Phycochemical Characterization of Marine Macroalga Sargassum Tenerrimum Collected from Beyt Dwarka, Western Coast of Gujarat, India
Microbiology Department, Smt. S. S. Patel Nootan Science and Commerce College, Sankalchand Patel University, Visnagar 384315, India.
Corresponding Author E-mail: hskalasariya.fsh@spu.ac.in
DOI : http://dx.doi.org/10.13005/ojc/380218
Article Received on : 03-Mar-2022
Article Accepted on : 04-Apr-2021
Article Published : 14 Apr 2022
Reviewed by: Dr. Md Nurul Islam Siddique
Second Review by: Dr. I. Nizamov
Final Approval by: Dr. Tawkir Sheikh
Industries are moving towards finding a natural source of functionally active constituents which is better and safer materials to fulfill customers’ demand. Marine algae contain a huge variety of biologically active compounds and express a promising role in different applications. Hence, the present study was carried out to characterize different biochemicals from brown alga Sargassum tenerrimum by FTIR, GCMS, HRLCMS Q-TOF, and ICP AES technique. First, the macroalga was collected from the Beyt Dwarka sea site, the Western coast of Gujarat, India. In FTIR, different types of bioactive functional groups were characterized as accountable for different beneficial components whereas ethanolic and methanolic extracts of S. tenerrimum reported fourteen and nineteen different beneficial phycocompounds in GCMS analysis, respectively. In HRLCMS Q-TOF analysis, two compounds were found carbohydrate derivatives and fifty-nine different compounds were determined to be different types of phycocompounds. Additionally, in the ICP AES study, Silicon was found to be high up in amount whereas Copper remained the minimum among studied elements. Moreover, the HRLCMS-QTOF study of amino acids reported that Glutamic acid (132.13 nmol/mL) was found to be the highest whereas Isoleucine (0.70 nmol/mL) was found to be the least amidst measured twenty-one amino acids. Bioactive potentials of these compounds have been reported in many previous studies. The inclusion of macroalgae-derived constituents in different applications has been broadly rising due to their bioactive potentials.
KEYWORDS:Biochemical; FTIR; GCMS; HRLCMS; Macroalga; Sargassum tenerrimum
Download this article as:| Copy the following to cite this article: Kalasariya H. S, Patel N. B. Phycochemical Characterization of Marine Macroalga Sargassum Tenerrimum Collected from Beyt Dwarka, Western Coast of Gujarat, India. Orient J Chem 2022;38(2). |
| Copy the following to cite this URL: Kalasariya H. S, Patel N. B. Phycochemical Characterization of Marine Macroalga Sargassum Tenerrimum Collected from Beyt Dwarka, Western Coast of Gujarat, India. Orient J Chem 2022;38(2).Available from: https://bit.ly/3jF0yL8 |
Introduction
High energy requirements, natural, nutritious, harmless, and nontoxic compounds in different types of products such as food, cosmetics, dairy, pharma as well as aquaculture supplements promote the search for alternative natural renewable and sustainable materials 1. These compounds are eco-friendly, sustainable, and economically cheaper. Mainly, great attention towards using biomass for overcoming the demerits of harmful and synthetic components. There are different types of natural resources utilized for different applications but among them, marine macroalgae are well known due to having high nutritional compositions as well as the presence of bioactive compounds than terrestrial plants and animals 2. Marine macroalgae are similarly known as seaweeds, which are macroscopic, multicellular, eukaryotic, marine photosynthetic microorganisms. It is highly diversified in length and morphology 3. They are mainly growing in brackish water as well as coastal zone (water close to the sea coast). It is found attached to rocky sea fronts, sand gravels, or floating freely 4. Some species of macroalgae can reach up to 65 meters in length. Seaweed species occupy several ecological areas. Some algal species are found wet in seafoam whereas other algae can attach to substratum several meter deeps. Its growth expands for miles to the deeper sea 5. Moreover, other species have become to live in tide pools and must tolerate hasty changes in environments such as temperature, drying, and salinity 6. According to pigment composition, it can be divided into three types: red algae, brown algae, and green algae belong to Rhodophyta, Phaeophyta, and Chlorophyta, respectively. Among these three types, the Plantae kingdom comprises Chlorophyta and Rhodophyta whereas the Chromista kingdom comprises Phaeophyta 7-10. Red algae mainly contain phycocyanin, phycoerythrin, chlorophyll a, and carotenoids such as lutein, zeaxanthin, and beta carotene. Brown algae are well known for chlorophyll (a & c), and carotenoids such as fucoxanthin whereas chlorophyll a, b, and carotenoids are found in green algae 11. As a result of the diversified components, macroalgae are extensively useful in many regions of the world as an ingredient of human food and animal feed, pharmaceutical, cosmeceutical, agriculture, and many more 12. Marine algae produce proteins, amino acids, carbohydrates, fatty acids (primary metabolites) which play their role in reproduction and normal growth as well as phenols, sterols, minerals, vitamins, and some other active constituents (secondary metabolites) found under stressful environmental factors such as Ultraviolet exposure, temperature, high salinity or pollutants 13-16.
Many marine algae have been completely practiced by humans for different uses as food for humans, animal feed, fertilizers, dairy and food industries, etc. China and Europe have had old practices to collect marine algae for nutritional food purposes as long as 500 B.C. 17 Macroalgae can be used as an alternative to today’s foods that we consume. It gives us high nutritional content, high caloric, and lesser fat. Macroalgae provide a good amount of carbohydrates, vitamins, minerals, and fibers that improve intestinal transition and reduce the cholesterol amount in the blood 18. They also have an old use as an animal food but it depends on the algal species, variable composition, time of collection, and habitat as well as some other atmospheric factors such as light intensity, temperature, and nutrient availability 19. Macroalgae have wide application in the agriculture sector to improve crop productivity. It contributes certain elements such as nitrogen, phosphorus, potassium, magnesium, iron, and calcium. Besides, it is utilized to regulate the pH of the soils and possesses plant growth-promoting components such as auxins, gibberellin, cytokinin, and others 20. It is also utilized to combat different plant infections caused by different insects and fungal pathogens 21. Marine algae-derived bioactive molecules are widely used in various industrial applications like, shows anticoagulant, antitumor, anti-inflammatory, antimicrobial, antithrombotic, and immunomodulatory 22,23 Seaweed based bioactive compounds are useful in making soft drinks, glass, soaps, shampoos, dyes as well as biopolymers 24-26. The phycocolloids exhibit an important action in pharmaceutical applications as a stabilizer, a thickener, as well as an, impart antimicrobial component 27. Macroalgae are widely advantageous in the cure and hair washing due to their biding ability with hair proteins 28. Previous studies reported that it can be used to treat warms infection, wound healing, treatment of gastritis, diarrhea, menstrual disorders, hypertension, skin disorders, ulcers, and syphilis 29-31. Moreover, it is also useful in the skin cosmeceutical applications such as antiwrinkle, photoprotection, skin whitening, anti-aging, moisturizer, antiacne, and other skin benefits 32,33.
Brown macroalga Sargassum tenerrimum is very commonly found on the western coast of Gujarat, India. The thallus of S. tenerrimum is soft, slender, light brown to greening brown in the color of about 30-40 cm in height. It has a very short main axis, terete, and alternatively arranged primary cylindrical branches with branchlets. Vesicles of S. tenerrimum are stalked, spherical, pointed, and ovate about 3-4 mm broad. It is fusiform, slightly compressive, edged, and possess toothed at the margin. Its holdfast discoid and grow on rocks in subtidal regions 34,35. The present study aims to carry out the characterization of brown alga S. tenerrimum derived bioactive components such as fatty acids, amino acids, carbohydrates derivatives, as well as mineral content analysis by using different characterization techniques to show its phycochemical profile.
