Thermoanalytical and Kinetic Studies for the Thermal Stability of Nimesulide under Different Heating Rates
1Deanship of Common First Year, Jouf University, P.O. Box 2014, Sakaka, Saudi Arabia.
2Pharmaceutical Chemistry Department, National Organization for Drug Control and Research, Egyptian Drug Authority (EDA), Giza, Egypt.
Corresponding Author E-mail: monagamall77@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380215
Article Received on : 06-Jan-2022
Article Accepted on :
Article Published : 09 Mar 2022
Reviewed by: Dr. D. Ramachandran
Second Review by: Dr. Sripal Reddy
Final Approval by: Dr. Tanay Pramanik
The elucidation of the thermal stability and degradation of pharmaceutical drugs utilized in medication is commonly conducted using thermal examination techniques. Kinetic studies have emerged as a critical component of thermal analysis, with the primary goal of determining the kinetic approach of degradation and calculating the Arrhenius equation parameters. The current study describes the thermal behaviour of nimesulide, and the calculation of the melting point using thermoanalytical graphs, as well as the identification of parameters of the decomposition kinetic. The kinetic investigation of nimesulide's active substance's, thermal degradation was carried out in a nitrogen environment via non-isothermal parameters at four rates of heating: 5, 10, 15, 20, and 20 °C/min. The kinetic models of the thermal breakdown pathway were calculated from TG/DTG graphs using differential approaches of Friedman isoconversional method and integral approaches such as the Ozawa, and Starink method.
KEYWORDS:Decomposition; Kinetic Parameters; Nimesulide; Quality Control; Thermal Stability
Download this article as:| Copy the following to cite this article: Ahmed A. M. A, Asran A. M, Mohamed M. A. Thermoanalytical and Kinetic Studies for the Thermal Stability of Nimesulide under Different Heating Rates. Orient J Chem 2022;38(2). |
| Copy the following to cite this URL: Ahmed A. M. A, Asran A. M, Mohamed M. A. Thermoanalytical and Kinetic Studies for the Thermal Stability of Nimesulide under Different Heating Rates. Orient J Chem 2022;38(2). Available from: https://bit.ly/3pLi8jS |
Introduction
Nimesulide (4-nitro-2-phenoxymethanesulfonanilide) has a nitroaromatic ring and is extensively used as a nonsteroidal anti-inflammatory (NSAID) medication that specifically inhibits prostaglandin formation1. Furthermore, nimesulide contains antipyretic and analgesic characteristics that may be used to treat rheumatoid arthritis, prostatovesiculitis, and osteoarthritis 2.
Thermal analysis is a common quality control tool for pharmaceutical medications and chemicals that offers important facts regarding the physical characteristics of materials 3, 4
Thermogravimetric analysis (TG) is frequently the gateway in the comprehensive exploration for a specific drug’s characteristic and for determining its compatibility in single form or in the presence of other constituents, thermal stability, and kinetic examination. Additionally, with minimal amounts of material and without the use of solvents, results may be reached rapidly. 5. The thermogravimetry data collected by thermal decomposition may be evaluated to derive kinetic parameters. The use of thermoanalytical methods may yield fresh insights into the temperature and energy involved with phenomena like melting, glass transition, oxidation reactions, decomposition, crystallisation, or crystal transition 6.
The thermal degradation of pharmaceuticals is significant because it may be used to anticipate degradation (decomposition) rates at lower values of heating using data from accelerated routes investigated at high temperatures. The temperature might raise the temperature of chemical processes, supplying enough activation energy to disruption the chemical links and start the degradation pathway. Understanding of such variables are useful for elucidating incompatibility and the influence on the stability in both pure pharmaceuticals and drug–excipient combinations.
Nimesulide is determined using different techniques including liquid chromatography7-9, spectrophotometry10-12, and electrochemical methods 13-17.
The purpose of this research is to look at the thermal behaviour and kinetics of nimesulide decomposition using non-isothermal settings at changed heating rates. Moreover, to the best of our knowledge, no information regarding the nimesulide’s thermal characteristics or degradation kinetics.
Experimental
Materials
Nimesulide was offered from Pharaonia Co., Alex, Egypt, and the purity is 99.36%.
Apparatus
Simultaneous Shimadzu Thermogravimetric Analyzer TGA-60 H with TA 60 software was used to perform thermogravimetry and differential thermal analysis in a platinum crucible in a dry nitrogen environment via a flow rate of 30 mL min-1. The studies were carried out at various heating speeds from room temperature to 800 oC. (5, 10, 15 and 20 oC min-1). Without any additional treatment, the drug mass was around 5 mg of the nimesulide. TG/DTG curves were used to derive decomposition kinetic parameters.
Results and discussion
Thermal behavior of nimesulide
The thermoanalytic graphs that define the thermal demeanor of nimesulide in raised temperature setting with a rate of heating of 5 °C/min under N2 gas environment are displayed in Figure1. By depicting the TG/DTG plots, two distinct steps with different mass loss are presented. The first stage corresponds to the loss of -C12H9N2O3 (Δm = 74.35%) and happens between 210 °C to 385 °C, with Tpeak DTG = 305 °C.
 |
Figure 1: Thermogravimetric analysis, TG/DTG with DTA graphs of nimesulide under dynamic N2 gas atmosphere (30 mL min-1) using heating rate at 5 oC/min. |
In the temperature range 386–680 oC, the second disintegration phase shows a mass loss (m = 25.65%), indicating full nimesulide decomposition.
