Adsorption Isotherm Study of Crystal Violet Dye on to Biochar Prepared from Agriculture Waste
School of Environmental and Earth Sciences,Kavayitri Bahinabai Chaudhari North Maharashtra University, Jalgaon Maharashtra, India.
Corresponding Author E-mail: profskjadhav@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380234
Article Received on : 18 Feb 2022
Article Accepted on : 20 Mar 2022
Article Published : 22 Apr 2022
Reviewed by: Dr. Makeswari R
Second Review by: Dr. Buhani
Final Approval by: Dr. Bal krishan
In the present investigation, crystal violet dye adsorption on biochar was prepared from the banana stem (Musa Acuminata) to know its capacity for adsorption of colour dyes from synthetic aqueous solution. The banana stem biochar (BSB) was prepared using pyrolysis method at 3500C and 4500C. The characterization was carried out to know its morphology and chemical composition using scanning electron microscopy for BSB-350 and BSB-450. Present work was carried out to examined surface characteristics and the batch experiment parameters such as pH, contact time, concentration and amount of dose which depends on adsorption kinetic model Langmuir, Freundlich, Temkin and Dubinin Radakovich. In our findings, it shows maximum adsorption of crystal violet dye on BSB 350 - 208.33mg/g and BSB 450 - 153.50mg/g which reveals that adsorption of dyes by using biochar is a cost-effective, environment-friendly practice and will be helpful to reduce pollutant from industrial effluent treatment plant. (ETP).
KEYWORDS:Adsorption; Biochar; Batch Study; Crystal Violet; Isotherm Model
Download this article as:| Copy the following to cite this article: Jadhav S. K, Thorat S. R. Adsorption Isotherm Study of Crystal Violet Dye on to Biochar Prepared from Agriculture Waste. Orient J Chem 2022;38 (2). |
| Copy the following to cite this URL: Jadhav S. K, Thorat S. R. Adsorption Isotherm Study of Crystal Violet Dye on to Biochar Prepared from Agriculture Waste. Orient J Chem 2022;38 (2).Available from: https://bit.ly/3xH5qrf |
Introduction
Banana is originated from southeast Asia and grows mainly in the humid tropical climate of Asia. Indian subcontinental producing high amount of banana crop. In the historical context, banana cultivation was found during the 7th century AD, and its spread very fastly to Africa and Egypt. The present scenario of banana cultivation is all over the globe and mainly in the equatorial region of 300N and 300S of the warm tropical climate regions. Colour in wastewater or effluent is one of the most perceptible markers of water contamination and the extremely coloured artificial dye discharge is aesthetically very unpleasing and can harms the receiving waterbody by hampering the dispersion of light. Industrial effluents like textile, leather, plastic food, paper, cosmetics, pharmaceuticals and dyes manufacturing are full of dyes which, are usually complex and synthetic origin which chemically makes them stable and hard to biodegradate10,14. which is having a hazardous impact on human and aquatic life. Dyes are highly toxic and carcinogenic compounds; they are also recalcitrant and thus stable in the receiving environment causing a serious threat to human and environmental health3,5,8,18. The removal of dyes from wastewater for Several physicals, chemical, and biological treatment techniques have been applied mainly photochemical degradation, biological degradation, coagulation, chemical oxidation, reverse osmosis, flotation, and adsorption1,3,14 but the most feasible economical and environmentally among the methods such as adsorption on pyrolyzed biomass. There are so many techniques and treatments methods to remove colure dye effluent, but these seems to be conventional treatment techniques and are expensive compare to adsorption process which creates to be more effective we observed in our study. Biochar is a carbon-rich adsorbent that produced pyrolysis of plant waste material in the minimum presence of oxygen4,17. Biochar is prepared as a carbon-rich, porous and low-cost product obtained from the pyrolyzing of biomass in an oxygen-limited atmosphere at moderately low temperatures less than 700 °C and it has been used as fertilizer, carbon extraction and pollutant removal6,9,16. Biochar’s porous property is expressed in the size of the pores, which is between nanometers and micrometers, based on the Standard of IUPAC. Macropores are larger than 50 nanometers, Mesosphere is smaller than 50 nanometers, and Micropores are smaller than 2 nanometers. To understand Biochar’s internal composition, use a distribution of pores. However, this is based on assumptions. To suggest a true complex and solid structure, use a regular equivalent pore model with interaction and shape19.
