Ethnomedicinal Uses, Pharmacological and Phytochemical Studies of Bambusa Arundinaceae Retz (A Review)
Department of Chemistry, Faculty of Science, Al Baha University, Al Baha, Kingdom of Saudi Arabia.
Corresponding Author E-mail: syed.nazreen@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380204
Article Received on : 17-Jan-2022
Article Accepted on :
Article Published : 23 Mar 2022
Reviewed by: Dr. Mingjing Sun
Second Review by: Dr. D.Gnanasangeetha
Final Approval by: Dr. Ayssar Nahle
Medicinal plants are a source of different types of natural products which are used in different illness. Bambusa arundinacea, commonly known as Bamboo belongs to family Poaceae (Gramineae). In folk medicine, it has been utilized for various inflammatory disorder, strangury, wounds, piles, dislodgement of worms, cirrhosis, hard tumour and diabetes mellitus. This plant possess antiinflammatory, antidiabetic, antimicrobial, antioxidant, anticancer, antiglycation, laxative, antifertility, antihelmintic, insecticidal, antiarthritic and neuroprotective activities. B. arundinacea extract has also been utilized in the synthesis of nanoparticles which have enhanced the biological activities such as anticancer, antidiabetic, antimicrobial and antiglycation. The phytochemical analysis of this plant has afforded important classes of natural products such as terpenoids, steroids, flavonoids, phenols and glycosides, etc. These natural products have been reported for various pharmacological activities which may be responsible for the different biological activities of this plant. Although, this plant has not been explored much scientifically, very few reports are available on this plant. However, this plant is gaining interest in nanomedicine field as the nanoparticles synthesized using this plant extract have shown enhancement in antidiabetic, anticancer and antimicrobial activities. The present review article will help the readers to explore more on this plant in various filed of nanotechnology, biotechnology and phytochemistry as it contains promising bioactive molecules. Moreover, some phytoconstituents especially flavonoids may be used for semisynthetic modification for development of lead molecules.
KEYWORDS:Bambusa arundinacea; ethnomedicinal; pharmacology; phytochemistry
Download this article as:| Copy the following to cite this article: Nazreen S. Ethnomedicinal Uses, Pharmacological and Phytochemical Studies of Bambusa Arundinaceae Retz (A Review). Orient J Chem 2022;38(2). |
| Copy the following to cite this URL: Nazreen S. Ethnomedicinal Uses, Pharmacological and Phytochemical Studies of Bambusa Arundinaceae Retz (A Review). Orient J Chem 2022;38(2).Available from: https://bit.ly/3unFFJ6 |
Introduction
Bambusa arundinacea, commonly known as Bamboo belongs to family Poaceae (Gramineae). It is known by various names as Bnah in Assamese, Spanish bamboo in English, Wans in Gujarati, Kanta bans in Hindi, Bansa in Urdu, Vansa in Sanskrit and Bilawar in Kashmiri1. It is situated upto height of 1250 m on the hills of Andhra Pradesh, Tamil Nadu, Karnataka in India, Peru, China, Sri Lanka, Bangladesh, and Malaysia2, 3. The plant consists of hard stem with internode and solid node that gives strength to this plant. The nodes bears many buds from which branches evolve, this makes Bamboo different from other grass family. The plant stems are thorny tufted on a stout rootstock, with a height of 240-280cm and diameter of 150-170 mm. It has elongated and lanceolate leaves and flowers are in the form of panicles4.
Bambusa arundinacea has been reported to possess important pharmacological activities such as antiinflammatoy5, antidiabetic6, antioxidant7, antimicrobial8, insecticidal9, antihelmintic10, anticancer11, protective effect12, antiarthritis13, etc. Muniappan et al5 reported that the methanolic leaves extract exhibited promising antiinflammatory activity on carrageenan and immunologicaly induced paw edema in Albino wistar rats14. B. arundinacea has been found to possess antimicrobial activity against a number of bacterial and fungal strains hence it could be a possible source to obtain new and effective compound to treat bacterial and fungal infections. Zubair et al14 reported that n-hexane and chloroform extract displayed significant antimicrobial activity against the tested pathogens while acetone, methanol and butanol extract were found to be inactive. Thamizarasan et al8 reported that hexane, acetone and hydroethanolic extract of this plant seeds have significant antimicrobial effect against various microorganisms. Jayarambabu et. al15 reported the antimicrobial activity of ZnO nanoparticles using Bambusa arundinacea extract by Agar disc diffusion method against S. aureus and B. subtilis. The ethanolic extract of tender shoots of the plant possess antifertility effect as reported by Vanithakumari et al16.
B. arundinacea is endowed with antihyperglycemic potential. Joshi et al17 reported the aqueous ethanolic extract of leaves of B. arundinacea to possess hypoglycemic activity, Nazreen et al6 reported the hypoglycemic effect of B. arundinacea leaves three fractions viz. ethanolic petroleum ether, chloroform in STZ induced diabetic rats. Kumar et al23 reported that roots ethanolic extract of this plant displayed lowering in the blood glucose level in normal and hyperglycemic rats in alloxan and OGTT methods. Macharla et al18 reported that the aqueous ethanolic stem extract (200mg/kg) of Bambusa arundinaceae showed decrease in glucose level in alloxan induced diabetic rats. Jayarambabu et al15 reported the antidiabetic effect of Bambusa arundinaceae (BA) extract and its ZnO nanoparticles in normal and STZ induced rats.
