Removal of Cadmium, Copper and Lead from Aqueous Solution Using Activated Carbon Prepared from Avocado Kernel
Chemistry department, Dilla University, P.o.Box. 419, Dilla, Ethiopia.
Corresponding Author E-mail: mitiku.abdisa@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/380107
Article Received on : 22-11-2021
Article Accepted on :
Article Published : 05 Feb 2022
Reviewed by: Dr. Muwafaq Rabeea
Second Review by: Dr. A. Gupta
Final Approval by: Dr. Tawkir Sheikh
Toxic heavy metal ions are extremely harmful to living things and the environment due to their toxicity, carcinogenicity, ability to collect in nature, and capacity to contaminate surface and ground water. The focus of this research was to develop an appropriate and low cost adsorbent for the removal heavy metal ions from aqueous solutions. The activated carbon was prepared from avocado kernels and characterized by using XRD, FTIR, and pHPZC. From the XRD spectra the crystal size of AC-1 and AC-2 were calculated. The sample with the smallest crystallite size (40.05 nm) was found to be AC-1 and chosen for the further characterization and sorption experiments. Batch studies on synthetic samples were performed at room temperature. pH effect, initial concentration, adsorbent dosage, initial concentration, and contact time were investigated and also heavy metal ion adsorption isotherms were calculated. At pH 7, the highest removal effectiveness of metals (copper, cadmium, and lead) by activated carbon adsorbent made from avocado kernel was achieved. The best adsorbent dose for cadmium and lead was 500 mg, 700 mg for copper, and the optimum contact times for lead, cadmium and copper ions were 120, 60 minutes, respectively. In these optimum conditions the removal efficiencies were 87%, 89.4%, and 99.5% for copper, Lead, and Cadmium ions respectively. The removal efficiencies for copper, lead, and cadmium ions were 87 %, 89.4 %, and 99.5 %, respectively, under these optimal conditions. The Langmuir isotherm model has the strongest correlation with the obtained results. According to the findings, activated carbon made from avocado kernels has high adsorption capacity for removing heavy metal ions from aqueous solutions.
KEYWORDS:Activated Carbon; Adsorption; Avocado Kernel; Heavy Metal
Download this article as:| Copy the following to cite this article: Chimdessa M. A, Ejeta B. A. Removal of Cadmium, Copper and Lead from Aqueous Solution Using Activated Carbon Prepared from Avocado Kernel. Orient J Chem 2022;38(1). |
| Copy the following to cite this URL: Chimdessa M. A, Ejeta B. A. Removal of Cadmium, Copper and Lead from Aqueous Solution Using Activated Carbon Prepared from Avocado Kernel. Orient J Chem 2022;38(1). Available from: https://bit.ly/3Gv4lnd |
Introduction
Chemists and environmental engineers have long been concerned about heavy metal contamination of water. As a result of industrial and metallurgical activities that release the majority of toxic substances into the environment, heavy metal pollution has become a worldwide issue.1
Nowadays, if industrial and home wastewater is not adequately handled, it causes significant environmental harm and has a negative impact on human health. Heavy metals are dispersed throughout the environment by industrial wastewater, causing major difficulties and harming living species. Heavy metals have caused a variety of diseases and disorders in many living organisms as a result of their widespread industrial applications.2 Although heavy metal pollution is not a new problem, its management and prevention remain a major concern around the world. When these toxic metals are found above the tolerance level, they can cause accumulative poisoning, cancer and brain damage.