Materials and methods
 |
Figure 1: Flow chart of the present study. |
Sample collection
The sample of marine macroalga Sargassum tenerrimum was done in sterile plastic bags from Beyt Dwarka sea site (22°28’37.7″N 69°07’48.2″E), the West coast of Gujarat, India. (Figure 2-a) After collection, the sample was transferred (10°C) to the laboratory. This sample collection was carried out by handpicking from the low tide zone and environmental surroundings as follows: 0% Precipitation, 55% Humidity, 12 km/h Wind, and 27°C temperature. Then, this sample was cleaned with deionized water to separate extraneous materials and debris and kept shed dry at room temperature for six to seven days. This dried material was reduced to a fine powder by a mechanical grinder and preserved at freezing temperature until the next analysis.
Identification of Sargassum tenerrimum
The sample identification was accomplished by taking the help of Dr. N. Joshi, at the Aquaculture department, Veraval, Gujarat-India. The authenticated image is illustrated in Figure 2-b.
 |
Figure 2: (a) The geographical location of the sample collection site (in respect to Gujarat state, India); (b) Isolated sample of Sargassum tenerrimum (Chlorophyta). |
Phycocompounds characterization study by GCMS technique
Extract Preparation
0.5 kg of dry S. tenerrimum’sdried powder was added in 80% ethanol for 24 hours by the continuous hot percolation in the Soxhlet procedure. Afterward, the extract was then filtered and dried at 40°C for 24 hours in a Hot air oven (REMI, India) to remove the excessive ethanol solvent (Sigma-Aldrich, India). This obtained filtrate was fixated to dryness at 20°C in reduced pressure (150 mbar) by using a vacuum evaporator (Sigma scientific, India). The above-concentrated extract was isolated in the air-free vessel and kept at freezing temperature (-20°C, Esquire Biotech, India).
GCMS Characterization study
For the characterization study, the T100GCV GC model and EB 5 column were used for chromatographic separation. GC specifications such as Helium (He) used as a Carrier gas; 1 mL/min flow rate; 200°C Injector operation; 50–250°C Column oven temp.; Injection mode: 10 °C/min.
AccuTof Mass from the jeol model was used in mass spectrometry analysis. MS specifications are as follows: 70 eV Ionization voltage; 250 °C ion source temperature; 250 °C interface temperature; 50–600 mass units mass range. The obtained gas chromatogram and mass spectra of the screened compounds were put in comparison with the available data of known compounds in the National Institute of Standard and Technology (NIST) library ver. 2005.
Characterization of Fatty Acids
The methanol solvent (99.8%) was used to prepare an extract from the shed dried fine powder of S. tenerrimum. This extract was prepared 10% (ratio 1:10) in a flask for three days. The above mixture was filtered out in another sterile vessel. After filtrates collection, the surplus solvent was eliminated by vacuum evaporator using fresh methanol solvent. This process was carried out twice with the same sample residues using fresh methanol. And this extract was used for further study. The GCMS characterization study was carried out by fixing the HP-5 column. Other specifications of GC and MS are used the same as above. The obtained gas chromatogram and mass spectra of each phycocompounds were put in comparison with known data in the NIST library (ver. 2005). The chemical identity, mol. weight and chemical framework of all obtained phycocompounds were regained and the percentage peak area was also calculated.
Functional Groups characterization study by FTIR
Total 5 mg of shed dried S. tenerrimum’s a fine powder added with FTIR grade KBr (Potassium bromide) and evenly mixed to get a homogenized texture. This mixture was then placed in the mechanical mold using a sterile spatula and pressed by mechanical support for 30 seconds. This pellet of the mixture was conditioned on the pan proceeded for IR study. In this FTIR study, 3000 Hyperion Microscope with Vertex 80 FTIR model (Bruker, Germany) and 400–4000 cm–1 scanning range were used. The peak ratio (the peak value in the IR spectrum) was used to separate the functional groups of components.
Elemental analysis by ICP AES procedure
Digestion and extraction procedure
All standard grade reagents (purchased from Sigma-Aldrich, India) were used in this analysis. About 50 milligrams of S. tenerrimum’s powder was added into the TFM (modify PTFE-PolyTetraFluoroEthylene-) vessels. Following this, the mixture of reagents (3 mL HCl + 1 mL HNO3 + 1 mL HF + 1 mL H2O2) was added and the vessel was closed immediately. The digestion of this mixture was carried out in a microwave digester (Titan Microwave system, India) based on the below specifications: 15 minutes hold time 10 min with 130 degrees, and following 190 degrees ramp time. After cooling this hot vessel at 70°C, the sample mixture was vented and opened. By using Milli-Q water, made total volume up to 25 mL and then shaken completely to dilute the rest of the particles adhered to the vessel’s wall. Blank was also kept for hydrolysis without adding the sample. The ICP-AES (Inductive Couple Plasma-Atomic Absorption Emission Spectrometer) instrument was considered to measure the amount of different elements. Model of the instrument: ARCOS, Simultaneous ICP Spectrometer;
Amino acid analysisAcid digestion and detection.
0.1 gram of S. tenerrimum’s fine powder was weighed and added into the 12 mL of 6N hydrochloric acid in it and the mixture was tightly packed after adding pure N2 gas. Put this test tube at 120°C temperature for16 hours in a hot air oven (REMI, India) for digestion. After hydrolysis, filtration was carried out and flash evaporation was done to eliminate excess hydrochloric acid. 0.05 N HCl was used to make a definite amount. Its filtration was carried out by a Whatman filter (0.45µ size). The filtrate was used as a sample for analysis. Moreover, standard amino acids were also run to get a standard chromatogram. Specifications such as, model of the instrument: 6550 iFunnel QTOFs (Quadrupole Time of Flights), Agilent Technologies, USA; Column details: Poroshell HPH C18 (4.6 ×100 mm), 2.7 µ; 60°C temp. in the oven; analysis mode: non-switch flow.
Phycocompounds analysis by HRLCMS-QTOF
Acid Hydrolysis and detection
The uniform fine powder was obtained by drying the algal sample at 40 °C for 48 hours. A total of 0.1 gram of the selected macroalgal sample was placed in a sterile airtight tube and add 10 mL of 2M HCl containing 1% phenol. Then, the tube was tightly closed in presence of N2 gas and kept at 80°C for 3 hours in a Hot air oven, allowing it to cool and Whatman no. 41 paper was used to carry out vacuum filtration. The obtained filtrate was diluted to make a final volume of 25 mL with ultrapure water which was again filtered to get the hydrolysate. Different phycocompounds were analyzed by the HRLCMS QTOF technique. Specifications include,
Model of the instrument: 6550 iFunnel Q-TOFs, Agilent Technologies, USA; Column detail: Hypersil GOLD C18 (100 mm × 2.1 mm × 3 μm); 250°C Gas Temp.; 3 L/min Flow of gas; 35 psig Nebulizer; 300°C Sheath Gas Temperature;
Results
Characterization of phycocompounds
Total fourteen different phycocompounds were identified in S. tenerrimum’sethanolic extractbased on retention time (RT) in minutes, percentage peak area (PA), mol. formula, and mol. weight. A gas chromatogram of S. tenerrimum’s ethanolic extract is illustrated in figure 3. PubChem ID, mol. formula, Kovats Index, mol. weight, % peak area, RT (min), and SMILE structure for each compound are reported in table 1. Among identified phycocompounds, 1-O-butyl 2-O-(6-ethyloctan-3-yl) benzene-1,2-dicarboxylate is showed the highest percentage peak area (23.33%) with 23.23 minutes retention time whereas Propanoic acid, 2-[(1-cyclohexylethyl)carbamonyl]- methyl ester compound reported the lowest percentage peak area (0.47%) with 21.19 minutes retention time. The chemical framework of each compound is illustrated in supplementary data Table S1.