B. arundinacea has been reported to possess insecticidal9 and antihelmintic10 properties. B. arundinacea has been used for the treatment of rheumatoid arthritis. Anti-arthritic effect against complete Freund’s Adjuvant (CFA) induced arthritis in female rat were investigated by Rathod et al13. B. arundinacea has been found to possess protecting properties as well as anti-plasmin activity in cortical neuron which were induced by N-methyldaspartate and fibrinogen and fibrin degradation (FDPs) assay, respectively as reported by Hong et al12. B. arundinacea is a rich source of antioxidants. Chauhan et al7 reported that the methanolic extract and different fractions of young shoots of Bambusa arundinace have prominent antioxidant property. B. arundinacea synthesized nanoparticles further enhanced the biological activities. For example, Kalaiarasi et al11 have synthesized the silver nanoparticles (AgNPs) using B. arundinacea leaf extract which showed enhanced cytotoxicity on PC-3 (lung cancer) and Vero normal cell lines. Jayarambabu et al15 prepared zinc oxide nanoparticles (ZnO NPs) of BA extract exhibited strong anticancer activity against MCF-7 cell line. Patel et al19 reported antiglycation potential of zinc oxide nanoparticles utilizing B. arundinacea leaf extract, inhibited the formation of AGEs, decreased the level of fructosamine and formation of glycosylated Hb.
Ethnomedicinal Uses
Bambusa arundinacea are arboreous grasses known to mankind since a long time and is utilized as a food and shelter by native people20, 21. It is used in various application such as making traditional and musical instruments, boat rafts, construction, furnitures and flooring, fencing and fodder for cattles, utensils for cooking, and in management of waste water22.
In folk medicine, Bambusa arundinacea is used for the treatment of many inflammatory disorders23. The plant stem and leaves are acidic and used as a laxative, in blood diseases, kapha, inflammation, piles and bile disorders24. It is also used in constringent and kidney disorders in Ayurveda. The extract of leaves bud is used in menstrual discharge, leaves infusion for eye wash and used internally for bronchitis, gonorrhoea and fever9. The acrid seed are used for liver dysfunction and urinary discharges2. The root ointment is a remedy for liver cirrhosis and tumors1. The leaves are emmenagogue and used for sciatica, fibrositis, gastic and liver diseases, lung bronchitis, and gonorrhea whereas flower juices is used in deafness and ear pain. The bark is used as a cure for eruptions25. The manna is sweet, acrid, tonic which is used for blood diseases, lung diseases such as asthma, bronchitis and tuberculosis. Besides this, it is also used for fever, haemoglobin deficient anaemia, hepatitis and leprosy. Tabasheer (dried bamboo sap) containing 97% silicon dioxide is used as a tonic for cough and asthma. The leaves of this plant are used by tribal women in Madras for abortion of a child by chewing it two times a day26. In Kanyakumari Kani tribes, the seeds of this plant increase the fertility and therefore it is in large demands for the improvement of fertility in this area27. The leaf juice is used for osteoarthritis, osteoporosis for making the cartilage and bones strong, for strengthening arterial walls, teeth and nails and reduces psoriasis and dermatitis. Traditional practitioner’s uses 2-3 cups of B. arundinacea Retz. leaf decoction three times a day for months to treat diabetes mellitus5.
Pharmacology
Bambusa arundinacea has been reported to exhibit important pharmacological activities viz. antiinflammatory, antidiabetic, anticancer, antifertility, antihelmintic, insecticidal, antimicrobial, etc. (Figure 1). These activities are discussed below.
 |
Figure 1: Biological activities exhibited by B. arundinacea |
Antiinflammatory, analgesic and antiulcer activity
B. arundinacea is reported for the treatment of many inflammatory conditions. It has been found that the methanolic leaves extract exhibited antiinflammatory activity on carrageenan and immunologicaly induced paw edema in Albino wistar rats5. The effect was found to be significant, compared to positive control, phenylbutazone. However, amalgamation of extract and phenylbutazone when administered orally in Albino rats resulted in enhanced antiinflammatory activity experimentally without any ulcerogenic effect. Therefore, the amalgamation of extract of this plant with modern medicationcould produce antiinflammatory drugs that could be of advantage in treating inflammatory problems such as rheumatoid and osteoarthritis. B. arundinacea ethanolic and hydroalcoholic extract displayed in vitro anti-inflammatory activity with IC50 of 700 and 212 μg/mL, respectively whereas ibuprofen displayed IC50 of 118.33 μg/mL28. Also, the ethanolic leaves extract at 100 mg/kg and 200 mg/kg exhibited promising analgesic and antipyretic effect in dose dependent manner29. These results indicate that B. arundinacea has the potential to reduce inflammation with less side effects.