Pb2+, Cr3+, As3+, Zn2+, Cd2+, Cu2+, and Hg2+ are the heavy metals most typically discovered in contaminated streams.3 These poisonous metals have currently poisoned our environment, water, soil, and food chain, and have been found to be slightly dangerous even at low concentrations.4
Heavy metals are removed from aqueous solutions using ion exchange, chemical precipitation/co-precipitation, filtering, coagulation, membrane technology, and commercial activated carbon, among other methods. 2 The expense of these methods, the effectiveness of the operations, and the disposal of waste generated are the key drawbacks.5 As a result of these shortcomings, researchers are looking for alternative heavy metal treatment strategies.6 A variety of sorbents can be used for adsorption, such as activated carbon, phosphate rocks, carbonates, zeolites, and alkaline agents, but recently low-cost sorbents have been studied .7,8,9
Adsorption of dangerous metals from industrial effluents using naturally available materials has been shown to be effective. Microbes and naturally available plant materials, as well as dead waste biomass, are widely used in this process.10,11 Some of biosorbent have a sorption capacity due to the existence of functional groups.5 The utilization of adsorption is low cost, ecologically beneficial, and readily available technology. Maize tassels, 11 watermelon shell, 12 and coffee beans 13 are some of the plant-based adsorbents that have already been employed and updated for heavy metal removal from wastewater and aqueous solutions. For seeds, stones, and pits, only carbonized samples adsorption capabilities have been investigated. Although there is no indication that non-carbonized forms are inefficient, new research suggests that high specific surface area magnitude carbonization is the only way to generate efficient adsorbents from naturally existing materials. Fruit seeds haven’t been given much thought as sorbents. Human consumption or food preparation generates a significant amount of waste.14 Therefore the aim of this study was to evaluate the removal efficiency of activated carbon prepared from avocado kernel for the removal heavy metals (cadmium, copper and lead) from aqueous solutions.
Materials and Method
Materials
The adsorbent material, avocado kernels were collected from local markets of Dilla town, south Ethiopia. O.1M of NaOH and HCl (BDH, England) for pH regulating. The aqueous solutions containing Cd (II), Cu (II) and Pb (II) were prepared from Cd(NO3)2, Cu(NO3)2.3H2O, and Pb(NO3)2 respectively. Deionized water was used for the preparation of standard stock solution. Using a version 520 Atomic absorption spectrometer (Perking Elmer, USA), the concentration of Cadmium (II), copper (II) and lead (II) ions in the aqueous solution were determined. For calcination, an electrical furnace (Model Nabertherm) was used.
Activated carbon preparation
Avocado kernels (persea americana) were gathered and cut into approximately 2-3 mm pieces. The avocado kernels were cleaned in distilled water, dried for three days in the sun, and ground with a mortar and pestle. The impregnation procedure was carried out by mixing 100 g of avocado kernels with a 43 % K2CO3 solution (AC-1) and 2M of HCl solution in 1:2 (W:V) ratio (AC-2). This mixture was dried in an oven at 50°C for 12 hours and sieved, then washed with distilled water until the pH of the solution reached 7.0 and dried in an oven at 100°C overnight. The sample was heated in an electric furnace for 160 minutes at 800 oC at a rate of 5 oC. The product was allowed to cool to room temperature and again washed with distilled water and dried in an oven at 100°C for 3 hours.15
Characterization of the adsorbents
Using a Fourier transforms infrared [FT-IR] spectrophotometer (Perkin Elmer model Spectrum 65 FT-IR), the functional groups of produced activated carbon were identified before adsorption. A X-ray diffractometer was used to analyze the X-ray diffraction of activated carbon. A reported method was used to determine the biosorbent’s point zero charge (pHzpc).16 The crystallite domain size activated carbon (AC) was calculated from the width of the peaks, using Scherrer’s formula.
D = K λ / β Cos θ (1)
Where D is the average crystallite domain size, K is the dimension shape factor has a typical value of about 0.9, λ is wavelength; β is the full width at half maximum (FWHM) and θ is the diffraction angle.17
Adsorption study
The effects of several parameters on the adsorption process were investigated, including the initial concentration of heavy metal ions, the amount of adsorbent, contact time, and the effect pH. The value of 0.1 g activated carbon made from avocado kernel was put in 100 mL of the metal ions solution with a concentration of 60 ppm to determine the optimum pH. The experiment was then repeated with contact time of 30, 60, 90, 120, and 150 minutes at various pHs (1, 3, 5, 7, and 9) and the adsorbent was exposed to pollutant metal ions in ambient temperature. The samples were then filtered using 0.45-inch filter paper, and the residual metal ions were measured using an atomic absorption instrument. The procedure was repeated separately for each metal ion. The percent adsorption (%) is calculated using the following equations:
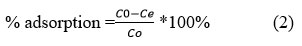
Where Co is the initial concentration of the metal ions in the solution (mg/L) and Ce is the equilibrium concentration or final concentration of metal ions in the solution (mg/L).