 |
Figure 3: The Gas chromatogram was obtained from the ethanolic extract of S. tenerrimum. |
Table 1: Chemical information of phycochemicals found in ethanolic extract of S. tenerrimum.
|
No. |
Name |
PubChem Id |
Mol. Formula |
Mol. Wt. (g/mol) |
Retention Time (Min) |
Kovats Index (Iu) |
Peak Area % |
SMILE |
|
1 |
1-O-tetradecyl 4-O-(2,3,6-trichlorophenyl) butanedioate |
91701556 |
C24H35Cl3O4 |
493.9 |
8.19 |
3358 |
9.75 |
CCCCCCCCCCC |
|
2 |
3-Hexanone, 2,5-dimethyl- |
15901 |
C8H16O |
128.21 |
12.41 |
8241 |
8.22 |
CC(C)C |
|
3 |
Pentanal |
8063 |
C5H10O |
86.13 |
16.42 |
707 |
4.25 |
CCCCC=O |
|
4 |
1,3-Propanediamine, N-methyl- |
80511 |
C4H12N2 |
88.15 |
20.7 |
860 |
1.72 |
CNCCCN |
|
5 |
Pentadecanal- |
17697 |
C15H30O |
226.4 |
20.77 |
1701 |
13.56 |
CCCCCCCC |
|
6 |
Propanoic acid, 2-[(1-cyclohexylethyl)carbamonyl]- methyl ester |
541974 |
C13H23NO3 |
241 |
21.19 |
1816 |
0.47 |
CC(C1CCCCC1) |
|
7 |
[s]-{+}-1-cyclohexylethylamine |
5325951 |
C8H17N |
127.23 |
21.52 |
1059 |
1.91 |
CC(C1CCCCC1)N |
|
8 |
1-O-butyl 2-O-(6-ethyloctan-3-yl) benzene-1,2-dicarboxylate |
6423866 |
C22H34O4 |
362.5 |
23.23 |
2505 |
23.33 |
CCCCOC(=O) |
|
9 |
t- Boc-sarcosine |
83692 |
C8H15NO4 |
189.21 |
23.77 |
1305 |
1.95 |
CC(C)(C)OC(=O) |
|
10 |
2-Pyrrolidinone,1-phenyl-4-[1-(phenylmethyl)-1H-1,3-benzimidazol-2-yl]- |
2947212 |
C24H21N3O |
367.4 |
26.02 |
3277 |
1.95 |
C1C(CN(C1=O) |
|
11 |
Benzyl icosanoate |
562252 |
C27H46O2 |
402.7 |
31.54 |
2949 |
4.24 |
CCCCCCCCCCCCC |
|
12 |
Benzyl oleate |
5368218 |
C25H40O2 |
372.6 |
34.09 |
2758 |
0.56 |
CCCCCCCCC |
|
13 |
1,1,3,3,5,5,7,7,9,9,11,11-Dodecamethylhexasiloxane |
6329090 |
C12H36O5Si6 |
428.92 |
34.33 |
1341 |
3.99 |
C[Si](C)O[Si] |
|
14 |
Propane, 1,3-bis(octadecyloxy)- |
624534 |
C39H80O2 |
581.1 |
34.60 |
4050 |
6.22 |
CCCCCCCCCCCC |
1-O-tetradecyl 4-O-(2,3,6-trichlorophenyl) butanedioate and 1-O-butyl 2-O-(6-ethyloctan-3-yl) benzene-1,2-dicarboxylate are found to be carboxylic acid derivatives. 1,3-Propanediamine, N-methyl- & [s]-{+}-1- undecyl ester are amine derivatives and primary aliphatic amine, respectively. Moreover, t- Boc-sarcosine and 2-Pyrrolidinone,1-phenyl-4-[1-(phenylmethyl)-1H-1,3-benzimidazol-2-yl]- are N-methylglycine and 2-Pyrrolidone derivative, respectively. Hydrocarbon derivative Propane, 1,3-bis(octadecyloxy)- was found whereas Pentanal; Benzyl icosanoate; Benzyl oleate; Propanoic acid, 2-[(1-cyclohexylethyl)carbamonyl]- methyl ester; Pentadecanal-; and 3-Hexanone, 2,5-dimethyl- were also detected that belong to class fatty acid and fatty acid like molecule. Lastly, 1,1,3,3,5,5,7,7,9,9,11,11-Dodecamethylhexasiloxane; is found to be Polymers (Siloxanes).
Characterization of fatty acids and derivatives
Different fatty acids and derivatives are identified in this characterization depending on RT (in min), % peak area, mol. formula, and mol. weight. The gas chromatogram for the methanolic extract is illustrated in figure 4 whereas nineteen different phycocompounds were identified which are tabulated in table 2 with its chemical information. Among identified phycocompounds, cis-Vaccenic acid is found to be the considerable compound that had the large value of % peak area (52.96%) with 19.68 minutes retention time whereas 9-Octadecenamide compound reported the lowest percentage peak area (0.29%) with 27.41 minutes retention time.
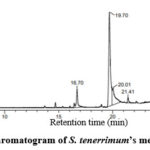 |
Figure 4: The Gas chromatogram of S. tenerrimum’s methanolic extract. |
Table 2: Chemical information of phycochemicals found in S. tenerrimum’s methanolic extract.
