Synthesis of Insulin Loaded Nanoparticles and their Antidiabetic Studies
Department of Chemistry and Research Centre, Scott Christian College (Autonomous), Nagercoil- 629 003. Affiliated to Manonmaniam Sundaranar University, Abhishekapatti, Tirunelveli-627 012. Tamil Nadu, India
Corresponding Author E-mail: ashaanbaianmsc@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370627
Article Received on : 16-Sep-2021
Article Accepted on :
Article Published : 16 Nov 2021
Reviewed by: Dr. Krishnasamy K

Second Review by: Dr. Rabiu Garba Ahmad

Final Approval by: Dr. Bal Krishna
The goal of this present work turned into synthesis of polymeric nanoparticles from natural polymeric material. Natural polymeric nanoparticles have some unique physicochemical properties like stability, biocompatibility, biodegradability, and manage the drug release, so it’s used for drug transport. On this look natural polymeric material Casein and Chitosan are used for the synthesis of nanoparticles. Insulin drug was introduced to these polymeric nanoparticles, which are used for the drug delivery. These drug loaded polymeric nanoparticles are synthesised by nano precipitation technique. The particles size of drug loaded polymeric nanoparticles based totally the rate of stirring and time of nanoparticles synthesised. Insulin was used as a drug and Glutaraldehyde was used as a linking agent. This insulin drug becomes adsorbed on the surface of the nanoparticles. Those drug loaded nanoparticles are characterized by FT-IR spectrum and physical status is analysis by means of XRD pattern. The morphology of the drug loaded nanoparticles is studied by using Scanning Electron Microscopy (SEM). From those characterisation studies insulin drug was efficaciously loaded with the nanoparticles. It has high inhibitory property towards kind II diabetics.
KEYWORDS:Anti-diabetic; Casein; Chitosan; Insulin; Nanoparticles
Download this article as:| Copy the following to cite this article: Asha A, Malar G. S. P. L. Synthesis of Insulin Loaded Nanoparticles and their Antidiabetic Studies. Orient J Chem 2021;37(6). |
| Copy the following to cite this URL: Asha A, Malar G. S. P. L. Synthesis of Insulin Loaded Nanoparticles and their Antidiabetic Studies. Orient J Chem 2021;37(6). Available from: https://bit.ly/3qHgykc |
Introduction
Diabetes is the continual disease. Now a day’s diabetes mellitus is the widespread metabolic disease. Around 463 million humans dwelling with diabetics. In 2045 the number of suffers will increase to 700 million. People with kind II diabetic affected person’s wide variety accelerated in maximum of the countries1. Insulin is the important key to glucose of physical body. However kind I diabetes didn’t produce the insulin and type II didn’t responds to the insulin. So it leads to increase the blood sugar level2.
Natural polymeric nanoparticles derived from dwelling organism. And it has a few special residences like non poisonous, low immunogenicity, biodegradability, biocompatibility and so on. So this biopolymer like casein chitosan becomes used as a natural drug delivery vehicle3. Casein was a protein, which was found in milk. Because of the biological function, nutrients transfer from the mother to her youngsters4. It is a non toxic and high stable milk protein. This casein is proline- wealthy and has lacks of disulfide bonds and additionally poor secondary and tertiary structures5. Casein has four fundamental phosphate-rich sub-units, that are amphiphilic and assembled them self into a bilayer structure in the size 50-300 nm. But for hydrophobic interactions, the bilayers are also held collectively with the aid of calcium phosphate nanoclusters performing as bridges connecting those subunits4.
Chitosan is a linear polysaccharide; it’s acquired through partial deacetylation of chitin. It is received from the exoskeletons of marine arthropod including crabs, shrimps, lobsters, prawns and fungi6. Chitosan consisting 2-amino-2-deoxy-D-glucose units joined with glycosidic linkages7,8. Like casein, chitosan additionally have some special characters like non toxic, high drug maintaining ability, low immunogenicity, biodegradability, biocompatibility etc9. Chitosan is used in bandages, haemostatic vehicle, and wound recuperation belongings and also used as a anti microbial agent10-12. It is insoluble in water, but soluble in acids.
Material and Methods
Low molecular weight chitosan (LMW), Casein powder was purchased from Merck. Novolin R human insulin was purchased from Novo Nordisk. PBS, Glacial acetic acid and Glutaraldehyde was purchased from Sigma Aldrich. α-amylase, α-glucosidase enzymes was purchased from SRL Pvt. Ltd., Mumbai. Calcium Chloride and NaOH also purchased from Merck.