In the present work, we have used a Musa Acuminata stem (Banana) for the preparation of biochar at 3500C and 4500C Though, ample banana cultivation is done Khandesh region of Maharashtra and it is easily available as a waste product in the farms of Khandesh region all seasons throughout the year. The adsorption by using biochar is a cost-effective and eco-friendly technique which easily available and applicable for dyes removal with no harmful effect to the environment. The work we have conducted was for synthetic dye and was focused for different parameters. We observed that percentage removal efficiency by powder biochar is more efficient.
Materials and Method
The material used in the present study is crystal violet, the molecular formula is C25H30C1N3, and the molecular weight is 407.99. A stock solution of crystal violet was prepared in 1000 mg/l was prepared by dissolving a 1.0g of Crystal violet dye in 1000ml double distilled water. Subsequently, all required working solutions are prepared from the parent solution were prepared from the parent solution.
Preparation of Biochar
The sample of the banana stem was collected from a nearby village farm known as Takhrkheda connected with the campus of Kavayitri Bahinabai Chaudhari North Maharashtra University Jalgaon. After collection of the sample, the banana stem biomass was pyrolysis at 3500C and 4500C respectively and converted as a final product in the form of Biochar.
Method used for the preparation of biochar
The air-dried fresh banana stems were cut into pieces and airtight in the aluminum foil and wrapped with aluminum foil and placed in the muffle furnace. The material was slowly pyrolyzed for up to 2 hours at 3500C and 4500C. After the pyrolysis, the biomass was grind in the mixer and sieved more than at <250µ particle size to get powdered material. The prepared biochar final product of banana stem was named BSB 350 and BSB 450 and was utilized for the batch adsorption study. More information about the preparation of BSB 350 and BSB 450 biochar detail available8.
Why Banana (Musa Accuminata)
Banana is widely available in the study area as well as its second most important fruit crop in the Indian agricultural crop pattern. Statistical data shows that Banana cultivation is above 20% of the area under the whole crop; among the fruit production, it is above 37% in the Indian subcontinental. As per the state of Maharashtra is concern it shows the second rank. The study area also shows that Jalgaon in Maharashtra is a key Banana producing district. which covered 50,000 hectares of area in the Khandesh region.
Batch experiment for adsorption of crystal violet dyes
The adsorption experiments of the crystal violet have been assessed in the triplicate batch method in the 100 ml flask. The control findings were conducted with 20 mg/l of 25 ml volume with CV dyes with the addition of 25 to 220mg/l adsorbent at pH 3.0. Moreover, these CV+BSB-350 and CV+BSB-450 mixture was shacked thoroughly by using an orbital shaker (Steelmet Novatech Model Table-Top) at 150 rpm for 1hr. The mixture of CV+BSB-350 and CV+BSB-450 was filtered through a Whatman’s filter paper number 42 and the filtered solutions were used to estimate the removal capacity of BSB-350 and BSB-450. Analyzing the concentration of CV was accomplished by taking an absorption using UV-spectroscopy (SL-159, Elico, India). The different batch studies i.e., the concentration of CV (60-220 mg/l) was respectively, pH of CV (3-10), and Dose of BSB-350 and BSB 450 (25-450 mg/L).
Result and Discussion
Physical Characteristics of BSB-350 and BSB-450
To analyze the characteristics of BSB-350 and BSB-450 surface structure the FESEM images were done by SEM analyzer (FESEM, S-4800, Hitachi, USA). The first images (a) clearly show in Figures 1 and 2 the porous smooth and adsorptive structure. The second image (b) shows both adsorbents and shrink surfaces with blocked pores.
 |
Figure 1: FESEM images for BSB-350 adsorbent (a) before and (b) after CV adsorption. |
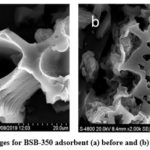 |
Figure 2: FESEM images for BSB-350 adsorbent (a) before and (b) after CV adsorption. |
Table 1: Physico-chemical Characteristics of Banana Stem Biochar (BSB).