|
No. |
Name |
PubChem ID |
Mol. Formula |
Mol. Wt. (g/mol) |
RT (Min) |
Kovats Index (Iu) |
Peak Area % |
SMILE |
|
1. |
Phytol |
5366244 |
C20H40O |
296.5 |
14.69 |
2045 |
1.36 |
CC(C)CCCC(C) |
|
2 |
Methyl 3,5-bis(tert-butyl)-4-hydroxyhydrocinnamate |
62603 |
C18H28O3 |
292.4 |
16.43 |
2134 |
1.45 |
CC(C)(C)C1= |
|
3 |
n-Hexadecanoic acid |
985 |
C16H32O2 |
256.42 |
16.70 |
1968 |
6.69 |
CCCCCCCCCC |
|
4 |
cis-Vaccenic acid |
5282761 |
C18H34O2 |
282.5 |
19.68 |
2175 |
52.96 |
CCCCCC/C=C\CC |
|
5 |
Erucic acid |
5281116 |
C22H42O2 |
338.6 |
20.02 |
2572 |
16.67 |
CCCCCCCCC=CCC |
|
6 |
4-Tridecene, (Z)- |
5362712 |
C13H26 |
182.35 |
21.41 |
1321 |
1.93 |
CCCCCC |
|
7 |
9,17-Octadecadienal, [Z]- |
5365667 |
C18H32O |
264.4 |
24.31 |
1997 |
3.62 |
C=CCCCCCC |
|
8 |
Pentanoic acid,10-undecenyl ester |
543363 |
C16H30O2 |
254.41 |
25.04 |
1769 |
0.92 |
CCCCC(=O) |
|
9 |
Cyclopentaneundecanoic acid, methyl ester |
535041 |
C17H32O2 |
268.4 |
25.85 |
1921 |
0.83 |
COC(=O)CCCCC |
|
10 |
9-Octadecenal |
5283381 |
C18H34O |
266.5 |
26.17 |
2007 |
0.42 |
CCCCCCCCC=C |
|
11 |
Cyclooctane acetic acid,2-oxo- |
536995 |
C10H16O3 |
184.23 |
26.68 |
1647 |
0.38 |
C1CCCC(=O) |
|
12 |
9-Octadecenamide |
5353370 |
C18H35NO |
281.5 |
27.41 |
2228 |
0.29 |
CCCCCCCCC=C |
|
13 |
17-Octadecynoic acid |
1449 |
C18H32O2 |
280.4 |
27.95 |
2165 |
1.84 |
C#CCCCCCCCC |
|
14 |
Oleic acid |
445639 |
C18H34O2 |
282.5 |
28.83 |
2140 |
0.62 |
CCCCCCCCC=C |
|
15 |
1-(4-bromobutyl)-2-piperidone |
536377 |
C9H16BrNO |
234.13 |
30.67 |
1635 |
2.08 |
C1CCN(C(=O) |
|
16 |
2-Monolinolenin, 2TMS derivative |
5362857 |
C27H52O4Si2 |
496.9 |
30.94 |
2804 |
0.83 |
CCC=CCC=CCC=C |
|
17 |
Linoleic acid, phenylmethyl ester |
5368290 |
C25H38O2 |
370.6 |
33.20 |
2760 |
0.69 |
CCCCCC=CCC= |
|
18 |
Patchouli alcohol |
10955174 |
C15H26O |
222.37 |
34.27 |
1420 |
1.72 |
CC1CCC2 |
|
19 |
9-Octadecadienoic acid[Z]-, phenylmethyl ester |
5368218 |
C25H40O2 |
372.6 |
34.60 |
2758 |
0.47 |
CCCCCCCCC=C |
2-Monolinolenin, 2TMS derivative; is a lipid-like compound whereas cis-Vaccenic acid; Erucic acid; 4-Tridecene, (Z)-; 10-Undecenyl pentanoate; Methyl dihydrohydnocarpate; 9-Octadecenal; 9 Octadecenamide; 17-Octadecynoic acid; Linoleic acid, phenylmethyl ester; Oleic acid benzyl ester and Oleic acid are fatty acyl compounds which belong to class fatty acids or fatty acid derivatives. In addition, n-Hexadecanoic acid; is a saturated fatty acid. Some other compounds such as 9,17-Octadecadienal, [Z]- and 1-(4-Bromobutyl)-2-piperidinone are aldehyde derivative and organonitrogen heterocyclic compounds, respectively. In addition, carboxylic acid derivative cyclooctane acetic acid,2-oxo- was found. Lastly, Phytol and Patchouli alcohol is found that belong to terpenes (Diterpenes) whereas hydrocarbon derivative methyl di-t-butyl hydroxy-hydro cinnamate was also detected. The chemical framework of each compound is revealed in supplementary data Table S2.
FTIR characterization study
Characterization of the functional groups of an active constituent was done by FTIR depending on the value of peaks in the IR spectrum. The FTIR spectrum of S. tenerrimum is depicted in figure 5. Peak values, functional group, and compound class are revealed in table 3. The bands found at different intensities such as 669.92 cm-1, 820.73 cm-1, 1064.33 cm-1, 1091.40 cm-1, 1338.88 cm-1, 1420.08 cm-1, 1509.02 cm-1, 1543.82 cm-1, 1655.96 cm-1, 1702.36 cm-1, 1733.29 cm-1, 1771.96 cm-1, 1872.50 cm-1, 1992.37 cm-1, 2928.14 cm-1, 3450.16 cm-1, and 3651.23 cm-1 corresponding to C-Br/C-Cl/C=C/C-H/C-F/S=O/C-O/C-N/O-H/N-O/C=N/C=C=N/N=C=S/N-H. These functional groups revealed the presence of halo, alkene, hydrocarbon, fluoro, sulfoxide, amine, Imino/oxime, alkene, isothiocyanate, amine salt, alcohol, and substituted constituents in the extracts of S. tenerrimum.
 |
Figure 5: FTIR spectrum of Sargassum tenerrimum. |
Table 3: Characterization offunctional groups of S. tenerrimum.
|
Absorption |
Appearance |
Group |
Compound Class |
|
669.92 |
Strong Strong Strong |
C-Br (S) C-Cl (S) C=C (B) |
halo compound halo compound alkene(disubstituted) |
|
820.73 |
Strong Medium Strong |
C-Cl (S) C=C (B) C-H (B) |
halo compound alkene(trisubstituted) 1,4 disubstituted or 1,2,3,4 tetra substituted |
|
1064.33 |
Strong Strong Strong Medium |
C-F (S) S=O (S) C-O (S) C-N (S) |
Fluoro compound Sulfoxide Primary alcohol Amine |
|
1091.40 |
Strong Medium Strong Strong |
C-F (S) C-N (S) C-O (S) C-O (S) |
Fluoro compound amine Secondary alcohol aliphatic ether |
|
1338.88 |
Strong Strong strong Medium Strong Strong |
C-F (S) S=O (S) C-N (S) O-H (B) S=O (S) S=O (S) |
Fluoro compound Sulfone aromatic amine Phenol Sulfonamide Sulfonate |
|
1420.08 |
Medium medium |
O-H (B) O-H (B) |
alcohol Carboxylic acid |
|
1509.02 |
Strong |
N-O (S) |
nitro compound |
|
1543.82 |
strong |
N-O (S) |
nitro compound |
|
1655.96 |
Weak Medium Medium medium |
C-H (B) C=N (S) C=C (S) C=C (S) |
aromatic imine/oxime alkene(vinylidene) alkene(distributed) |
|
1702.36 |
Weak Strong strong |
C-H (B) C=O (S) C=O (S) |
aromatic Conjugated aldehyde Conjugated acid |
|
1733.29 |
Weak Strong |
C-H (B) C=O (S) |
aromatic aldehyde |
|
1771.96 |
Weak Strong Strong |
C-H (B) C=O (S) C=O (S) |
aromatic Conjugated acid halide Vinyl/ phenol ester |
|
1872.50 |
Weak |
C-H (B) |
aromatic |
|
1992.37 |
Medium Strong Weak |
C=C=N (S) N=C=S (S) C-H (B) |
alkene isothiocyanate aromatic |
|
2928.14 |
Strong broad Weak broad Strong broad medium |
O-H (S) O-H (S) N-H (S) C-H (S) |
Carboxylic acid alcohol amine salt alkene |
|
3450.16 |
Strong broad |
O-H (S) |
alcohol |
|
3651.23 |
Medium sharp |
O-H (S) |
alcohol |
Bond type: (S) Stretching; (B) bending;
Determination of Elements
Among different mineral elements, Silicon, Potassium, Calcium, and Magnesium were found in large proportion as compared to Sodium, Iron, Boron, and Copper in water extract of S. tenerrimum in ICP AES analysis.The result reported that Silicon (13.67%) was found in the highest amount whereas Boron (0.01 %) remained at the lowest. The mineral % follows Silicon > Potassium > Calcium > Magnesium > Sodium > Iron> Boron order. The amount of each element (%) is tabulated in table 4.