The following equation was used to determine the adsorption capacity, which is defined as the amount of ions adsorbed per mass unit of activated carbon (mg/g).
Adsorption isotherm
To characterize the adsorption process of Cu(II), Cd(II), and Pb(II) onto activated carbon prepared from avocado kernel, the two empirical models of Langmuir and Freundlich isotherms were studied.
The Langmuir equation expressed as follows:

Where Ce is the equilibrium concentration of solute (mmol L−1), qe is the amount of solute adsorbed per unit weight of adsorbent (mmol g−1), Qm is the adsorption capacity (mmol g−1), or monolayer capacity, and b is a constant (L mmol−1).
The slope and intersect of the linear plots of Ce/qe vs Ce determine the values of Qm and b. The Freundlich equation is written as follows:
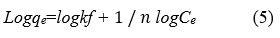
Where k is adsorption capacity (mmol g−1) and n is degree of nonlinearity between solution concentration and adsorption respectively. These constants can be obtained from the plot of log qe versus log Ce. 18
Result and discussion
X-ray diffraction (XRD)
The intensity of the measured rays with respect to scattering angle (2θ) was used to determine the amorphous nature of the activated carbon. Figure 1 shows the X-ray diffractometer pattern of prepared activated carbons AC-1 and AC-2.
The XRD pattern of typical amorphous carbon showed broad asymmetric peaks corresponding to 2θ = 23.05º for AC-1 with sharp peaks and 22.0º for AC-2 with absence peak, which confirm that the samples are amorphous or may have microporous structure, which is considered as suitable for adsorption. The lack of sharp peaks in activated carbon indicates that it has a mostly amorphous structure, which is a good thing for well-defined adsorbents.19 The presence of sharp peaks in the AC-2 around 43.93º offers indication of more crystal structure of carbon and less amorphousness structure when compared with AC-1 activated carbon structure. 20, 21
The XRD spectra were examined using origin 8.0 software and the crystallite size was calculated from the width of the peaks, using Scherrer’s formula and crystal size for AC-1 and AC-2 was 40.05 and 44.84 respectively. The sample with the smallest crystallite size (40.05) was found to be AC-1. This sample was chosen for the further characterization and sorption experiments.
 |
Figure 1: XRD analysis of activated carbon of AC-1 and AC-2 |
Determination of pH of point zero charge (pHPZC)
The PHpzc of an adsorbent is a critical property that indicates the pH at which the adsorbent is neutral, whereas beyond this pH, the adsorbent becomes positively or negatively charged. 22 Figure 2 shows the pHPZC sorbent, which was found to be around 6.5. At pH less than 6.5, the adsorbent surface was charged positively, and the cation was repelled from the surface, but at pH greater than 6.5, the adsorbent surface was charged negatively, and electrostatic attraction occurred between the surface of the adsorbent and the metal ion. As a result, when the surface of the adsorbents is negatively charged, metal ion biosorption occurs. At pH > 6.5, the electrostatic interactions of AC enhance the accumulation of metal ions on the adsorbent surface.
 |
Figure 2: pH point zero charge (pHPZC) of synthesized activated carbon |
FTIR analysis
The FTIR spectra in Figure 3 show a lot of peaks, indicating that the adsorbent is complex.23 The FTIR results revealed an absorption band of 3430 cm-1, which can suggest either -OH or N-H groups, and bands of 1400 to 1420 cm-1, which were attributed to aromatic C=C stretch vibration.24 Figure 1 show that the band at 2944 cm-1 is caused by C-H stretching vibrations in saturated aliphatic compounds, while the band at 1654 cm-1 is caused by -NH bending vibrations in primary amines. At 1420 cm-1, a small peak was found, that corresponds to aromatic ring C-C stretching vibrations. 25 the peak observed at 1007 corresponds to the C-O stretch of at 1007 correspond to the C-O stretch of alcohols, carboxylic acids, esters, and ethers. 26 The absorption bands at 668 cm-1 and 703 cm-1 could be attributed to the existence of an alkyl halide.