Antimicrobial effect
B.arundinacea could be a possible source to obtain new and effective compound to treat bacterial and fungal infections. B. arundinacea has been found to possess antimicrobial activity against a number of bacterial and fungal strains. 2,6 dimethoxy-p-benzoquinone isolated from bamboo extract inhibits the growth of Candida albicans, Trichophyton interdigitale, Microsporum gypseum, Peniciilium. chrysogenumStaphylococcus aureus, Bacillus subtilis, and P. aeruginosa30.
Zubair et al14 reported that n-hexane and chloroform extract displayed significant antimicrobial activity against the tested pathogens while acetone, methanol and butanol extract were found to be inactive. Table 1 showed that n-hexane extract showed good inhibitory activity against E. coli, P. multocida, and B. subtilis with MIC 3.81 μg/mL, 5.28 μg/mL and 5.26 μg/mL, respectively whereas it was inactive on tested fungal strains. Chloroform extract displayed moderate inhibition on S. aureus and B. subtilis with MIC 15.5 μg/mL and 10.7 μg/mL, respectively while it was inactive against E. coli.Acetone extract was found to be active against G. lucidum with MIC = 4.00 μg/mL only while it did not show any inhibitory activity against A. alternata, E. coli, and S. aureus.
Table 1: Antimicrobial activity in terms of inhibition zones and minimum inhibitory concentration of B. arundinaceae leaves against selected bacterial and fungal strains..
|
Tested microorganisms |
||||||
|
Different organic extracts |
B. subtilis |
P. multocida |
(Diameter of inhibition Zone (IZ) S. aureus E. coli |
, mm) A. alternata |
G. lucidum |
|
|
n-hexane |
17.0±0.81b |
19.0±0.70b |
15.5±1.11d |
22.2±1.47b |
n.d |
n.d |
|
Chloroform |
17.0±0.70b |
16.0±1.80d |
19.0±0.70c |
n.d |
8.5±1.23c |
9.0±1.11c |
|
Ethyl acetate |
22.7±2.27b |
18.0±0.70bc |
n.d |
n.d |
n.d |
n.d |
|
Acetone |
18.5±0.5b |
16.2±1.47cd |
n.d |
n.d |
n.d |
24.2±5.26a |
|
n-butanol |
n.d |
n.d |
19.5±1.08c |
22.0±3.11b |
6.5±0.03d |
6.5±1.11b |
|
Absolute Methanol |
n.d |
n.d |
n.d |
n.d |
11±1b |
8.5±0.02b |
|
(0.5:9.5) water: methanol |
17.7±0.82b |
n.d |
22.0±1.82b |
n.d |
7.75±1.47cd |
8.5±1.11b |
|
(1:9) water: methanol |
n.d |
n.d |
n.d |
n.d |
6.0±1.58d |
n.d |
|
Control |
29.0±1.08a |
34±1.08a |
28.5±0.82a |
28.5±1.08a |
26.0±5.71a |
26±2.38a |
|
Minimum inhibitory concentration (MIC) mg/mL. |
||||||
|
Extracts |
B. subtilis |
P. multocida |
S. aureus |
E. coli |
A. alternata |
G. lucidum |
|
n-hexane |
5.62±0.12 |
5.28±0.16 |
22.4±0.32 |
3.81±0.5 |
n.d |
n.d |
|
Chloroform |
10.7±0.07 |
12.5±0.21 |
15.5±0.21 |
n.d |
10.6±0.38 |
10.5±0.40 |
|
Ethyl acetate |
3.02±0.25 |
6.48±0.29 |
n.d |
n.d |
n.d |
n.d |
|
Acetone |
12.4±0.11 |
12.1±0.12 |
n.d |
n.d |
n.d |
4.00±0.36 |
|
n-butanol |
n.d |
n.d |
15.8±0.22 |
2.81±0.23 |
18.4 |
15.0±0.04 |
|
Absolute Methanol |
n.d |
n.d |
n.d |
n.d |
7.4±0.40 |
11.4±0.04 |
|
(0.5:9.5) water: methanol |
10.4±0.17 |
n.d |
5.89±0.10 |
n.d |
11.8±0.07 |
11.4±0.46 |
|
(1:9) water: methanol |
n.d |
n.d |
n.d |
n.d |
18.4±0.36 |
n.d |
|
Control |
2.04±0.32 |
2.04±0.32 |
2.14±0.32 |
2.04±0.32 |
3.05±0.04 |
3.05±0.04 |
Data are expressed as the mean ± standard deviation; values having different letters differ significantly (p <0.05). n.d= not detected. Rifampcin and Fluconazole used as control for bacterial and fungal strains respectively
The hexane, acetone and hydroethanolic extract of this plant seeds have shown significant antimicrobial effect against various microorganisms8. Jayarambabu et. al15 reported the antimicrobial activity of ZnO nanoparticles using Bambusa arundinacea extract by Agar disc diffusion method against S. aureus and B. subtilis. As observed from figure 2, the synthesized ZnO nanoparticles showed enhanced zone of inhibition of 14, 16, 17, 19 mm and 12, 14, 15, 17 mm against S. aureus and B. subtilis, respectively. The ZnO NPs interaction between S.aureus and B. Substilis was studied by SEM to observe the morphology changes of bacteria and under ZnO NPs treatment. The normal S.aureus and B. substilis shows spherical and rod shape with smooth surface area, but ZnO NPs treated bacteria displayed irregular morphology and ruptured bacterial cell walls which indicate the interaction of bacteria and ZnO NPs, leading to demolition of bacterial cells.
 |
Figure 2: Antimicrobial activity of Bambusa Arundinacea mediated ZnO NPs against S. aureus and B.subtilis and SEM images of a) Control S. aureus b) ZnO NPs treated c) Control B.subtilis and d) ZnO NPs treated. |
Antifertility effect
The ethanolic extract of tender shoots of the plant exhibits antifertility effect16. The administration of plant extract at 300 mg/kg in male rats for 7 days caused a fertility reduction in male rats and the reduction was 15% for control rats and 23% for tested rats after a seven day recovery period. The protein serum as well as effect of oxaloacetic and pyruvic transaminase was non toxic on the rats.