The DTA plot represents a significant thermal occurrence across the studied range of temperature.
The endothermic event at 145 oC 18 is most probable owing to nimesulide melting, whereas the exothermic event at 320 oC is described to the first breakdown course linked with the 1st mass loss shown in the TG/DTG curve as shown in Figure 2. A significant exothermic peak is detected at 610 oC due to the complete pyrolysis of nimesulide. The proposed nimesulide thermal breakdown is best exemplified in Scheme 1.
 |
Scheme 1: The suggested thermal degradation of nimesulide |
Effect of heating rate
Figure 2 depicts nimesulide weight-loss curves at various heating speeds ranging from 5 to 20 °C/min. When shown in the graph, as the heating rate rises, the Tmax values move to higher values. Furthermore, the curves show that nimesulide breakdown occurs in a two phase, with no evolution of water contents at the start of the process.
 |
Figure 2: Thermograms of nimesulide in nitrogen atmosphere at different heating rates. |
DTA data for the decomposition of nimesulide at various rates of heating is depicted in Figure 3. The melting point events and disintegration temperatures of the medications were found to be moved to upper temperatures by increasing the heating rate.
 |
Figure 3: DTA curves of nimesulide at different heating rates. |
Kinetic models
The kinetic factors found from the current studies done using non-isothermal circumstances were utilized to evaluate the nimesulide thermal stability. The following kinetic models were used to determine the moving strictures using the TG/DTG graphs: Friedman (Fd) 19, 20, Flynn–Wall–Ozawa (FWO) 21-23, and Starink (St)24 .
Usually, the kinetics of processes (such as polymerization, crystallisation, decomposition, etc) may be delineated using rate equations 25, 26:
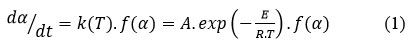
where t is referring to the time, k(T) is referring to the Arrhenius rate constant, α is referring to the degree of conversion, A is referring to the pre-exponential factor and E is defining the activation energy, R is referring to the universal gas constant, and f (α) is defining the reaction model related to a the persuaded decomposing pathway. Utilizing non-isothermal settings, the term dα/dt is converted with βdα/dT, where β is referring to the rate of heating, yielding.
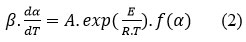
Friedman (Fd) method
The isoconversional Friedman technique is dependent on the generic reaction rate relation 3
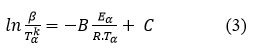
where B and C are constants. The rate equation is transformed into:
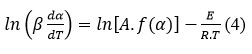
The graphical depiction of da / dt versus 1/T for = const. and varying heating rates (β) give rise to straight lines, their slope is defined E. A sequence of straight lines, known as the Friedman diagram, is obtained for various values of (Fig. 3).
Flynn–Wall–Ozawa (F–W–O) approach
Flynn–Wall–Ozawa is considered as one of the integral isoconversional approaches that uses the integral relation of the rate as well as the Doyle approximation for p (x):
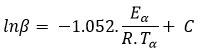
For α= const., plotting lnβ according to 1/T yields a straight line relation and the energy of activation (E) could be obtained from the slope. A straight family is obtained for various values of (Figure 4). When the heating rate is increased, there is a variation in the mechanism or the rate-determining step. Table 1 shows the activation energy (E) values.
 |
Figure 4: Plot of Friedman’s for nimesulide at variant heating rates (A), The Flynn–Wall–Ozawa (B), and Starink (C) plots |
Starink (St) method
St proposes the following estimation for the integral temperature:
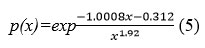
The values of E were estimated using the Starink technique from the slope of the fitted relation of ln/
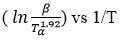
(Figure 4C) and are presented in Table 1.
Table 1: nimesulide calculated activation energy values using Friedman (Fd), Flynn–Wall–Ozawa (FWO), and Starink (ST) approaches
|
Technique |
E/kJ mol-1, for conversion degree, α |
||||||||||
|
0.05 |
0.1 |
0.2 |
0.3 |
0.4 |
0.5 |
0.6 |
0.7 |
0.8 |
0.9 |
||
|
FR |
133.0 |
138.1 |
137.1 |
138.8 |
137.1 |
139.6 |
137.5 |
139.1 |
139.2 |
133.2 |
|
|
FW |
129.6 |
129.94 |
130.19 |
130.61 |
130.61 |
130.77 |
130.94 |
131.07 |
131.19 |
129.69 |
|
|
Starink |
131.6 |
130.7 |
130.9 |
131.1 |
130.6 |
131.3 |
132.1 |
132.1 |
132.2 |
131.6 |
|
The obtained observations have been made based on the collected results include that the activation energies determined via the Fd approach, FWO, and Starink techniques are quite similar throughout the whole decomposition fraction range. These findings are consistent with the published data on the accuracy offered by these approaches. The activation energy (E) values obtained by these approaches are in worthy convention, and the low fluctuation of E vs. designates a unistadial, yet complicated decomposition pathway. Given that 65% of the E vs. α amount identified in the literature are in the 100 to 230 kJ mol-1 range, the E values revealed a significant thermal stability of the nimesulide -active chemical 27.