Its adsorptive property is due to the presence of acidic functional groups.27 Natural plant materials that include acidic functional groups in their biochemistry are responsible for metal ion uptake. 28
 |
Figure 3: FTIR spectra of synthesized activated carbon (AC-1). |
Optimum Conditions for heavy metals (cadmium, copper, lead) Removal by the Sorbent
pH effect
The optimum pH for the removal of Cd (II), Cu (II) and Pb (II) was 7. At pH greater than 7, due to the formation of hydroxide metals were precipitated and sorption removal was low. Because of positive charge density on the metal binding site at lower pH, metal ions biosorption is reduced and hydrogen ions effectively compete with metal ions for binding. When a pH of the biosorbent raised, deprotonation increased the negative charge density on the cell surface.29 While maintaining the other optimal conditions constant, the percentage adsorption of copper, lead, and cadmium was determined to be 75 %, 86 %, and 96 % at pH 7 as shown in figure 4.
 |
Figure 4: Effect of pH on the removal of metal ions Cu (II), Pb (II) and Cd (II) |
Effect of dosage of adsorbent
Figure 5 shows the patterns in metal ion adsorption, which indicate a direct relationship with biomass quantity. The adsorption of metal ions rises as the amount of biomass increases. 30 This is because the more biomass, the more links there are for the production of metal ion complexes. Further increasing does not result in a substantial change, indicating the presence of ions-saturated connections and the establishment of equilibrium between biomass and ions. While maintaining the other optimal circumstances constant, the percentage adsorption of lead and cadmium was found to be 89% and 98.8%, respectively, in 500 mg of adsorbent dosage, while the percentage adsorption of copper ions was found to be 83% in 700 mg of adsorbent dosage as shown in Figure 5.
 |
Figure 5: Effect of dosage of adsorbent on the removal of Cu2+, Pb2+and Cd2+. |
Effect of initial concentration of metal ions
Metal ions are adsorbed by specific active site of the adsorbent at low concentration, but as metal ions concentration increase, binding sites becomes more quickly saturated while the biomass concentration remains constant.31 As indicated in Figure 5, initial metal ions concentrations of Pb2+, Cu2+, and Cd2+ were 60 ppm, 80 ppm, and their adsorption was found to be 85 %, 80 %, and 99 %, respectively, indicating that Cd2+ ions have a greater extracted than Cu2+ and Pb2+ ions when optimized parameters were kept constant and initial ion concentrations ranged from 20 to 100 mg/L. Figure 6 shows the effect of initial ion concentration on the biosorption process.
 |
Figure 6: Effect of initial concentration on the removal of Cu2+, Pb2+and Cd2+ |
Effect of contact time
Contact time influence on the rate of metal ion uptake onto activated carbon from avocado kernels. The values of percent removal grew rapidly at the start of adsorption. The rapid absorption was attributable to the metal ion’s rapid transfer to the vacant adsorption sites on the adsorbent’s surface, and after 60 minutes for lead and 120 minutes for cadmium and copper, percent removal slowed. As a result, the three metal ions adsorb quickly on the biosorbent. The percent removal of Lead, copper and cadmium ions was determined to be 85 %, 87 %, and 99.5 %, respectively, while the other parameters remained at their optimal values, as shown in Figure 7. The adsorbed quantity of the three metal ions remained practically unchanged after 120 minutes. The two-stage sorption mechanism has been extensively reported in the literature, with the first being fast and quantitatively dominant and the second being slower and quantitatively insignificant. 32,33
 |
Figure 7: Effect of contact time on the removal of metal ions Cu2+, Pb2+and Cd2+. |