Experimental Methods
Syntheise of nanoparticles
2 grm of Casein was dissolved in 10mM calcium chloride salt solution with stirring then heating to 40°c for 15 mints. 2.5 grm of chitosan was dissolved in 1% acetic acid. This chitosan solution was added to casein solution as drop wise, with continuous stirring. 0.1 ml of glutaraldehyde was added to this mixture and stirrer for 3 hrs. Now cross linking Casein chitosan nanoparticles are formed.
Synthesis of Insulin loaded nanoparticles
0.1 ml of insulin delivered to that casein chitosan nanoparticles combination and nonstop stirred. Ultimately insulin drug loaded casein chitosan polymeric nanoparticles are synthesised. This aggregate become centrifuged at 7000 rpm for 30 mints and washed with water. Store that product as powder form for future research.
Characterization
Fourier Transform- Infrared (FT-IR) Spectroscopy
FT-IR spectrumbecome received from a PerkinElmer 1000 paragon FT-IR spectrometer. FT-IR spectrum was used to study the interplay between the drug and the polymeric nanoparticles.
Surface morphology and physical state analysis
Scanning electron microscopy becomes used to look at the morphology of synthesised dug loaded polymeric nanoparticles. The physical state of insulin loaded casein chitosan nanoparticles become studied the usage of XRD analysis.
Invitro Anti-Diabetic Assay
Inhibition of α- amylase assay
Different concentration of (100,200,400,600,800,1000µl/ml) drug loaded nanoparticles and standard drug was added to 500 µl of 0.20 mM phosphate buffer (pH6.9), which contains α- amylase solution and it had been incubated at 25°C for 10 min. 500µl of starch solution in phosphate buffer become added to the mixture again incubated at 25°C for 10 mints. The reaction was stopped with the aid of the addition of 1 ml of 3,5 dinitrosalicylic acid colour reagent. Again it had been incubated in boiling water and allows cooling at temperature. The reaction combination turned into diluted with water and absorbance was measure at 520nm against positive control.
Inhibition of α- glucosidase assay
To determine the inhibition of α- glucosidase activity incubating 1 ml of starch solution with various concentration (100, 200, 400, 600, 800,1000µl/ml) of drug loaded nanoparticles for 5 mints at 37°C. Add 200µl of α- glucosidase (0.1U/ml) to initiate the response and incubated for 5 mints at 37°C.To forstall the response the reaction mixture turned into heated with boiling water both for 2 mints. Using glucose oxidase peroxidise approach the amount of liberated glucose become measured.
Calculation of IC50 value
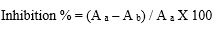
Whereas,
A a =Absorbance of control reaction
A b =Absorbance in the presence of sample of drug loaded nanoparticles
Using this formula IC50 value for both inhibition of α- amylase and Inhibition of α- glucosidase was calculated.
Result and Discussion
FT-IR spectroscopy
Fig 1. The peak at 1598 cm-1 is determined in the chitosan spectrum; it is the amide II group of bending vibration. In casein spectrum, the peak at 1650 cm-1 is absorbed it is denoted the carboxyl group of casein.
 |
Figure 1: FT-IR spectrum of Insulin, Casein, Chitosan |
Fig 2. The spectrum of insulin loaded chitosan casein nanoparticles. In this spectrum, the peak at 1598 cm-1 of chitosan was shifted to 1524 cm-1. The peak at 1650 cm-1 of casein was disappearing. The cause of the change of peak position is the interaction between the opposite groups of the nanoparticles. Those peaks display the formation of the casein chitosan nanoparticles. The peak at 1660 cm-1 shows the interaction between the drug insulin and the nanoparticles. Consequently this FTIR spectrum confirmed the formation of casein chitosan nanoparticles due to the interaction between the protonated amino group of chitosan and carboxyl group of casein13.
 |
Figure 2: FT-IR spectrum of Insulin loaded casein chitosan nanoparticles. |
X- Ray Diffraction Studie
Fig 3. Shows XRD diffraction studies of drug loaded chitosan casein nanoparticles. The XRD pattern of drug loaded nanoparticles showed the broad diffractions peaks at 2Ɵ values is 19.45°. The lower intensity shows via the drug loaded nanoparticles, that they’re amorphous in nature. Due to the absence of another diffraction peaks means, no impurities found in the drug loaded nanoparticles14.
 |
Figure 3: XRD pattern of Insulin loaded casein chitosan nanoparticles |
Scanning Electron Microscopy
Scanning electron microscopy is used to study the morphologies of the nanoparticles. Fig 4. shows the insulin loaded polymeric nanoparticles are spherical in shapes and uniform distributions.
 |
Figure 4: SEM image of Insulin loaded casein chitosan nanoparticles |
In vitro Anti-Diabetic Assay
Invitro anti-diabetic activity was carried out in insulin loaded casein chitosan polymeric nanoparticles using α- amylase and α- glucosidase enzymes.