Table 4: Element contents of S. tenerrimum.
|
Minerals |
Amount in % (Mean± S.E.M.) |
|
Boron (B) |
0.01±0.01 |
|
Calcium (C) |
1.36±0.09 |
|
Copper (Cu) |
NO |
|
Iron (Fe) |
0.10±0.02 |
|
Potassium (K) |
10.31±0.13 |
|
Magnesium (Mg) |
0.66±0.06 |
|
Zinc (Zn) |
NO |
|
Sodium (Na) |
0.12±0.03 |
|
Silicon (Si) |
13.67±0.28 |
|
Selenium (Se) |
NO |
“NO” means less than 0.01 PPM;
Determination of Amino Acids
In this analysis, a total of twenty-one different types of amino acids were measured in S. tenerrimum by the HRLCMS-QTOF technique. A gas chromatogram containing different peaks for amino acids is illustrated in fig. 6. Among 21 amino acids, Glutamic acid, Alanine, Glycine, and Aspartic acid were determined to be higher than 100 nmol/ml whereas Leucine, Serine, Arginine, Threonine, Tyrosine, Lysine, Phenylalanine, valine, and Isoleucine were found to be lower than 100 nmol/ml. Content (nmol/mL) of these amino acids were detected in the below order: Glutamic acid > Alanine > Glycine > Aspartic acid > Leucine > Serine > Arginine > Threonine > Tyrosine > Lysine > Phenylalanine > Valine > Isoleucine. Concentrations of detected amino acids (in nmol/ml) are presented in Table 5.
 |
Figure 6: Chromatogram for different amino acids by HRLCMSQTOF. |
Table 5: The total amino acid content of S. tenerrimum.
|
Sr.no |
Amino Acids |
Amount in (nmol/ml) |
|
1 |
Aspartic acid |
106.13 |
|
2 |
Glutamic Acid |
132.13 |
|
3 |
Methionine |
ND |
|
4 |
Asparagine |
ND |
|
5 |
Serine |
55.45 |
|
6 |
Valine |
9.37 |
|
7 |
Glutamine |
ND |
|
8 |
Histidine |
ND |
|
9 |
Isoleucine |
0.70 |
|
10 |
Glycine |
110.18 |
|
11 |
Threonine |
28.35 |
|
12 |
Norvaline |
ND |
|
13 |
Arginine |
34.67 |
|
14 |
Alanine |
129.05 |
|
15 |
Tryptophan |
ND |
|
16 |
Tyrosine |
25.46 |
|
17 |
Cystine |
ND |
|
18 |
leucine |
63.82 |
|
19 |
Lysine |
17.16 |
|
20 |
Hydroxproline |
ND |
|
21 |
phenylalanine |
11.90 |
ND: Not detectable
Characterization of phycocompounds and derivatives
Total sixty-one compounds were found in methanolic extract of Sargassum tenerrimum by HR-LCMS Q-TOF analysis at different RT. In HRLCMS-QTOF analysis, obtained liquid chromatogram for this analysis is illustrated in fig. 7. The different types of phycocompounds and their chemical information were revealed in table 6. By comparing obtained data with the main library, all these compounds were characterized and identified. The chemical frameworks of compounds are illustrated in supplementary table S2. In this analysis, 9Z,11E-Hexadecadienal; 9,12,13-trihydroxy-10,15octadecadienoic acid; formyl 2E,4E,6Z-decatrienoate; 12-deoxy-J2-IsoP; 2(R)-HPOT; 5,8,11-Octadecatriynoic acid and 5-deoxy-J2-IsoP were belong to Fatty Acyls, Fatty acids and derivatives. Likewise, C16 Sphinganine; Phytosphingosine and 17-phenyl trinor Prostaglandin E2 serinol amide are amino alcohol that belongs to lipid and lipid-like molecule. In addition, organic heterocyclic compounds such as Isopiperolein B; Diversifolide; 2-Morpholinomethylestrone; Hydroxyatrazine; 2,4,6-Trimethyl-4-phenyl-1,3dioxane; Phendimetrazine; Imiquimod and 2-Phenylethyl propanoate were found whereas two carbohydrate derivatives 25-O-(2”-beta-Dglucopyranosyl-beta-Dglucopyranosyl)-25-hydroxy11E-eicosenoic acid and (1RS,2RS)-Guaiacylglycerol 1glucoside were found. Benzyl alcohol, Benzenemethanol, 2-(2hydroxypropoxy)-3-methyl-; secondary alcohol, Convallasaponin A; carboxylic ester, Heptyl p-hydroxybenzoate; carboxylic acid derivative, Procaine; aldehyde derivative, 2,3-Butanedione trimer; amino acid derivative, Alpha,beta-Didehydrotryptophan; Amine, Benzonatate, and Nylidrin; alkaloid, Codonocarpine; Narwedine; Isocorydine (+); 3-Ethylethcathinone and Salsolidine; organophosphorus compound, 13-Octadecene-9,11-diynoic acid, (Z)- and Triphenylphosphine oxide; terpenes (Diterpenes) like compound, Cyathin A3 and 18-Nor-4(19),8,11,13abietatetraene were identified. Vitamin B complex, (nicotinic acid), Beta-Butoxyethyl nicotinate was also identified in S. tenerrimum. An antibiotic N2′-Acetylgentamicin C1a (gentamycin) and peptide Tumonoic Acid I were also detected. Moreover, hydrocarbon derivatives, 1,4-Diethylbenzene and Benzo[a]fluorene; an aromatic ether, Juvocimene 2; organic amino compound, Pirimicarb; organic compounds, Buspirone; Dehydroxyzyleuton; (E)-1-Cinnamoylpyrrolidine and Ecklonialactone A; organonitrogen compounds, PAC-1; Distichonic acid A; 5-(alpha-Phenylethyl)semioxamazide and Istamycin C1; as well as organosulfur compounds, Sulfabenzamide; 3-(2-Methyl-3-furylthio)-2-butanone and Dicyclohexyl disulfide were identified in S. tenerrimum.
 |
Figure 7: Chromatogram for phycocompounds’ of S. tenerrimum by HRLCMSQTOF. |
Table 6: Chemical information ofidentified phycocompounds and derivatives by HRLCMS-QTOF.
|
No. |
Name |
PubChem ID |
Molecular Formula |
RT (min) |
Mass (Da) |
Hits (DB) |
|
1 |
3-(2-Methyl-3-furylthio)-2-butanone |
12980878 |
C9H12O2S |
0.79 |
184.0576 |
3 |
|
2 |
Sulfabenzamide |
5319 |
C13H12N2O3S |
0.795 |
276.0554 |
3 |
|
3 |
9Z,11E-Hexadecadienal |
11172431 |
C16H28O |
1.945 |
236.2118 |
10 |
|
4 |
1,4-Diethylbenzene |
7734 |
C10H14 |
1.995 |
134.1074 |
10 |
|
5 |
Benzo[a]fluorene |
9195 |
C17H12 |
2.235 |
216.0966 |
5 |
|
6 |
5-(alpha- Phenylethyl)semioxamazide |
249877 |
C10H13N3O2 |
2.3 |
207.1003 |
6 |
|
7 |
Hydroxyatrazine |
135398733 |
C8H15N5O |
2.384 |
197.1273 |
9 |
|
8 |
Salsolidine |
10302 |
C12H17NO2 |
2.618 |
207.125 |
10 |
|
9 |
2,3-Butanedione trimer |
193527 |
C12H18O6 |
3.203 |
258.1092 |
9 |
|
10 |
Procaine |
4914 |
C13H20N2O2 |
3.487 |
236.1518 |
3 |
|
11 |
Nylidrin |
4567 |
C19H25NO2 |
3.842 |
299.1929 |
2 |
|
12 |
Distichonic acid A |
85405750 |
C10H18N2O8 |
3.897 |
294.1069 |
10 |
|
13 |
Isopiperolein B |
16041826 |
C21H29NO3 |
4.264 |
343.2197 |
5 |
|
14 |
Diversifolide |
73232471 |
C13H20O3 |
4.535 |
224.1406 |
10 |
|
15 |
Isocorydine (+) |
10143 |
C20H23NO4 |
4.637 |
341.1619 |