|
Samples |
BSB-350 |
BSB-450 |
|
Yield (%) |
45.30% |
39.49% |
|
Ash (%) |
51.87% |
47.17% |
|
Volatile Matter (%) |
50.13% |
53.64% |
|
Fixed Carbon (%) |
99.93% |
93.53% |
|
pH in ultrapure water (1:5 w/w) |
8.75 |
8.61 |
|
Electrical Conductivity (EC) |
56.81mS/cm |
48.26mS/cm |
|
Cation Exchange Capacity (CEC) |
132.9cmol-kg-1 |
132.7cmol-kg-1 |
|
Organic carbon (%) |
28.74% |
19.98% |
|
Organic Matter (%) |
49.54% |
34.44% |
|
Metals in Extracted |
(µg/g) |
|
|
Sodium (Na) |
205.3 |
205 |
|
Potassium (K) |
2815 |
2551 |
|
Calcium (Ca) |
240.48 |
160.32 |
|
Magnesium (Mg) |
559.36 |
729.6 |
Effect of pH
pH is the most important and effective factor for the adsorption process, surface chemistry of sorbent and sorbet as well the media pH also effective and shows the efficiency and productiveness in the adsorption process. Crystal violet adsorption on BSB-350 and BSB-450 triplicate for pH batch study was carried out (pH 3 to pH 10) and the results result observed in figure no 3 seems to be maximum adsorption of CV to BSB-350 is 95.71% at 3 pH and BSB-450 is having maximum adsorption i.e., 99.21% at 10 pH. Similar findings were observed by6,7,10.
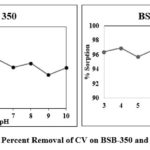 |
Figure 3: Percent Removal of CV on BSB-350 and BSB-450 |
Dose
The process of adsorption is consequently depending on the amount or dose of sorbent and sorbet present in the solution. The present study carried out in batch which has a dose of BSB-350 and BSB-450 is 25 mg/l to 200 mg/l at an interval of 25mg/l means 25,50,75 up to 200 were used in the triplicate study for obtaining higher adsorption dose. In BSB-350 dose, study obtaining data shows the higher adsorption at a 25mg/l dose having higher adsorption is 92.87% after that its slow decline up to 200mg dose and its 64.15% means the higher adsorption at 25 mg dose and whenever increasing doses, the saturation of adsorbent will occur, and the efficiency of adsorption will be decreased. As per literature, similar results were obtained from16,18,20. CV adsorption on BSB450 shows the maximum adsorption at a 25 mg dose is 95.11% and also shows the same pattern of adsorption as BSB-350 for increasing dose. As shown in figure 4.
 |
Figure 4: Showing Dose effect of BSB-350 and BSB-450 on percent adsorption of CV Click here to View figure |
Time study
The adsorption process is one of the most important factors to find and obtain accurate contact time for the sorption batch process. The present study is a triplicate batch time variation experiment that was carried out at contact time from 5 min to 60 min. Which was applied to find maximum sorption at a particular time, as shown in figure 5. In BSB-350 maximum adsorption at 1hr contact time is 95.60% but contact time 25min minute it shows 95.21% adsorption and 25 to 50 it’s a slightly increased 0.5% so 25min its also applicable for maximum adsorption; in BSB-450 experiment obtained data shows the maximum adsorption with contact time is obtained at 1hr it is 94.5% has observed by the various researcher study in the filled that is2,11,14.
 |
Figure 5: Contact time ratio of BSB-350 and BSB- 450 for CV adsorption |
Concentration study
The adsorption capacity of BSB-350 and BSB-450 was determined by using various initial concentrations. By using constant dosage (25 mg/l), the concentration effect of initial CV dye was examined by using the range of 60 -200 mg/l which shows adsorption study on biochar quoted in Figure 6. The obtained data shows CV adsorption onto BSB-350 using an initial concentration of CV is 60 mg/l to 200 mg/l triplicate data shows maximum adsorption is 158.38 mg/g of banana stem biochar prepared at 3500C and a similar experimental set up was used to obtained data of BSB-450. Which shows maximum adsorption of CV 124.05 mg/g. thus it observed that pyrolysis temperature varying is proportional to the adsorption capacity.
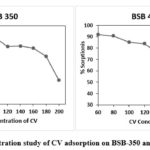 |
Figure 6: Concentration study of CV adsorption on BSB-350 and BSB-450 |
Isotherm Study
The adsorption isotherm study which was carried out shows the applicability of adsorbents in the sorption process. In the present investigation, the study was carried out for Freundlich, Langmuir, Temkin, and Dubinin–Radushkevich Isotherm model. using the nonlinear equations and R2 value which was obtained from the data were following equation was used. Figure 7 shows the Adsorption isotherm model plot of CV dyes on BSB-350 and BSB-450 with the isotherm plots which were plotted using Ce (mg/l) Vs Qe (mg/g) have compared with exponential data. In the present work maximum adsorption of CV on BSB-350 is 208.33 mg/l and the most applicable model is Langmuir because its R2 is 0.7761 which shows the nearest to 1 and high among both four models’ R2 value similar results have been obtained by various workers which shown in Table No 3. To conduct an empirical study of adsorption isotherms, we used four basic models, namely Langmuir, Freundlich, Temkin, and Dublin-Radushkevich, which were evaluated and quantified based on sorption data obtained on BSB 350 and BSB-450. We evaluated each of these variables using the following equations shown in the following table21:
 |
Table 1: Click here to View table |
The results obtained from our study are quoted in table 3 for these results Langmuir model was applied. In batch adsorption CV dye on BSB-450 experimental data interpreted for maximum adsorption 153.50mg/l and as per our study we suggest that best-fitted model is Temkin because of the estimated value of R2 is 0.9645 but, simultaneously there is the applicability of Freundlich and Langmuir model due to the R2 value 0.9425 and 0.9472 respectively.
