inorganic material, in XRD to identify the purity and crystal nature. Crystal Impact Match-2 and Diamond-3 software are used to draw the crystal structure of pure and doped ZnO. The blue lines indicate observed data and the red line stasis matched data, and various colors show doped sample. The observed XRD is shows in Fig-1.
 |
Figure 1: Observed XRD for Pure Sample. |
 |
Figure 2: Interpretation XRD for Pure ZnO. Click here to View figure |
The pure ZnO has hexagonal structure and P63mc space group. The atomic distance between Zn-Zn band is 0.33Ǻ and for Zn-O band is 0.38Ǻ. The d-value is 2.8902Ǻ of the sample indicates that the sample is pure (98-900-4181 ref data number).
Table 2: Cell parameter for Pure Sample.
|
Cell Parameter |
Angstrom[Ǻ] |
|
a |
3.2994Ǻ |
|
b |
3.2994Ǻ |
|
c |
5.2038Ǻ |
Table 3: d-value and hkl value of pure sample.
|
2 theta |
d[Ǻ] |
hkl value |
|
31.91 |
2.8019 |
100 |
|
34.43 |
2.6028 |
002 |
|
36.09 |
2.4868 |
101 |
|
47.46 |
1.9028 |
012 |
|
56.65 |
1.6236 |
110 |
|
62.94 |
1.4756 |
013 |
|
66.43 |
1.4062 |
200 |
 |
Figure 3: Structure of Pure ZnO Drawn by Crystal Impact Match-2 and Diamond-3. |
Effect of Doping
In our study we used two doping with pure sample Al and Mg were used as doping materials, the blue lines in Fig-5 shows the observed data the red line shows matched data. The observed XRD is shows in Fig-4 the doped sample shows the cubic shape which is shows in Fig-6 where the lattice parameter of the doped materials is changed. The lattice cell parameter of the doped sample is a = 8.066 Ǻ and shows the Fd-3 space group the atomic distance of doped material is Zn-O = 1.7463Ǻ, Al-O = 2.0165Ǻ, Mg-O = 0.0051Ǻ. The doping of Al is 65.5 % and Mg is 34.5%. The 2 theta value of 56.23Ǻ, and 59.57Ǻ are indicated for doping with is common to Al and Mg, The doublet peaks show the doping exists in the sample it because of lattice order displacement. The hkl value of doped material in the particular point is.
 |
Figure 4: Observed XRD Data for Doped Sample. Click here to View figure |
 |
Figure 5: Interpretations XRD for Doped Sample. |
 |
Figure 6: Structure of Pure ZnO doped material |
Theoretical magnetism based on magnetic symmetry space group for pure zno
Magnetic nature is studied through Magnetic Group Table, 1-, 2- and 3-Dimensional Magnetic Sub periodic Groups and Magnetic Space Groups Daniel B. Litvin., book. The pure sample shows P63mc space group, the magnetic symmetry origin of center arranges 31m at 63 cm asymmetric unit which shows no magnetic moment (9,10). The doped sample shows magnetic symmetry shows in Fig-7 the space group as m which origin of center arranged as 3m at – 1/8 -1/18 from center m (11,12,13). Asymmetric unit with the ferromagnetic moment the XRD shows the arrangement of the atom in this symmetric nature and confirms the theoretical magnetism changed from diamagnetic nature to ferromagnetic nature.
 |
Figure 7: Magnetic Symmetry for Zn, Al, and Mg doped Metal Complexes. |
 |
Figure 8: Pure Sample. Click here to View figure |
 |
Figure 9: Doped Sample. Click here to View figure |
 |
Figure 10: Doped Sample. |
Scanning Electron Microscopy (SEM)
The SEM images shows in Fig-8, 9, 10, from the image. The size varies from 20-60nm. It may be due to environmental factors like far atmospheric moisture etc. From the image the average particle size is 20-60nm. As doping happens, the addition of impurity to the parent material. The parent materials lattice arrangement is changed, as there is also a change in lattice disorder of parent material and the morphology of parent material is also changed f(9). In the present study, Al and Mg used as a doping material and zinc oxide were used as the parent material. The atomic radius of Zn is more than Al, so the doping takes place in a good manner. The lower atomic radius gives the best result in doping and from lattice disorder this lattice disorder gives the change in morphology.
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR can find functional groups in Nanoparticle sample. Adsorption signals are found 2978.09 cm-1, 1512.19 cm-1, 864.11 cm-1, 902.64 cm-1, 948.98 cm-1, 1342.46 cm-1, 1396.46 cm-1,678.94 cm-1, 1072.42 cm-1,439.77 cm-1, by removing OH molecule the observation is observed in 2800 -3600 cm-1. 682 cm-1, 518 cm-1, 457 cm-1 was allotted for ZnO microcrystals. The positions of the peaks are depend on the axial ratio (c\a) of the crystal. Spectra is observed in 3649.32 cm-1 and it converted into ZnO, CH3 Stretching mode is found in 2978.09 cm-1, C=C Stretching mode is observed in 1512.19 cm-1, , C-H-O bonding mode asymmetrical and symmetrical stretching of zinc carboxylate is found in 1396.46 cm-1, C-H-O bonding mode is found in 1342.46 cm-1 1072.42 cm-1 was assigned for Zn-O Stretching bond, 864.11 cm-1,902.64 cm-1,948.98 cm-1 was assigned for C-H out of plane mode, 678.94 cm-1 was assigned for Zn-O Stretching and deformation vibration, 439.77 cm-1 was assigned for E2 mode Zn-O (hexagonal) bond, these peaks confirm the presence of PEG in the sample which is used as a capping agent or surfactant, the doped material for the following peaks are assigned and confirmed the doping of 1249.87 cm-1,1411.89 cm-1 was assigned for C-H-O bonding mode, 1087.85 cm-1 was assigned for C-O Stretching mode (alcohol), 555.50 cm-1 was assigned for Mg-O Lo phonon mode of MgO lattice, 439.77 cm-1 was assigned for E2 mode of morphed ZnO and the Vibration of Mg-O also. The SEM of this sample shows the morphology change of ZnO nanoparticles.