10 |
|
16 |
(1RS,2RS)-Guaiacylglycerol 1glucoside |
131751407 |
C16H24O10 |
4.671 |
376.1377 |
10 |
|
17 |
2-Morpholinomethylestrone |
235463 |
C23H31NO3 |
4.675 |
369.2368 |
10 |
|
18 |
Istamycin C1 |
46174030 |
C19H37N5O6 |
4.87 |
431.2721 |
6 |
|
19 |
Dehydroxyzyleuton |
10104880 |
C11H12N2OS |
5.186 |
220.0666 |
10 |
|
20 |
17-phenyl trinor Prostaglandin E2 serinol amide |
35026314 |
C26H37NO6 |
5.199 |
459.2662 |
10 |
|
21 |
Tumonoic Acid I |
2505812 |
C27H47NO7 |
5.344 |
497.3423 |
1 |
|
22 |
Benzenemethanol, 2-(2hydroxypropoxy)-3-methyl- |
85909061 |
C11H16O3 |
5.623 |
196.109 |
10 |
|
23 |
Imiquimod |
57469 |
C14H16N4 |
5.821 |
240.1355 |
4 |
|
24 |
beta-Butoxyethyl nicotinate |
14866 |
C12H17NO3 |
5.984 |
223.1212 |
7 |
|
25 |
Benzenemethanol, 2-(2hydroxypropoxy)-3-methyl- |
85909061 |
C11H16O3 |
6.089 |
196.1092 |
10 |
|
26 |
2-Phenylethyl propanoate |
31225 |
C11H14O2 |
6.092 |
178.0989 |
10 |
|
27 |
2,4,6-Trimethyl-4-phenyl-1,3dioxane |
107381 |
C13H18O2 |
6.466 |
206.1301 |
10 |
|
28 |
Convallasaponin A |
441883 |
C32H52O9 |
6.854 |
580.3744 |
2 |
|
29 |
N2′-Acetylgentamicin C1a |
16069998 |
C21H41N5O8 |
6.901 |
491.2955 |
4 |
|
30 |
(E)-1-Cinnamoylpyrrolidine |
2056198 |
C13H15NO |
7.597 |
201.115 |
6 |
|
31 |
Heptyl p-hydroxybenzoate |
14138 |
C14H20O3 |
7.639 |
236.1408 |
10 |
|
32 |
13-Octadecene-9,11-diynoic acid, (Z)- |
5312685 |
C18H26O2 |
8.297 |
274.1925 |
10 |
|
33 |
Phendimetrazine |
30487 |
C12H17NO |
8.355 |
191.1307 |
10 |
|
34 |
9,12,13-trihydroxy-10,15octadecadienoic acid |
5312876 |
C18H32O5 |
8.419 |
328.2252 |
10 |
|
35 |
13-Octadecene-9,11-diynoic acid, (Z)- |
5312685 |
C18H26O2 |
8.655 |
274.193 |
10 |
|
36 |
Benzonatate |
7699 |
C30H53NO11 |
15.582 |
603.3757 |
2 |
|
37 |
alpha,beta- Didehydrotryptophan |
5280990 |
C11H10N2O2 |
8.71 |
202.076 |
5 |
|
38 |
Codonocarpine |
5281820 |
C26H31N3O5 |
8.856 |
465.22 |
3 |
|
39 |
beta-Butoxyethyl nicotinate |
14866 |
C12H17NO3 |
8.878 |
223.1213 |
7 |
|
40 |
13-Octadecene-9,11-diynoic acid, (Z)- |
5312685 |
C18H26O2 |
8.995 |
274.1926 |
10 |
|
41 |
9,12,13-trihydroxy-10,15octadecadienoic acid |
5312876 |
C18H32O5 |
9.118 |
328.2252 |
10 |
|
42 |
3-Ethylethcathinone |
91696115 |
C13H19NO |
9.291 |
205.1462 |
8 |
|
43 |
formyl 2E,4E,6Z-decatrienoate |
11052245 |
C11H16O2 |
9.468 |
180.114 |
10 |
|
44 |
12-deoxy-J2-IsoP |
52921986 |
C20H28O3 |
9.519 |
316.2034 |
10 |
|
45 |
2(R)-HPOT |
23724727 |
C18H30O4 |
9.522 |
310.2137 |
10 |
|
46 |
5,8,11-Octadecatriynoic acid |
5312681 |
C18H24O2 |
9.581 |
272.1769 |
10 |
|
47 |
Ecklonialactone A |
23428255 |
C18H26O3 |
9.719 |
290.1876 |
10 |
|
48 |
C16 Sphinganine |
656816 |
C16H35NO2 |
9.989 |
273.2665 |
1 |
|
49 |
Phytosphingosine |
122121 |
C18H39NO3 |
10.013 |
317.2925 |
1 |
|
50 |
Triphenylphosphine oxide |
13097 |
C18H15OP |
10.096 |
278.0855 |
10 |
|
51 |
17beta-Hydroxy-2-oxa-5alphaandrostan-3-one |
252289 |
C18H28O3 |
10.155 |
292.2033 |
10 |
|
52 |
Cyathin A3 |
442017 |
C20H30O3 |
10.22 |
318.2185 |
10 |
|
53 |
18-Nor-4(19),8,11,13abietatetraene |
5320205 |
C19H26 |
10.593 |
254.2084 |
7 |
|
54 |
Buspirone |
2477 |
C21H31N5O2 |
10.667 |
385.2457 |
3 |
|
55 |
Juvocimene 2 |
131752882 |
C20H26O2 |
10.913 |
298.1926 |
10 |
|
56 |
5-deoxy-J2-IsoP |
52921971 |
C20H28O3 |
10.977 |
316.2031 |
10 |
|
57 |
Narwedine |
10356588 |
C17H19NO3 |
11.148 |
285.136 |
10 |
|
58 |
PAC-1 |
135421197 |
C23H28N4O2 |
12.134 |
392.2216 |
10 |
|
59 |
25-O-(2”-beta-Dglucopyranosyl-beta-Dglucopyranosyl)-25-hydroxy11E-eicosenoic acid |
42607366 |
C32H58O13 |
14.394 |
650.3867 |
9 |
|
60 |
Pirimicarb |
31645 |
C11H18N4O2 |
26.713 |
238.1402 |
4 |
|
61 |
Dicyclohexyl disulfide |
17356 |
C12H22S2 |
26.721 |
230.1177 |
1 |
Many seaweeds derived bioactive compounds have been applicable in skin cosmetic applications for varieties of skin benefits. Many previous studies reported that Dicarboxylic acids and derivatives reported benefits as a pH adjuster, fragrance enhancer as well as emollient [36]. Phthalic acid ester showed antibacterial activities against different bacterial species such as Streptococcus epidermidis, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Bacillus subtilis [37]. Different commercially available products were reported containing Hexadecane. The former commercially available products can be used as a softener, emollients, stabilizers, and thickeners. Likewise, Fatty acids and derivatives have an important role in regulation and healthy benefits for the skin. First, fatty acids are important useful in normal skin barrier functions. Long-chain fatty acids have become helpful in skin hydration, protective barrier, and metabolic regulators. Medium-chain fatty acids show antiinflammation as well as antitumor activity. In addition, short-chain fatty acids promote the immune system and anti-inflammatory benefits [38]. Moreover, fatty acids have antibacterial properties which can be used as preservatives and lubricants [39,40]. Ahmad et al. [41] revealed the presence of different bioactive constituents such as alcohol, carboxylic acids, ethers, esters, ketones, amides, alkanes, and aldehydes by FTIR characterization study. These types of compounds are beneficial for various applications mainly antimicrobial, photoprotection, food flavoring, color enhancer, preservatives as well as anticancer activity [42-45] Halogenated compounds are reported to use in face care products, antiseptic and antiacne, deodorants, antiperspirant, as well as antimicrobial benefits [46,47]. Many previously existing studies demonstrated the benefits of minerals and amino acids for skin health benefits. Mainly, it plays its role in the regulation of epithelial layers, skin nourishment, skin repair, topical formulations as well as skin protectants [48-50]. Except for skin benefits, it is helpful in nutraceutical and pharmaceutical formulation, in food and dairy applications, as well as in animal feed supplements.