Antidiabetic effect
B. arundinacea is endowed with antihyperglycemic potential. The aqueous ethanolic extract of leaves of B. arundinacea have been found to possess hypoglycemic activity. Joshi et al17 reported that BA leaves aqueous extract at 1000 mg/kg produced a promising decrease in blood glucose level to 260 mg/dL at 3 hr in STZ induced diabetic rats. Whereas in OGTT method, the extract increased blood glucose significantly at 30 minutes as shown figure 3.
 |
Figure 3: OGTT in euglycemic rats treated |
Nazreen et al6 reported the hypoglycemic effect of B. arundinacea leaves three fractions viz. ethanolic petroleum ether, chloroform in STZ induced diabetic rats showed that chloroform was the most potent in lowering the blood glucose at 150 mg/kg b.w., compared to glibenclamide, positive control. Also, this fraction did not cause any ulceration, decreased GSH level and increased LPO, AST and ALP enzymes (Figure 4).
 |
Figure 4: Effect of chloroform fractions and ethyl acetate fraction of Bambusa on blood glucose levels of diabetic rats (A). Effect of fractions on lipid peroxidation (B) |
Kumar et al31 reported that roots ethanolic extract of this plant displayed lowering in the blood glucose level in normal and hyperglycemic rats in alloxan and OGTT methods. Macharla et al18 reported that the aqueous ethanolic stem extract (200mg/kg) of Bambusa arundinaceae showed decrease in glucose level in alloxan induced diabetic rats. As observed from figure 5, Group II, III, IV, V and VII showed suppression of blood glucose level at 6 hrs significantly (p<0.01) compared to zero hour to its respective group.
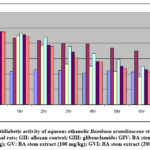 |
Figure 5: Antidiabetic activity of aqueous ethanolic Bambusa arundinaceae stem extracts. GI: Normal rats; GII: alloxan control; GIII: glibenclamide; GIV: BA stem extract (50 mg/kg); GV: BA stem extract (100 mg/kg); GVI: BA stem extract (200 mg/kg) |
Jayarambabu et al15 reported the antidiabetic effect of Bambusa arundinaceae (BA) extract and its ZnO nanoparticles. As shown in figure 6, BA extract and ZnO-NPs did not produce reduction in blood glucose levels in normal rats. Whereas in STZ induced diabetic rats, ZnO NPs significantly dropped the blood glucose levels at 200 mg/kg after 14 days of oral administration than BA extract. Moreover, the histopathology of liver after treatment with BA extract and ZnO-NPs displayed normal architecture, and the hepatocytes were observed (figure 7).
 |
Figure 6: (a) Effect of Bambusa arundinacea and ZnO NPs on fasted normal Wister rats, (b) Effect of Bambusa arundinacea and ZnO NPs on blood glucose levels in STZ induced diabetic Wister rats. |
 |
Figure 7: (a) Normal control, (b) Diabetic control, (c) Metformin standard, (d) Bambusa arundinacea group, (e) Zinc oxide nanoparticles group. |
Insecticidal activity
B. arundinacea has been reported to possess insecticidal property. The young shoots contain 0.03% hydrogen cyanide, benzene carboxylic acid and cyanogenic glucosides viz. taxiphyllin which are reported to be fatal on mosquito larvae. Bamboo shoots can resists insects, laterites, pH and hemp thus they have antiseptic and antilarval properties9.
Antiarthritic activity
B. arundinacea has been used for the treatment of rheumatoid arthritis. Anti-arthritic effect against complete Freund’s Adjuvant (CFA) induced arthritis in female rat were investigated13. The methanolic plant extract at 100, 200 and 300 mg/kg b.w. for 21 days displayed dose dependent reduction in erosion of bone and enlargement of spleen, compared to control, But the reduction was not as significant as dexamethasone (5 mg/kg i.p.)
Antihelmintic Activity
B. arundinacea root ethanolic extract has been endowed with antihelmintic property. The anthelmintic activity of the extract has been investigated in Pheretima posthuma. It has been observed that the extract at 10, 20 and 50 mg/mL were found to be lethal for Pheritima posthuma. The antihemintic effect was comparable to Albendazole, (10 mg/mL) and Piperazine citrate (15 mg/mL) positive control10.