Inhibition of α- amylase activity
Gra 1. Shows Inhibition of α- amylase activity of Insulin loaded polymeric nanoparticles, it has more effect on α- amylase enzyme. Various concentrations (100, 200, 400, 600, 800, 1000 µl/ml) of drug loaded nanoparticles are subjected to α- amylase inhibitory assay. The maximum inhibition value 51.70 was obtained in 1000 µl/ml. The IC 50 value of α- amylase inhibition assay is 36.193.
 |
Graph 1: % of inhibition of α- amylase against various concentration of Insulin loaded |
Inhibition of α- glucosidase activity
Gra 2. ShowsInhibition of α- glucosidase activityInsulin loaded polymeric nanoparticles, it has more effect on α- glucosidase enzyme. Various concentrations (100, 200, 400, 600, 800, 1000 µl/ml) of drug loaded nanoparticles are subjected to α- glucosidase inhibitory assay. The maximum inhibition value 80.40 was obtained in 1000 µl/ml. The IC 50 value of α- glucosidase inhibition assay is 40.20.
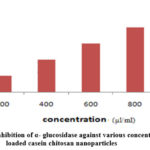 |
Graph 2: % of inhibition of α- glucosidase against various concentration of Insulin loaded casein chitosan nanoparticles |
Conclusion
The Insulin loaded casein chitosan nanoparticles was efficaciously synthesised by nanoprecipitation technique. The functional group of drug loaded polymeric nanoparticles was confirmed with the aid of FT-IR spectrum. The synthesised nanoparticles are amorphous state, which was confirmed by XRD evaluation. SEM morphologies research showed that nanoparticles are spherical in shape. The synthesised insulin loaded casein chitosan nanoparticles have more effective on α- glucosidase assay whilst examine with α- amylase assay.
Acknowledgement
The author has great thankful to Scott Christian College (Autonomous), Nagercoil, Tamilnadu, India and STIC, Cochin University, Kochi.
Conflict of Interest
The authors declare no conflict of interest.
Referance
- https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.
- https://www.healthline.com/health/difference-between-type-1-and-type-2-diabetes.
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. J. Cont. Rele. 2012,161, 38–49.
CrossRef - De Kruif, C.G.; Holt, C.; Adv. Dairy Chem. 2003, 1, 233-276.
CrossRef - Livney, Y.D. Cur. Opi. Colloid & Inter. Sci. 2010, 15, 73–83.
CrossRef - Ilangumaran, G.; Stratton, G.; Ravichandran, S.; Shukla, P. S.; Potin, P.; Asiedu, S.; Prithiviraj, B. Front. Micro. 2017; 8, 781
CrossRef - Dev, A.; Mohan, J.C.; Sreeja, V.; Tamura, H.; Patzke, Greta R.; Hussain, F.; Weyeneth, S.; Nair, S.V.; Jayakumar, R. Carbo. Poly. 2010, 79, 1073–1079.
CrossRef - Felt, O.; Buri, P.; Gurny, R. Drug. Dev. Ind. Pharm. 1998, 24, 979–993.
CrossRef - Yadollahi, M.; Namazi, H. J. Nano. Res. 2013,15,1563.
CrossRef - Kozen, B.G.; Kircher, S.J.; Henao, J.; Godinez, F.S.; Johnson, A.S. Offi. J. Acad. Emerg. Med. 2008, 15, 74-81.
CrosssRef - Ueno,H.; Mori, T.; Fujinaga, T. Adv. Drug. Deliv. Rev. 2001, 52, 105-15.
CrossRef - Robson, M.C. Surg. Clin. North. Am. 1997, 77,637-50.
CrossRef - Grasianto .; Siswanta .; Mudasir .; Yosie Andriani. Int.J. Dru. Delv. 2015, 7, 167-173.
- Anupama Ammulu Manne .; Vinay Viswanath, K.; Ajay Kumar, G.; Ushakiranmayi Mangamuri.; Sudhakar Podha. J. Gent. Eng. Bio. Tech. 2020,18, 1-13.

This work is licensed under a Creative Commons Attribution 4.0 International License.