Conclusion
Oceans contain a huge diversified marine organism, comprise the majority area of the earth. Among them, marine macroalgae offer a wide variety of bioactive ingredients such as polysaccharides, amino acids, proteins, vitamins, fatty acids, bioactive peptides, etc. It confers a broader range of beneficial actions such as antimicrobial, anticancer, antioxidant, anti-inflammation, antiaging, anticancer as well as other benefits in food and dairy sectors, medicinal and pharmaceutical sectors, fuel and remediation, agricultural benefits, etc. Researchers from around the world have demonstrated the biological potential of different macroalgae and derived compounds. The present findings showed that the Phaeophyta S. tenerrimum is a valuable source of bioactive ingredients. In the finding, 1-O-butyl 2-O-(6-ethyloctan-3-yl) benzene-1,2-dicarboxylate; Pentadecanal-; 1-O-tetradecyl 4-O-(2,3,6-trichlorophenyl) butanedioate; 3-Hexanone, 2,5-dimethyl-; Propane, 1,3-bis(octadecyloxy)-; Pentanal; Benzyl icosanoate, 1,1,3,3,5,5,7,7,9,9,11,11-Dodecamethylhexasiloxane, and t- Boc-sarcosine, etc. compounds in ethanolic extracts whereas cis-Vaccenic acid, Erucic acid, n-Hexadecanoic acid, 9,17-Octadecadienal, [Z]-, 1-(4-bromobutyl)-2-piperidone, 4-Tridecene, (Z)-, 17-Octadecynoic acid and Oleic acid were majorly found in methanolic extract of S. tenerrimum. Likewise, Lipid and lipid-like molecules, Carboxylic acid derivatives, Carbohydrate derivatives, Terpenes like compounds, alkaloids as well as some other organic compounds were screened in the HRLCMS characterization study. In addition, Glutamic Acid, Alanine, Glycine, Aspartic acid, Leucine, Serine, Arginine, Threonine, Tyrosine, and Lysineamino acids were obtained in larger amounts whereas Silicon, Potassium, Calcium, Magnesium, and Sodium elements were measured higher in amount. This finding revealed a very good phycochemical profile and that compounds can be applied in different types of applications after successful experimentation further. These compounds are natural, less toxic, economical, almost inexhaustible, and safer than synthetic ingredients. By different characterization studies, selected marine alga reported a good type of potentially active components. S. tenerrimum or derived bioactive constituents can be utilized whole or part in various applications such as food, beauty enhancer, medicinal and pharmacological properties, antioxidant activities, antimicrobial, anti-inflammatory, anticancer, antidiabetic activity, antiviral activity, cellular growth, plant growth promotion, vermifuge activity, antitumor activity, antiulcer, wound healing treatment, Goitre treatment, industrial for fuel production, renewable energy suppliers, animal feed preparation, organic manure preparation, domestic sewage treatment, wastewater treatment, etc. Along with its large availability of biomass (particularly on the Beyt Dwarka sea site), no need to worry about its cultivation, it could be utilized in various applications or product preparations after successful in vitro and in vivo evaluation as well as clinical assessment.
Additional Supplementary Files
Supplementary Tables: https://www.orientjchem.org/pdf/Additional%20Table%20S1-%20S2.pdf
Acknowledgment
Authors are thankful to Microbiology Department, Sankalchand Patel University (SPU), Visnagar for providing the platform and support to carry out this work. We are also thankful to Dr. N. H. Joshi, Junagadh Agriculture University, Veraval for helping in the identification of selected alga.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding sources.
References
- Katiyar, N.K., Goel, G., Hawi, S. and Goel, S. Nature-inspired materials: Emerging trends and prospects. NPG Asia Materials. 2021, 13(1), pp.1-16.
CrossRef - Øverland, M., Mydland, L.T. and Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. Journal of the Science of Food and Agriculture. 2019, 99(1), pp.13-24.
CrossRef - Pereira, L. Macroalgae. Encyclopedia. 2021, 1(1), pp.177-188.
CrossRef - Pereira, L. and Correia, F. Algas marinhas da costa Portuguesa—ecologia, biodiversidade e utilizações. Nota de Rodapé Edições, Paris. 2015, p.340.
- Kalasariya, H.S.; Patel, N.B.; Yadav, A.; Perveen, K.; Yadav, V.K.; Munshi, F.M.; Yadav, K.K.; Alam, S.; Jung, Y.-K.; Jeon, B.-H. Characterization of Fatty Acids, Polysaccharides, Amino Acids, and Minerals in Marine Macroalga Chaetomorpha crassa and Evaluation of Their Potentials in Skin Cosmetics. Molecules. 2021, 26, 7515. https://doi.org/10.3390/molecules26247515
CrossRef - Pereira, L. Guia ilustrado das macroalgas. Imprensa da Universidade de Coimbra/Coimbra University Press. Coimbra, Portugal, 2009, p. 90, ISBN 978-989-26-0002-4.
- Pereira, L. ALGAE. Litoral of Viana do Castelo: Uses in Agriculture, Gastronomy and Food Industry (Bilingual). 2010, pp. 7–8. ISBN 978-972-588-217-7.
- Kalasariya, H.S.; Pereira, L.; Patel, N.B. Pioneering Role of Marine Macroalgae in Cosmeceuticals. Phycology. 2022, 2, 172-203. https://doi.org/10.3390/phycology2010010
CrossRef - Pereira, L. Cytological and cytochemical aspects in selected carrageenophytes (Gigartinales, Rhodophyta). Advances in algal cell biology. 2012, pp.81-104. ISBN 978-3-11-022960-8.
CrossRef - González-Minero, F.J. and Bravo-Díaz, L. The use of plants in skin-care products, cosmetics and fragrances: Past and present. Cosmetics. 2018, 5(3), p.50.
CrossRef - Alam, T., Najam, L., and Al Harrasi, A. Extraction of natural pigments from marine algae. J. Agric. Mar. Sci. 2018, 23, pp.81-91.
CrossRef - Pereira, L. Therapeutic and Nutritional Uses of Algae; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2018, p. 560, ISBN 9781498755382.
- Thomas, N.V. and Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Marine drugs. 2013, 11(1), pp.146-164.
CrossRef - Cikoš, A.M., Jokić, S., Šubarić, D. and Jerković, I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Marine Drugs. 2018, 16(10), p.348.
CrossRef - Zhao, C., Yang, C., Liu, B., Lin, L., Sarker, S.D., Nahar, L., Yu, H., Cao, H. and Xiao, J. Bioactive compounds from marine macroalgae and their hypoglycemic benefits. Trends in Food Science & Technology. 2018, 72, pp.1-12.
CrossRef - Malinowska, P. Algae extracts as active cosmetic ingredients. Zeszyty Naukowe/Uniwersytet Ekonomiczny w Poznaniu. 2011, (212), pp.123-129.
- Pereira, L. Edible Seaweeds of the World; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2016, p. 448, ISBN 9781498730471.
- Gaspar, R., Fonseca, R. and Pereira, L. Illustrated Guide to the Macroalgae of Buarcos Bay, Figueira da Foz, Portugal. MARE UC, DCV, FCT. Coimbra, Portugal, 2020, p. 128.
- Pereira, L.; Bahcevandziev, K.; Joshi, N.H. (Eds.) Seaweeds as Plant Fertilizer, Agricultural Biostimulants, and Animal Fodder; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2019, p. 232, ISBN 978-1-13-859706-8.
CrossRef - Muthezhilan, R., Jayaprakash, K., Parthiban, C. and Hussain, A.A.J. Plant Growth Promoting Effect of Seaweeds Collected from East Coast of Tamil Nadu, India. Biosci. Biotechnol. Res. Asia. 2014, 11, pp.53-58.