Protective effects
B. arundinacea has been found to possess protecting properties as well as anti-plasmin activity in cortical neuron which were induced by N-methyldaspartate and fibrinogen and fibrin degradation (FDPs) assay, respectively12. It has been observed that B. arundinacea pyrolyzates treated neuronal cells increases the cell viability, compared to untreated cells. Furthermore, B. arundinacea pyrolyzates treated cortical neurons when stained by Hoechst 33342 showed decline in apoptosis following NMDA exposure. Besides this, pyrolyzates of B. arundinacea showed anti-plasmin activity. Pyrolyzate isolated from bamboo have antiapoptotic effect and it can be used for ischemic injury treatment as the amalgamation of NMDA receptor antagonists, GABAergic drugs ,glucocorticosteroids, and heparin are used for delay in post ischemic injury
Antioxidant property
Chauhan et al7 reported that the methanolic extract and different fractions of young shoots of Bambusa arundinace have prominent antioxidant property. Among the tested fractions (Table 2), n-butanol fraction contained highest phenolic (56.6±2.4 mg gallic acid equivalent (GAE)/g DW) and ethylacetate fraction contain highest flavanoid (47±2.6 mg Quercetin equivalent/g DW) content. From the DPPH scavenging activity as shown in Figure 8, Ethylacetate fraction dispalyed highest scavenging with IC50= 40.33±0.63 μM, compared to ascorbic acid (IC50= 30.69±2.1 μM. Also, this fraction showed superior anti-lipid peroxidation (IC50= 61.89±0.44 μM and reducing power as shown in Figure 8.
Table 2: Total Phenolic and Flavonoid content of different fractions of young shoots of Bambusa arundinaceae by Chauhan et al.
|
|
Phenolic content |
Flavonoid content |
|
n-hexane fraction |
3.5±1.4 |
9.5±1.1 |
|
Chloroform fraction |
10.2±1.8 |
8.5±0.7 |
|
Ethyl acetate fraction |
30.8±1.5 |
47±2.6 |
|
n-butanol fraction |
56.6±2.4 |
20±1.6 |
 |
Figure 8: DPPH free radical scavenging, antilipid peroxidation and reducing power of different fractions of young shoots of Bambusa arundinaceae and Ascorbic acid. Data are represented as mean ± SD (n = 3). |
Anticancer activity
Kalaiarasi et al11 have synthesized the silver nanoparticles (AgNPs) using B. arundinacea leaf extract and screened for cytotoxicity on PC-3 (lung cancer) and Vero normal cell lines. The AgNPs were characterized by FTIR, TEM and SEM. The FTIR spectra of BaAgNPs showed intense peaks at 3396 cm-1, 3211 cm-1, 2286 cm-1,1402 cm-1for NH stretching, S–H stretching, C=C stretching (alkenes) and C=C stretching (Figure 9)
 |
Figure 9: FT IR of AgNPs of Bambusa arundinaceae extract |
SEM analysis confirmed the synthesis of AgNPs in the reaction mixture. SEM images of BaAgNPs is shown in Figures 10A. The size of the synthesized AgNPs obtained ranged between 32 to 46.65 nm. TEM images for the biosynthesized AgNPs showed that the particles were spherically shaped with an average size ranging from 30 nm to 90 nm for AgNPs (Figures 10B).
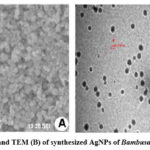 |
Figure 10: SEM (A) and TEM (B) of synthesized AgNPs of Bambusa arundinaceae extract |
The synthesized silver nanoparticles showed IC50 values of the PC3 cells and Vero cells were 73.57 and 84.88 µg/mL and 93.58 and 96.41µg/mL for BaAgNPs. The effect of NPs on the growth of normal cell lines (Vero) did not exhibit significant cytotoxicity at their lower concentrations (figure 11). The percentages of the apoptotic bodies by AO/EtBr staining were found to be 76% and 62% for BaAgNPs and BnAgNPs, respectively (figure 12)
 |
Figure 11: Cytotoxicity of synthesized AgNPs of Bambusa arundinaceae extract on PC-3 and Vero cell lines |
 |
Figure 12: Bright field and fluorescence microscopy image of IC50 concentration of AgNPs and in vitro leaf extracts of B. arundinacea and B. nutans applied to PC3 cell lines and incubated at 37 °C with 5% CO2 for 48 h. Cytotoxicity observed from bright field microscopy: |
Jayarambabu et al15 prepared zinc oxide nanoparticles (ZnO NPs) of BA extract exhibited strong anticancer activity against MCF-7 cell line. As shown in figure 13 (a,b), SEM images of the obtained nanoparticles were agglomerated, and most of them are in spherical. From TEM figure 13 (c,d), the sizes of the particles were observed in the range of ~7-20 nm.
 |
Figure 13: FESEM images of the ZnO NPs biosynthesized with plant extract (a-b) low and high magnifications. (c-d) TEM images of ZnO NPs at different magnifications |
Anticancer activity of Bambusa arundinacea mediated ZnO NPs against the MCF-7 cell lines is depicted in figure 14. It was observed that with an increase in ZnO NPs concentration the cell viability levels decreased. These results indicate that biosynthesized ZnO NPs exhibit good anticancer activity.