Adsorption isotherms analysis
The Langmuir and freundlich isotherm models were studied in this study to describe the link between the level of adsorption and the equilibrium concentration in the liquid phase. Table 1 shows this relationship as well as the adsorption parameters for each model. In this adsorption process, the amount of the correlation coefficient (R2) demonstrates that the Langmuir model states a better relationship adsorption process. Copper, lead, and cadmium ions had maximal adsorption capacities of 16.3, 7.9, and 142.85 mg/g, respectively. These values indicate high adsorption potential of the generated adsorbent in the adsorption of metal ions examined from aqueous solutions. The results of the adsorption isotherms are compatible with broad bean peel removal of cadmium from aqueous solution. 34,35
Table 1: Adsorption isotherm parameter for Cu, Pb and Cd by activated carbon produced from avocado kernel
|
Metal ions |
Langmuir parameters |
Freundlich parameters |
||||
|
Qm (mg/g) |
b |
R2 |
kf(mg/g) |
1/n |
R2 |
|
|
Cu |
16.13 |
0.05 |
0.998 |
1.3 |
0.589 |
0.988 |
|
Pb |
7.9 |
0.075 |
0.998 |
0.77 |
0.575 |
0.996 |
|
Cd |
142.85 |
0.189 |
0.999 |
37.32 |
0.361 |
0.996 |
Conclusion
The purpose of this work was to produce effective low-cost sorbents made from avocado kernels for the removal of heavy metal ions from aqueous solution. The adsorption of heavy metals ions like copper, lead, and cadmium on avocado kernel activated carbon showed a high efficiency. XRD, FTIR, and pHzc were used to characterize the activated carbon. The XRD results show that the materials are amorphous or have a microporous structure, making them appropriate for adsorption. The crystal size of activated carbon and its pHzc were found to be 40.05 nm and 6.5, respectively.
pH, concentrations, adsorbent dosage, and time were all adjusted to optimum. At optimized conditions (pH 7 with 120 min of contact time, 700 mg adsorbent dose, 60 ppm concentration), (pH 8 with 120 min of contact time, 700 mg adsorbent dose, 100 ppm of concentration), and (pH 8, with 120 min of contact time, 100 ppm of concentration, 500 mg adsorbent dose), respectively, the highest removal percentages of Pb2+, Cu 2+, and Cd2+ ions were 89.4%, 87%, 99.5% respectively. According to this research, the Langmuir model is better for the removal of metal ions. Avocado kernel can be employed as an effective adsorbent in the removal of heavy metal ions from aqueous solution due to its abundance and high adsorption ability.
Acknowledgment
For financial support and sponsorship, the authors are grateful to Dilla University’s Research Dissemination Office. We are also grateful to Dilla University’s Department of Chemistry for providing us with the research facility, as well as the necessary apparatus and other resources
Conflict of interest
The authors declare no conflict of interest
References
- Faisal, M. S. African Journal of Biotechnology., 2004, 3, 610-7.
- Mihajlovic, M.T.; Lazarevic, S.S.; Jankovic-Castvan, I.M.; Kovac, J.; Jokic, B.M., Janackovic, D.T.; Petrovic, R.D. Clean Technol.Environ. Policy.,2015, 17, 407–419.
- Stavropoulos, G.G.; Samaras, P.; Sakellaropoulos, G.P. J. Hazard. Mater., 2008, 151, 414-421.
CrossRef - Chehregani, A.; Malayeri, B.; Golmohammadi, R. P. J. Biol. Sci., 2005, 8 (4), 622–625.
- Wang, J.; Chen C. Biotechnol. Adv.,2009, 27, 195-226.
CrossRef - Forgacs, E.; Cserhti, T.; Oros, G. A review, Environ Int., 2004, 30, 953-971.
CrossRef - Hernandez-Ramirez, O.; Holmes, S.M. Journal of Material Chemistry., 2008, 18, 2751-2761.
CrossRef - Toohi, H. T.S A.; Rabeea, M. A.; Abdullah, J. A.; Farra, R. Carbon Letters., 2020, 1-13.
- Rabeea, M.; muslim, R. F.; Abdullah, J. Carbon letters., 2020, 31(06), 199-205
CrossRef - Al-Zboon, K.; Al-Harahsheh, M.S.; Hani, F.B. Journal of Hazardous Materials., 2011, 188, 414-421.