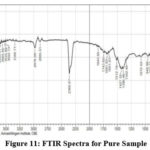 |
Figure 11: FTIR Spectra for Pure Sample. Click here to View figure |
 |
Figure 12: FTIR Spectra for Doped Sample. |
Conclusion
Zinc Oxide nanoparticles where prepared by using ultra sonicated method. The ZnO nanoparticles are characterized by using X-ray diffraction, SEM, and FTIR. The XRD confirmed the atomic distance, d-value and the hkl value of the sample for the pure zinc oxide and doped with Al and Mg. From X-RD results the shape of the pure sample is hexagonal and changed to the cubic structure. The magnetic symmetry is changed from diamagnetic to Asymmetric unit with ferromagnetic moment. X –RD shows the arrangement of the atom symmetric nature and confirms the theoretical magnetism changed from diamagnetic to ferromagnetic nature. The SEM image confirmed the spherical shape for Zinc Oxide nanoparticles and the hexagonal the Al and Mg doped Zinc Oxide nanoparticles was confirmed. The sample shows less than 100nm that shows in the report. The lower atomic radius gives best result in a doping and from lattice disorder this lattice disorder gives change in morphology. The FTIR confirmed the Zn-O stretching mode and 3649.32 cm-1 the elimination of OH in Zn (OH) converted into ZnO, while doping with Al and Mg confirmed by the doping 555.50 cm-1 is allotted for Mg-O Lo to phonon mode of MgO lattice, 439.77 cm-1is fit for E2 mode of morphed ZnO and the Vibration of Mg-O. These Nano particles are used to treat phenolic effluent like cresol, Ortho cresol, etc.,
Conflict of Interest
There is no conflict of interest.
Funding Sources
There are no funding source.
Reference
- Daniel B. Litvin., Magnetic Group Tables Book (Part-2).
- J.A. Cuervo Farfán, D.M. Aljure García, R. Cardona, D.A. Landínez Téllez and J. Roa-Rojas Structure, ferromagnetic, ferroelectric and electronic features of the LaBiFe2O6 biferroic material National University of Colombia.
- Kalyani Ghule, Anil Vithal Ghule, Bo-Jung Chen and Yong-Chien Ling* Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study, Green Chem., 2006, 8, 1034–1041.
CrossRef - Selvan, R. Shanmuga, and K. Gokulakrishnan. “Preparation and Characterization of Mg Doped γ-Fe2O3 Prepared by Self-Propagation Method.” International Journal of ChemTech Research june 2014 Vol.6(no3):2129-2131
- HongXin,QingPang,DangliGao,LongLi,WenChen,AipingZhang, Effect of Mn doping defect on 180° domain wall in ferroelectric PbTiO3, Physics Letters A, Volume 384, Issue 14, 18 May 2020, 126279.
CrossRef - R. Shanmuga selvan, and K. Gokulakrishnan. “Preparation and Characterization of Mg Doped γ-Fe2O3 Prepared by Self-Propagation Method.” International Journal of Applied Chemistry 9.3 2013: 291-297.
- Gyorgy deak1, florina diana dumitru1* , mihaela andreea moncea1, ana maria panait1, andreea georgiana baraitaru1, marius viorel olteanu1, madalina georgiana boboc1,2, silvius stanciu, synthesis of zno Nanoparticles For Water Treatment Applications, INTERNATIONAL Journal Of Conservation Science, Volume 10, Issue 2, April-June 2019: 343-350.
- Jared M. Hancock1, William M. Rankin1, Talaat M. Hammad, Jamil S. Salem,Karine Chesnel4, and Roger G. Harrison, Optical and Magnetic Properties of ZnO Nanoparticles Doped with Co, Ni and Mn and Synthesized at Low Temperature. Journal of Nanoscience and Nanotechnology Vol. 14, 1–7, 2014
- ErikaTóthováaMamoruSennaabAnatolyYermakovcdJozefKováčeErikaDutkováaMichalHegedüsfMáriaKaňuchovágMatejBalážaZdenkaLukáčováBujňákováaJaroslavBriančinaPetreMakreskih, Zn source-dependent magnetic properties of undoped ZnO nanoparticles from mechanochemically derived hydrozincite, Journal of Alloys and Compounds, Volume 787, 30 May 2019, Pages 1249-1259
CrossRef - Ekane Peter Etape ,1 Josepha Foba-Tendo ,1 Lambi John Ngolui,2 Beckley Victorine Namondo,1 Fomogne Cyrille Yollande,1 and Marius Borel Nguefack Nguimezong1, Structural Characterization and Magnetic Properties of Undoped and Ti-Doped ZnO Nanoparticles Prepared by Modified Oxalate Route, Volume 2018 |Article ID 9072325 | https://doi.org/10.1155/2018/9072325.
CrossRef - F.E Mabbs and D.T. Machin , Magnetism and Transition Metal Complexes , Published in 11 January (2008).
- J.M.G. Coey , Magnetism and Magnetic Materials. Cambridge University Press, UNITED KINGDOM, (2009).
- Stephen J.Blundell., Magnetsism : Avery Short Introduction , Published in ( 2 Jul 2012) OXFORD Publication.

This work is licensed under a Creative Commons Attribution 4.0 International License.