 |
Figure 14: Anticancer activity of Bambusa arundinacea mediated ZnO NPs. |
Miscellaneous
Zihad et al32 carried out the laxative activity of the ethanolic extract of shoot of B.arudinacea in mouse model. It was observed from figure 15 that the extract exhibited promising laxative potential by promoting watery faeces, increasing gastrointestinal motility and the amount of intestinal content, when compared with a negative control. The maximum activity (47.92%) was observed at 2h at the dose of 500 mg/kg b.w. Gastrointestinal transit at the dose of 250 and 500 mg/Kg was 67.18 and 60.03% respectively. This reports support the traditional use of shoot of B. arundinacea in constipation.
 |
Figure 15: Laxative activity of Bambusa arundinacea in mice. |
Patel et al19 reported that zinc oxide nanoparticles (ZnO NPs) utilizing B. arundinacea leaf extract inhibited the formation of AGEs, decreased the level of fructosamine and formation of glycosylated Hb. The antiglycation potential of zinc oxide nanoparticles, ZnO NPs which was prepared from zinc acetate using sodium hydroxide were investigated. The synthesized ZnO nanoparticles were characterized by FTIR, and TEM analysis as shown in Figure 16. The FT IR spectra of ZnO NPs showed a sharp and intense peak at 681cm-1 for Zn–O stretching, 1430 cm-1 for C-C stretching, 1600 to 1800 cm-1 for C=O, and 2854 cm-1 for O-H stretching of carboxylic acid. The Result of TEM showed that ZnO NPs are spherical and some oval particles with an aevarge size of 80 nm. The synthesized ZnO NPs were tested for degree of glycation of albumin by measuring fluorescence intensity. The increased fluorescent intensity in BSA/glucose reaction indicates the level of glycated BSA increased over a period of time. As observed from Figure 17, ZnO-Nps showed significant inhibition on glycation reaction , compared to control. Also, ZnO NPs treated glycation of albumin and AG treated glycation of albumin showed significant inhibition of glycated albumin (43.41% and 55.19% respectively) when compared to the untreated glycated control. Moreover, these nanoparticles inhibit the glycosylation of hemoglobin significantly when compared to the control (figure 18). Table 3 illustrates the preventive effect of green synthesized ZnO NPs on the formation of hemoglobin glycosylation. The study suggests biologically synthesized zinc nanoparticles showed the strong antiglycation potential and considered as a potential source of therapeutic agents for AGEs related disorder.
 |
Figure 16: (A) FT-IR spectrum of biologically synthesized ZnO NPs; (B) TEM images of ZnO NPs |
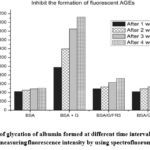 |
Figure 17: Degree of glycation of albumin formed at different time interval measured relatively by measuring fluorescence intensity by using spectro fluorometry. |
 |
Figure 18: Inhibitory effect of biosynthesized ZnO-NPs from bioactive fraction F3 obtained from crude Bambusa arundinacea leaf extract on glycosylated Hb. |
Table 3: Inhibitory effect of biologically synthesized ZnO NPs on the formation of glycosylated Hemoglobin expressed in percentage
|
No. |
Absorbance |
Percentage of Inhibition |
|
Control (Hb + glucose) |
0.723 ± 0.051 |
0 |
|
In presence of ZnO NPs with Hb + glucose |
0.448 ± 0.082 |
39.73% |
Phytochemistry
Phytochemical investigation of B. arundinacea Retz has afforded different type of chemical constituents (Figure 19). Tabasheer (dried bamboo sap) is white, crystalline and sweet siliceous matter near the shoots joint 33,34. Shoot of the plant contain various substances such as ethanedioic acid, hydrogen cyanide, waxes, and benzene carboxylic acid35, fiferuloyl arabinose hexasaccharide and taxiphyllin (1)36. The seed of the plant contains various amino acids and other essential organic molecules such as phenylalanine, thiamine, lysine, leucine, histidine, aginine, cysteine, isoleucine, lysine, valine, methionine, tyrosine, riboflavin, niacin and thiamine. The leaves of the plant contains mainly gluteline, betain, methionine, cholin, lysine, nuclease, urease and proteolytic enzymes.37
 |
Figure 19: Compounds isolated from B. arundinacea |
Tanaka et al38 have reported isoorientin (2), tricin glucoside (3), p-hydroxy cinnamic acid (4), lyoniresinol 3α-O- βD- glucopyranoside (5), 3, 4, 5-trimethoxyphenol-β-D-glucopyranoside (6), koaburaside (7), 2, 6 dimethoxy-p-benzoquinone (8) and sinapic aldehyde (9) from the leaves of the plant. The plant also contains 3′,3,6,7-tetramethoxy-4′,5,8-trihydroxy flavones (10), p-anisidic acid (11), 4′-hydroxy 3-hydroxy flavane (12) and hemicelluloses39, 40. Nazreen et al isolated 17, 20, 20-tri demethyl-20α-isopranyl oleanane, (13), eicosanyl dicarboxylic acid (14) stigmasterol (15), Stigmastrol glucopyranoside (16), α-amyrin acetic acid (17) and ursolic glucopyranoside (18) from the chloroform fraction of this plant41.