CrossRef - Chaturvedi, S., Kulshrestha, S. and Bhardwaj, K. Role of seaweeds in plant growth promotion and disease management. In New and Future Developments in Microbial Biotechnology and Bioengineering. 2022, (pp. 217-238). Elsevier.
CrossRef - Cotas, J., Leandro, A., Pacheco, D., Gonçalves, A.M. and Pereira, L. A comprehensive review of the nutraceutical and therapeutic applications of red seaweeds (Rhodophyta). Life. 2020, 10(3), p.19.
CrossRef - Pacheco, D., García-Poza, S., Cotas, J., Gonçalves, A.M. and Pereira, L. Fucoidan—A valuable source from the ocean to pharmaceutical. Front. Drug Chem. Clin. Res. 2020, 3, pp.1-4.
CrossRef - Boukid, F. and Castellari, M., 2021. Food and beverages containing algae and derived ingredients launched in the market from 2015 to 2019: a front-of-pack labeling perspective with a special focus on Spain. Foods. 2021, 10(1), p.173.
CrossRef - Sabaani, N.J., Peñaredondo, M.A.E. and Sepe, M.C. Antibacterial activity of liquid soap with combined Sargassum sp. and Eucheuma sp. seaweed extracts. Aquaculture, Aquarium, Conservation & Legislation. 2019, 12(5), pp.1514-1523.
- Michalak, I., Dmytryk, A. and Chojnacka, K. Algae Cosmetics. Encyclopedia of Marine Biotechnology, 2020, 1, pp.65-85.
CrossRef - Rajendran, I. Marine Algal Polysaccharides and Their Applications. Encyclopedia of Marine Biotechnology. 2020, 2, pp.1195-1208.
CrossRef - López-Hortas, L., Flórez-Fernández, N., Torres, M.D., Ferreira-Anta, T., Casas, M.P., Balboa, E.M., Falqué, E. and Domínguez, H. Applying seaweed compounds in cosmetics, cosmeceuticals and nutricosmetics. Marine drugs. 2021, 19(10), p.552.
CrossRef - Leandro, A., Pereira, L. and Gonçalves, A.M., 2020. Diverse applications of marine macroalgae. Marine drugs. 2020, 18(1), p.17.
CrossRef - Leandro, A., Pereira, L. and Gonçalves, A.M. Diverse applications of marine macroalgae. Marine drugs. 2019, 18(1), p.17.
CrossRef - Aditya, T., Bitu, G. and Mercy Eleanor, G. The role of algae in pharmaceutical development. Spec. Issue Rev. Pharm. Nanotechnol. Res. Rev. J. Pharm. Nanotechnol. 2016, 4, pp.82-89.
- Pimentel, F.B., Alves, R.C., Rodrigues, F. and PP Oliveira, M.B. Macroalgae-derived ingredients for cosmetic industry—An update. Cosmetics. 2018, 5(1), p.2.
CrossRef - López-Hortas, L., Flórez-Fernández, N., Torres, M.D., Ferreira-Anta, T., Casas, M.P., Balboa, E.M., Falqué, E. and Domínguez, H. Applying seaweed compounds in cosmetics, cosmeceuticals and nutricosmetics. Marine drugs. 2021, 19(10), p.552.
CrossRef - Abbott, I.A., Isabella, A. and Hollenberg, G.J. Marine algae of California. 1992, Stanford University Press.
CrossRef - Guiry, M.D. & Guiry, G.M. (2022). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway (taxonomic information republished from AlgaeBase with permission of M.D. Guiry). Sargassum tenerrimum J.Agardh, 1848. Accessed through: World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=494985 on 2022-03-15
- Fiume, M.M.; Eldreth, H.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Final Report of the Cosmetic Ingredient Review Expert Panel on the Safety Assessment of Dicarboxylic Acids, Salts, and Esters. Int. J. Toxicol. 2012, 31 (Suppl. 4), 5S–76S.
CrossRef - Rajamanikyam, M.; Vadlapudi, V.; Parvathaneni, S.P.; Koude, D.; Sripadi, P.; Misra, S.; Amanchy, R.; Upadhyayula, S.M. Isolation and characterization of phthalates from Brevibacterium mcbrellneri that cause cytotoxicity and cell cycle arrest. EXCLI J. 2017, 16, 375–387.
- Yang, M.; Zhou, M.; Song, L. A review of fatty acids influencing skin condition. J. Cosmet. Dermatol. 2020, 19, 3199–3204.
CrossRef - Cui, L.; He, C.; Fan, L.; Jia, Y. Application of lipidomics to reveal differences in facial skin surface lipids between males and females. J. Cosmet. Dermatol. 2018, 17, 1254–1261.
CrossRef - Cochran, S.; Anthonavage, M. Fatty Acids, Fatty Alcohols, Synthetic Esters and Glycerin Applications in the Cosmetic Industry. In Lipids and Skin Health. 2015, Springer: Cham, Switzerland, pp. 311–319.
CrossRef - Ahmad, S.; Ahmad, S.; Bibi, A.; Ishaq, M.S.; Afridi, M.S.; Kanwal, F.; Zakir, M.; Fatima, F. Phytochemical Analysis, Antioxidant Activity, Fatty Acids Composition, and Functional Group Analysis of Heliotropium bacciferum. Sci. World J. 2014, 2014, 829076.
CrossRef - Das, A.J.; Khawas, P.; Miyaji, T.; Deka, S.C. Phytochemical Constituents, Attenuated Total Reflectance Fourier Transform Infrared Analysis and Antimicrobial Activity of Four Plant Leaves Used for Preparing Rice Beer in Assam, India. Int. J. Food Prop. 2016, 19, 2087–2101.
CrossRef - Janakiraman, N.; Sahaya Sathish, S.; Johnson, M. UV-VIS and FTIR spectroscopic studies on Peristrophe bicalyculata (Retz.) Nees. Asian J. Pharm. Clin. Res. 2011, 4, 125–129.
- Burman, S.; Bhattacharya, K.; Mukherjee, D.; Chandra, G. Antibacterial efficacy of leaf extracts of Combretum album Pers. against some pathogenic bacteria. BMC Complement. Altern. Med. 2018, 18, 213.
CrossRef - Ambedkar, G.; Pragalathan, S.; Sabaridasan, A.; Gandhimaniyan, K.; Dineshkumar, S.; Balamurugan, V. FTIR Spectrum Analysis and Antibacterial Activity of Tribulus terrestris Leaves Extract. Res. Rev. A J. Microbiol. Virol. 2019, 9, 25–30.
- Gribble, G.W. Biological activity of recently discovered halogenated marine natural products. Marine drugs. 2015, 13(7), pp.4044-4136.
CrossRef - Wilcken, R., Zimmermann, M.O., Lange, A., Joerger, A.C. and Boeckler, F.M. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. Journal of medicinal chemistry. 2013, 56(4), pp.1363-1388.
CrossRef - Elias, P.M.; Ahn, S.K.; Denda, M.; Brown, B.E.; Crumrine, D.; Kimutai, L.K.; Kömüves, L.; Lee, S.H.; Feingold, K.R. Modulations in Epidermal Calcium Regulate the Expression of Differentiation-Specific Markers. J. Investig. Dermatol. 2002, 119, 1128–1136.
CrossRef - Matz, H.; Orion, E.;Wolf, R. Balneotherapy in dermatology. Dermatol. Ther. 2003, 16, 132–140.
CrossRef - Food and Drug Administration, HHS. Skin protectant drug products for over-the-counter human use; final monograph. Final rule. Fed. Regist. 2003, 68, 33362–33381.

This work is licensed under a Creative Commons Attribution 4.0 International License.