 |
Figure 7: Isotherm Model Plots for CV Adsorption. |
Table 3: Previous study on adsorption of CV on to various Adsorbent.
|
Sr. No |
Adsorbent |
Temperature |
Removal |
Reference |
|
1 |
Azolla pinnata |
250C |
194.2 mg g-1 |
[11] |
|
650C |
323.4 mg g-1 at |
|||
|
2 |
Peanut straw char |
3500C |
256.4 (mmol/kg) |
[12] |
|
3 |
Soybean straw char |
3500C |
178.6 (mmol/kg) |
|
|
4 |
Rice hull char |
3500C |
123.5 (mmol/kg) |
|
|
5 |
Granular activated carbon |
NA |
0.095 g/g |
[13] |
|
6 |
P. australis |
4500C |
500(mg/g) |
[14] |
|
7 |
Bagasse fly ash |
25-10000C |
41.90(1/mg) |
[7] |
|
8 |
Banana Stem Biochar |
3500C |
208.33 mg/L |
Present Study |
|
4500C |
153.50mg/L |
Present Study |
Relative study of various waste materials used for the preparation of biochar and its application for adsorption of crystal violet dye as well as isotherm model study are shown in table no 3. Subsequently, research findings on the capacity of adsorbent and its preparation for pyrolysis temperature show the variability for biochar and the raw biomass used for the activated charcoal preparation. Multiple linear reasons representing the external mass transfer followed by intraparticle or pore diffusion are similarly observed by11,12,13,14. Give a concrete base to our study.
Conclusion
From the research work, it may conclude that biochar prepared from the banana stem (Musa Acuminata) is a wholly available variety of bananas in the Khandesh region that can be utilized for the reduction of pollution from industrial wastewater as an adsorbent in the form of biochar. The highest removal of crystal violet dyes on the pH 3 is 95.71% and the minimum dose of 25mg/ contact time is also very low from 25min to 60min. From our study, it is also observed that the Langmuir isotherm model as compared to Freundlich, and Dubinin-Radakovich’s isotherm is best fitted to obtain the result. To disperse crystal violet blue adsorption to govern by adsorption isotherm kinetics and it was proved that the banana stem biochar can be applied for the adsorption of crystal violet. it can also be interpreted that the SEM images of BSB-350 and BSB-450 are smooth and porous structures, which seems to be helpful in the adsorption process. It was also recommended for future studies to investigate industrial applications and utilization of agricultural waste for pollutant removal in the environment.
Acknowledgment
Sincerly gratitude to BARTI, Pune for award of BANRF-2018, also thanks full to R&D section and Director SEES, KBCNMU, Jalgaon for providing TAP award as well research facility.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
References
- Ajji, Z. & Ali, A. M. Adsorption of methyl violet and brilliant blue onto poly (vinyl alcohol) membranes grafted with N-vinyl imidazole/acrylic acid. Nucl. Instrum. Methods Phys. Res. Sect. B. (2007) 265, 362–365.
CrossRef - Aladag, Erdinç, Fil, Baybars Ali, Boncukcuoglu, Recep Sozudogru, Onur Yılmaz, Alper Erdem Adsorption of Methyl Violet Dye, A Textile Industry Effluent onto Montmorillonite—Batch Study, Journal of Dispersion Science and Technology, (2014),35,12. https://doi.org/10.1080/01932691.2013.873865 1737-1744.
CrossRef - Azizian, Saeid & Haerifar, Monireh & Bashiri, Hadis.. Adsorption of methyl violet onto granular activated carbon: Equilibrium, kinetics and modeling. Chemical Engineering Journal. (2008) 146. 36-41. 10.1016/j.cej.2008.05.024.