Conclusions
Bambusa arundinacea is endowed with important pharmacological activities such as antiinflammatory, antidiabetic, antioxidant, antimicrobial, insecticidal, antihelmintic, and anticancer. It is also rich in phenolic and flavonoid bioactive molecules. Moreover, the nanoparticles using this plant extract have shown remarkable enhancement in various biological activities. Although, this plant has not been explored much scientifically, very few reports are available on this plant. The present review article will help the readers to explore more on this plant in various filed of nanotechnology, biotechnology and phytochemistry as it contains promising bioactive molecules.
Acknowledgments
Author acknowledges Departmemt of Chemistry, Al-Baha University for the required facilities.
Conflicts of Interest
There is no conflict of interest
Funding Sources
The review article received no funding
References
- Anonymous, The Wealth of India, Raw materials, Council of Scientific and Industrial Research, New Delhi, 1988, 2B, 1-38.
- Ghani, A. Medicinal Plants of Bangladesh, 2nd ed. 2003.The Asiatic Society of Bangladesh, Bangladesh.
- Ohrnberger, D. The Bamboos of the World: annonated nomenclature and literature of the species and the higher and lower taxa. 1st ed. Amsterdam,Elsevier, 1999, 1–6.
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants, International Book Distributers, Dehradun, India, 1990, 4, 2724–2727.
- Muniappan, M.; Sundararaj, T. Antiinflammatory and antiulcer activities of Bamboosa arundinaceaa. J. Ethnopharmacol. 2003, 188, 161-167.
CrossRef - Nazreen, S.; Kaur, G.; Alam, M.M.; Haider, S.; Hamid, H.; Alam, M.S. Hypoglycemic activity of Bambusa arundinacea leaf ethanolic extract in streptozotocin induced diabetic rats Pharmacologyonline 2011, 1, 964-972.
- Chauhan, K.N.; Shah, B.; Mehta, A.A. Antioxidant activity of different fractions of methanolic extract of young shoots of Bambusa arundinacea.World J. Pharmacy Pharma. Sci.2017, 6, 2492-2503.
CrossRef - Thamizharasan, S.; Umamaheswari, S.; Hari R.; S. Antimicrobial activity of Bambusa arundinacea seeds. Int. J. Pharm. Bio. Sci.2015, 6, 540-546
- Sharma, B.D. Anonymous. Flora of Maharashtra State, Monocotyledones, Botanical Survey of India, Calcutta, 1996, 412.
- Kumar, H.K.S.; Raju, M.B.V.; Dinda, S.C.; Sahu, S. Evaluation of anthelmintic activity of Bambusa arundinacea. Asian J. Pharm. Tech. 2012, 2, 62-63.
CrossRef - Kalairasi, K.; Prasannaraj, G.; Sahi, S.V.; Venkatachalam, P. Phytofabrication of biomolecule-coated metallic silver nanoparticles using leaf extracts of in vitro-raised bamboo species and its anticancer activity against human PC3 cell lines. Turk. J. Biol.2015, 39, 1406-1410.
CrossRef - Hong, E.J.; Jung, E.M.; Lee, G.S.; Kim, J.Y.; Na, K.J.; Park, M.J.; Kang, H.Y.; Choi, K.C.; Seong Y.H.; Choi, I.G.; Jeung E.B. Protective effects of the pyrolyzates derived from bamboo against neuronal damage and hematoaggregation. J. Ethnopharmacol. 2010, 2, 594–599.
CrossRef - Rathod, J.D.; Pathak, N.L.; Patel, R.G.; Jivani, N.P.; Patel, L.D.; Chauhan, V. Ameliorative effect of Bambusa arundinacea against adjuvant arthritis with special reference to bone erosion and tropical splenomegaly. J. Drug Deliv. Therapeu. 2012, 2, 141-145
- Zubair, M.; Bibi, Z.; Rizwan, K.; Rasool, N.; Zahoor, A.F.; Riaz, M. In Vitro Antimicrobial and Haemolytic Studies of Bambusa arundinaceae leaves. J. App. Pharma. Sci.2013, 3, 111-115.
CrossRef - Jayarambabu, N.; Rao, T.V.; Kumar, R. R.; Akshaykranth, A.; Shanker, K.; Suresh, V.Anti-hyperglycemic, pathogenic and anticancer activities of Bambusa arundinacea mediated Zinc Oxide nanoparticles. Mat. Today Commun. 2021, 26, 101688.
CrossRef - Vanithakumari, G.; Manonayagi, S.; Padma, S.; Malini, T. Antifertility effect of Bambusa arundinacea shoot extracts in male rats. J. Ethnopharmacol.1989, 25, 173-80.
- Joshi, R.K.; Patil, P.A.; Mujawar, M.H.K.; Kumar, D.; Kholkute, S.D. Hypoglycaemic activity of Bambusa arundinacea leaf aqueous extract in euglycemic and hyperglycaemic Wistar rats. Pharmacologyonline 2009, 3, 789-795.
- Macharla, S.P.; Goli, V.; Santhosha, D.; Ravindra Nath, A. Antidiabetic activity of Bambusa arundinacea stem extract on alloxan induced diabetic rats. Int. J. Chem. Biol. Phy.Sci. 2012,2, 832-835
- Patel, H.V.; Macwan, D.; Khambolja, D.B.; Bariya, H.S. In-vitro antiglycation activity of zinc oxide nanoparticles synthesized from the bioactive fraction of Bambusa arundinacea leaf extract. Res. J. Pharma. Biol. Chem. Sci. 2020, 11, 48-60.