Conclusion
Nimesulide was exposed to a thermal examination in an inert nitrogen environment under non-isothermal circumstances. The thermal stability of nimesulide was investigated in this work utilising thermal techniques and kinetic analysis. Its thermal behaviour was monitored for this purpose, and a kinetic analysis was carried out to estimate the activation energy. The way nimesulide reacts to heat demonstrates a high degree of thermal stability (up to 210 °C). Differential techniques (Fd technique) and integral approaches were utilized to calculate the kinetic parameter values. According to the findings, nimesulide decomposition happens in two steps. The values of thermodynamic functions calculated using differential and integral approaches were found to be in good agreement. This demonstrates the precision of the techniques used. The computed activation energy might be particularly valuable throughout the drug quality control and preformulation stages of manufacture.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare no conflict of interest.
Funding Sources
There is no funding sources.
References
- Davis R.; Brogden R. N., Drugs1994, 48, 431-54.
CrossRef - Haraoui B.; Pelletier J. P.; Cloutier J. M.; Faure M. P.; Martel‐Pelletier J., Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 1991, 34, 153-63.
CrossRef - Mohamed M. A.; Attia A. K., Journal of Thermal Analysis and Calorimetry 2017, 127, 1751-6.
CrossRef - Mohamed M. A.; Atty S. A.; Banks C. E.; Journal of Thermal Analysis and Calorimetry 2017, 130, 2359-67.
CrossRef - Fandaruff C.; Araya-Sibaja A.; Pereira R.; Hoffmeister C.; Rocha H.; Silva M., Journal of Thermal Analysis and Calorimetry 2014, 115, 2351-6.
CrossRef - Salama N. N.; Mohammad M. A.; Fattah T. A., Journal of Thermal Analysis and Calorimetry 2015, 120, 953-8.
CrossRef - Chandran S.; Ravi P.; Jadhav P. R.; Saha R. N., Analytical Letters 2008, 41, 2437-51
CrossRef - Khaksa G.; Udupa N., Journal of Chromatography B: Biomedical Sciences and Applications 1999, 727, 241-4.
CrossRef - Patravale V. B.; D’Souza S.; Narkar Y., Journal of Pharmaceutical and Biomedical Analysis 2001, 25, 685-8.
CrossRef - Altinöz S.; Dursun Ö. Ö., Journal of Pharmaceutical and Biomedical Analysis 2000, 22, 175-82.
CrossRef - Chandran S.; Saggar S.; Priya K. P.; Saha R. N., Drug Development and Industrial Pharmacy2000, 26, 229-34.
CrossRef - Upadhyay K.; Asthana A.; Tiwari N.; Mathew S. B., Research on Chemical Intermediates 2013, 39, 3553-63.
CrossRef - Deroco P. B.; Rocha-Filho R. C.; Fatibello-Filho O., Talanta 2018, 179, 115-23.
CrossRef - Łysoń M.; Górska A.; Paczosa-Bator B.; Piech R., Electrocatalysis 2021, 12, 641-9.
CrossRef - Shetti N. P.; Malode S. J.; Bukkitgar S. D.; Bagihalli G. B.; Kulkarni R. M.; Pujari S. B. et al., Materials Science for Energy Technologies 2019, 2, 396-400.
CrossRef - Wang C.; Shao X.; Liu Q.; Qu Q.; Yang G.; Hu X, Journal of Pharmaceutical and Biomedical Analysis 2006, 42, 237-44.
CrossRef - Wong A; Santos A. M.; Fatibello-Filho O, Journal of Electroanalytical Chemistry 2017, 799, 547-55.
CrossRef - Constantinescu I. C.; Florea M.; Arama C-C.; Nedelcu A.; Monciu C-M, Farmacia 2009, 57, 267-71.
- Friedman H. L., Journal of Polymer Science Part C: Polymer Symposia; 1964: Wiley Online Library.
- Friedman H. L., Journal of Polymer Science Part B: Polymer Letters 1969, 7, 41-6.
CrossRef - Flynn J. H.; Wall L. A., J Res Nat Bur Stand 1966, 70, 487-523.
CrossRef - Flynn J. H.; Wall L. A., Journal of Polymer Science Part B: Polymer Letters 1966, 4, 323-8.
CrossRef - Ozawa T., Bulletin of the chemical society of Japan1965, 38, 1881-6.
CrossRef - Starink M., Thermochimica Acta 2003, 404, 163-76.
CrossRef - Brown M., Thermal Decomposition of Ionic Solids. Elsevier Science; 1999.
- Brown M. E.; Dollimore D.; Galwey A. K., Reactions in the solid state. Elsevier; 1980.
- Galwey A. K., Thermochimica acta 1994, 242, 259-64.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