CrossRef - Gavrilescu, M. Eng. Life Sci.,2004, 4, 219-232
CrossRef - Elizalde-Gonzalez, M.P.; Mattusch, J.; Pelaez-cid, A.A.; Wennrich, R. J. Anal. Appl. Pyrolysis., 2007, 78: 185-193.
CrossRef - Moyo, M.; Chikazaza, L. Am. J. Anal. Chem., 2013, 4, 689-695.
CrossRef - Malik, R.; Ramteke, D.S.; Wate, R. A. Indian J.Chem. Technol., 2006, 13, 319-328.
- Kassahun, D.; Khalid, S, Shimeles, A. International Letters of Natural Sciences., 2016, 54, 42-57
- Prola, L.D.; Acayanka, T.E.; Lima, E.C.; Umpierres, C.S.; Vaghetti, J.C.P.; Santos,W.O.; Laminsi, S.; Djifon, P.T. Indian Crop Production., 2013, 46, 328–340.
CrossRef - Ceyhan, A. A.; Şahin, O.; Baytar, O., Saka, C. Journal of Analytical and Applied Pyrolysis., 2013, 104, 378–38
CrossRef - Mohamed N. R. 2013, http://dx.doi.org/10.5772/54048.
CrossRef - Dipa, D.; Debi, P.S.; Meikap, B.C. Journal of Chemical Engineering and Process Technology., 2015, 6, 1-7.
- Basker, A., Syed Shabude, P.S. International Journal of Research in Sciences., 2014, 2, 42-43.
- Jadhav, A.S.; Mohanra, G.T.J. Carbon Science and Technology., 2016, 8, 32-39.
- Malik, R., Ramteke, D.S.; Wate, R.A. Indian J.Chem. Technol., 2006, 13, 319-328.
- Alexandre, B.; Matthew, A.; Silvio, L.P.D.; Eder, L.; Julio, C.P.V; de olivera, E.R. Desalin. Water Treat., 2015, 51, 1-16.
- Prola, L.D.T.; Acayanka, E.; Lima, E.C, Umpierres, C.S.; Vaghetti, JCP, Santos, W.O. Indian Crop Production., 2013, 46, 328-340.
CrossRef - Khambhaty, Y.; Mody, K.; Basha, S.; Jha, B. World J. Microbiol. Biotechnol.,2009, 25:1413–1421.
CrossRef - Olorundare, O.F.; Krause, R.W.M.; Okonkwo, J.O.; Mamba, B.B. Environ Sci Pollut Res., 2015, 22, 5780–5792
CrossRef - Da´vila-Jime´nez, M.M.; Elizalde-Gonza´lez, M.P.; Pela´ez-Cid, A.A.; Colloid Surf. A: Physicochem. Eng. Aspects., 2005, 254, 107.
- Gilbert, A.; Emmanuel, I.; Adebanjo, A.; Olalere.; G. Biomass Bioenerg., 2011; 35:2517–2525
CrossRef - Ho, Y.S. Bioresource Tech., 2005, 96, 1292-1296
CrossRef - Ryu, T.G.; Ryu, J.C.; Choi, C.H.; Kim, C.G.; Yoo, S.J.; Yang, H.S. Journal of Industrial and Engineering Chemistry. 2006, 12: 401-407.
- Horsfall, M.; Spiff, A.I. Acta ChimicaSlovinica., 2005, 52, 174–181.
- Naiya, T.K.; Bhattacharya,A. K.; Das, S. K. Environmental Progress in Chemical Engineering., 2009, 28, 535-546.
CrossRef - Saeed, A.; Akhter, M.W.; Iqbal, M. Separation and Purification Technology. 2005, 45, 25-31.
CrossRef - Benaissa, H. J. Hazard. Mater.2006, 132, 189–195.
CrossRef - Pehlivan, E.; Yanik, B.H., Ahmetli, G.; Pehlivan, M. Bioresour. Technol., 2008, 99, 3520-3527.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