- Sastry, C. Bamboo and global development, special BAMTECH, Cane Bamboo News, 2003, 1, 18-19.
- Panigrahi, S.K. The role of bamboo in promotion of ecological security, special BAMTECH, Cane Bamboo News 2003, 1, 19-20.
- Sharma, Y.M.L. Bamboo in Asian Pacific Region, In. Bamboo research in Asia, World Publication, Singapore, 1980, 99-120.
- Yusuf, M.; Begum, J.; Hoque, M.N.; Chowdhury, J.U. Medicinal Plants of Bangladesh, Second edition. Bangladesh Council of Scientific and Industrial Research Laboratories Chittagong, Chittagong-4220, 2009, Bangladesh.
- Nadkarni, K.M. Indian Materia Medica. Popular Prakashan, 2000, Bombay, India.
- Chopra, R.N.; Chopra, I.C.; Handa, K.L.; Kapur, L.D. Indigenous Drugs of India, U.N. Dhur and Sons Pvt. Ltd., Calcutta, 1958, 289, 665.
- Bhaduri, B.; Ghose, C.R.; Bose, A.N.M.; Basu, U.P. Antifertility activity of some medicinal plants. Ind.J. Exp. Bio. 1968, 6, 252-253.
CrossRef - Kiruba, S.; Jeeve, S.; Manohardas, S.; Kannan, D. Bamboo seed as a mean to sustenance of the indigenous community. Ind. J. Trad. Knowl. 2007, 6, 199-203.
- Vanitha, B.; Kumar, R.R.; Duraiswamy, B.; Gnanavel, G.; Neelamma, G. Estimation of total phenol and total flavanoid content with Antioxidant and anti-inflammatory activity of Bambusa arundinacae (bamboo shoot) grown in Nilgiris. Der Pharma Chemica, 2016, 8, 307-316.
- Jawaid, T.; Kamal, M.; Singh, J. Analgesic and antipyretic activities of ethanolic extract of Bambusa arundinacea leaves.IJPSR2017, 8, 2867-287.
CrossRef - Nishina, A.; Uchibori, T. Agric. Biol. Chem. Antimicrobial Activity of 2,6-Dimethoxy-p-benzoquinone, Isolated from Thick-stemmed Bamboo, Its Analogs. 1991, 55, 2395-2398.
- Sundeepkumar, H.K.; Raju, M.B.V.; Dinda, S.V.;Sahu, S.K. Antihyperglycemic activity of Bambusa arundinacea. Rasayan J. Chem. 2012, 5, 112-116
CrossRef - Zihad, S.M.N.K.; Saha, S.; Rony, M.S.; Banu, S.; Uddin, S.J.; Shilpi, J.A.; Grice, I. D. Assessment of the laxative activity of an ethanolic extract of Bambusa arundinacea (Retz.) Willd. Shoot. J. Ethnopharmacol. 2018, 214, 8-12.
- Vaidya B., Some Controversial Drugs in Indian Medicine, Chaukhambha Orientalia, Varanasi, 1982, 2, 203-207.
- Watt G., A Dictionary of the Economic Products of India, Reprinted edition, Periodical Expert, Delhi, 1972, 1, 383-391.
CrossRef - Ghosh, N.N.; Ghosh, S.; Chopra, R.N. Chemical and pharmacological examination of the young sprouts of Bambusa arundinacea. Arch. Pharm. Berl. 1938, 276-351.
CrossRef - Ishii, T. Isolation and characterization of a diferuloyl arabinoxylan hexasaccharide from bamboo shoot cell-walls. Carbohydrate Res. 1991, 219, 15-22.
- Chatterjee, A.; Pakrashi, S. C. The Treatise on Indian Medicinal plants, National Institute of Science Communication, New Delhi, 2001, 6, 50-51
CrossRef - Tanaka, N.; Wada, H.; Fuchino, H.; Inoue, T.; Yasuda, D.; Kanda, S.; Kiyokawa, C.; Ashida, N.; Suzuki, T.; Yamazaki, K.; Hakamatsuka, T. Constituents of Bamboos and Bamboo grasses. Yakugaku Zashi. 1998, 118, 332-337.
- Guha, S.R.D.; Pant, P.C. 1967. Hemicelluloses from bamboo (Bambusa arundinacea). Indian Pulp Pap. 1967, 21, 439–440.
- Sundeep Kumar, H.K.; Raju, M.B.V.; Acharyya, S.; Mishra, B. Isolation of phytoconstituents from the roots of Bambusa arundinacea. Eur. J. Biomed. Pharm. Sci.2016, 3, 343–347.
- Nazreen, S.; Alam, M.S.; Hamid, H.; Kaur, G.; Alam, M.M., Haider, S.; Shafi, S. Phytochemical investigation of Bambusa arundinacea Retz. Int. J. Nat. Prod. Sci. 2011; 3, 1-7.

This work is licensed under a Creative Commons Attribution 4.0 International License.