CrossRef - Hao Zhu, Haiming Zou; Characterization of algae residue biochar and its application in methyl orange wastewater treatment. Water Sci Technol, (2021); 84 (12): 3716–3725. doi: https://doi.org/10.2166/wst.2021.473
CrossRef - Adsorption of Cationic and Anionic Dyes onto the activated Carbon Prepared from Grapevine Rhytidome J. Dispersion Sci. Technol., (2011), 33: 846–853. https://doi.org/10.1080/01932691.2011.579861
CrossRef - Hu, R., Xiao, J., Wang, T., Gong, Y., Chen, G., Chen, L., Tian, X.,. Highly concentrated amino-modified biochars using a plasma: evolution of surface composition and porosity for heavy metal capture. Carbon, (2020b) 168, 515–527.
CrossRef - Indra D. Mali, Vimal C. Srivastava, Nitin K. Agarwal, Removal of Orange-G and Methyl Violet dyes by adsorption onto bagasse fly ash kinetic study and equilibrium isotherm analyses; Dyes and Pigments, (2005), 69 (2006) 210-223.
CrossRef - Jadhav Shridhar K., More Ganpat B., and Thorat Sanjaykumar R.., Competitive adsorption of leather dyes by using biochar prepared from MUSA acuminata: a cost-effective technique., Res. J. Chem. Environ; (2021), Vol. 25(11); 110-118; doi: https://doi.org/10.25303/2511rjce110118
CrossRef - Li, H., Dong, X., da Silva, E.B., de Oliveira, L.M., Chen, Y., Ma, L.Q.,. Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere, (2017), 178, 466–478
CrossRef - More Ganpat B., Jadhav Shridhar K., and Thorat Sanjaykumar R., Development of a Novel Submerged Membrane Bioreactor (SMBR) for Treatment of Textile Wastewater, International Journal of Research in Advent Technology, (2019), 7(4), 35-46.
CrossRef - Muhammad Raziq Rahimi Kooh., Linda B. L. Lim., Muhammad Khairud Dahri.,Lee Hoon Lim., J. M. R. Sarath Bandara, azolla pinnata: An Efficient Low Cost Material for Removal of Methyl Violet 2B by Using Adsorption Method; Waste Biomass Valor.(2015), DOI 10.1007/s12649-015-9369-0
- Ren-kou Xu, Shuang-cheng Xiao, Jin-hua Yuan, An-zhen Zhao; Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues, Bioresource Technology 102, (2011) 10293–10298C, doi: 10.1016/j.biortech.2011.08.089
CrossRef - Saeid Azizian, Monireh Haerifar, Hadis Bashiri; Adsorption of methyl violet onto granular activated carbon: Equilibrium,kinetics and modeling, Chemical Engineering Journal 146 (2009) 36–41, doi:10.1016/j.cej.2008.05.024
CrossRef - Suhong Chen, Jian Zhang , Chenglu Zhang, Qinyan Yue, Yan Li, Chao Li, Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis; Desalination 252 (2010) 149–156, doi:10.1016/j.desal.2009.10.010
CrossRef - Suhong Chen, Jian Zhang, Chenglu Zhang, Qinyan Yue, Yan Li, Chao Li, Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis, Desalination, Volume 252, Issues 1–3, (2010), Pages 149-156, ISSN 0011-9164, https://doi.org/10.1016/j.desal.2009.10.010.
CrossRef - Xiang, W., Zhang, X., Chen, J., Zou, W., He, F., Hu, X., Tsang, D.C., Ok, Y.S., Gao, B., Biochar technology in wastewater treatment: a critical review. Chemosphere 252, (2020) 126539
CrossRef - Yu, J., Zhang, X., Wang, D. & Li, P. Adsorption of methyl orange dye onto biochar adsorbent prepared from chicken manure. Water Sci. Technol. (2018), 77 (5–6), 1303–1312.
CrossRef - Deniz, Fatih. Removal of an azo-metal complex textile dye from colored aqueous solutions using an agro-residue. Microchemical Journal (2011). 99. 296-302. 10.1016/j.microc.2011.05.021.
CrossRef - Hu, S., Xiang, J., Sun, L., Xu, M., Qiu, J., Fu, P.,. Characterization of char from rapid py-rolysis of rice husk. Fuel Process. Technol. (2008) 89, 1096–1105.https://doi.org/10.1016/j.fuproc.2008.05.001
CrossRef - A.C.Sonawane and S.R. Thorat, biculture characteristics of dye decolorizing bacteria isolated from textile industry treatment of wastewater.J.Pure&Appl.Microbiol. (2008),2,497-500.
- Choudhary, B., Paul, D., 2018. Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. J. Environ. Chem. Eng. (2018) 6, 2335–2343. https://doi.org/10.1016/j.jece.2018.03.028.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










