Studies of Substitution Effect of B2O3 on Structure and Properties of 1393 Bioactive Glass
1Department of Mechanical Engineering, National Institute of Technology, Uttarakhand, Uttarakhand -246174, India.
2Department of Ceramic Engineering, Indian Institute of Technology (Banaras Hindu University) Varanasi -221005, India.
Corresponding Author E-mail: ng99749974nituk@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370619
Article Received on : 17-Sep-2021
Article Accepted on : 15-Nov-2021
Article Published : 17 Nov 2021
Bioactive glass is mainly familiar for its outstanding biocompatibility and bioactive behavior and it’s known for important bone bonding ability. Bioactive glass is a reproduction fillet joint meant for orthopedic in addition to periodontal function of one of the leading applications. A silica based bioactive glass designated 1393 bio-glass® [wt. % (53) SiO2 – (6) Na2O – (12) K2O – (20) CaO – (5) MgO – (4)P2O5] 1393 is like 45S5 bio-glass®, other than it has a high SiO2 content and network modifiers, such as potassium oxide and magnesium oxide, bioactive glass, is also used clinically. In this communication, study of destructive (DT) & non-destructive (NDT) behavior of SiO2 replaced by boron trioxide (B2O3) in 1393 bioactive glass has been reported. The formed amorphous phase using x-ray diffraction (X-RD) analysis in bioactive glass will be identified. Density and mechanical properties measured using different types of instrument and using ultrasonic wave velocities study the elastic properties like young’s , shear, bulk modulus and Poisson’s ratio of bioactive glasses were reported. The results point to the substitution of boron trioxide in 1393 bioactive glass enhanced its density, mechanical properties and elastic properties, similarly for silica.
KEYWORDS:Bioactive Glass; Boron Trioxide; Destructive (Test); Density; mechanical properties; Non-destructive (Test)
Download this article as:| Copy the following to cite this article: Gupta N, Vyas V. K, Mandal A. Studies of Substitution Effect of B2O3 on Structure and Properties of 1393 Bioactive Glass. Orient J Chem 2021;37(6). |
| Copy the following to cite this URL: Gupta N, Vyas V. K, Mandal A. Studies of Substitution Effect of B2O3 on Structure and Properties of 1393 Bioactive Glass. Orient J Chem 2021;37(6). Available from: https://bit.ly/3HsX9cW |
Introduction
For substituted damaged or diseased tissues used biomaterials. This is a first generation material it’s considered as a property of bio-inertness. Bio-glass by means of the character of genetic material commencement was second-hand for tissue reinstatement and curative was chosen for the third age group of bio-materials 1. The thought of a protected bond between the bone and the artificial material that occurs on the surface of the bio-glass due to chemical reactions is presented by Hench. These chemical reactions are very useful in repairing the damaged bone which greatly helps in forming a bond between the bio-glass® and the bone. For medical applications for this characteristic quality bio-glass is considered one of the very stable biomaterials. The inside of the body usually contains silicon, potassium, sodium, magnesium, calcium, oxygen, and phosphorus, which are commonly found in bio-glass®, so there are no toxic effects being detected. The concentration levels of the ions do not increase to the extent that bio-glass® would affect the surrounding tissue during bone formation and binding. Several studies have shown 2. Bioactive-glass (BGs) such as “45S5 bio-glass®” (45) SiO2, (24.5) CaO, (24.5) Na2O, and (6) P2O5) weight 3 and “1393 bioactive glass” (53) SiO2, (6) Na2O, (12) K2O, (5)MgO, (20)CaO, and (4)P2O5) weight% 4, for tissue engineering applications compositions have been generally used for bone. Also various applications such as silicate glass, borate and have been generally used for bone. Also various applications such as silicate glass, borate and borosilicate glass have been found in biomedical and technical applications 5, 6, and 7. The hydroxyapatite (HA) layer is formed because bio-glass undergoes chemical degradation when exposed to biological conditions and facilitates bonding between bones and tissues 8. Improved convenient rate of degradation to form HA than silicate glass were In the middle of the previous mention bio-glass® borate based glasses. 9, 10 makes potential materials for prospect. 1393 bioactive glasses identify physically powerful bonding to rigid and spongy tissue and have been exposed to support orthogenesis via the establishment of more than a few pertinent genes 11. Boron is commonly established in minute amounts in the human body 12. In general, boron in bio-glass is either four- or three-fold coordinated. In a previous research, it was found that low amounts of boron trioxide (B2O3) are present in the form of [BO3] structural units, which leads to a rigid and more cross-linked structure. On the other hand, it has the possibility to be well thought-out as a catalyst in novel organic behavior and can be utilized in pharmaceutical remedies. For the most part, the boron (B) -containing bioactive molecules are of two types; first one is molecules containing a single boron atom and second is boron cluster. The research of this experiment is to provide information of (X-RD) analysis, Density and mechanical properties measured using different type of instrument and using ultrasonic wave velocities study the elastic properties like young’s , bulk, shear modulus, and Poisson’s ratio of bioactive glass were reported.
Methods of preparation materials
Bioactive glass preparation of different composition
Bio-glass samples replaced by boron trioxide (B2O3) are shown in Table 1. Raw resources were required to organize bioactive glass samples which have been taken in a systematic manner. Fine grained quartz has been taken in place of SiO2. Sodium carbonate (Na2CO3) has been taken in place of sodium oxide (Na2O).Calcium carbonate (CaCO3) has been used in place of calcium oxide (CaO). Phosphorus penta-oxide (P2O5) has been replaced by ammonium dihydrogen orthophosphate (NH4H2PO4). Magnesium carbonate (MgCO3) has been taken in place of magnesium oxide. Potassium carbonate (K2CO3) has been taken in place of potassium oxide. Boron trioxide has been taken up directly. We made 5 batches after weighing them according to their weight. The five batches were mixed separately by putting them in the agate jar. Now glass samples were prepared by putting one batch each in a muffle furnace at 35 to 1400 °C, alumina crucibles were used to prepare the glass samples. Then all the five samples were prepared for different measurements.
Table 1: Bioactive Glass Composition (weight %).
|
(SiO2) |
(Na2O) |
(CaO) |
(P2O5) |
(MgO) |
(K2O) |
(B2O3) |
|
|
1393 |
53 |
6 |
20 |
4 |
5 |
12 |
0 |
|
G-1 |
39.75 |
6 |
20 |
4 |
5 |
12 |
13.25 |
|
G-2 |
26.5 |
6 |
20 |
4 |
5 |
12 |
26.5 |
|
G-3 |
13.25 |
6 |
20 |
4 |
5 |
12 |
39.75 |
|
G-4 |
0 |
6 |
20 |
4 |
5 |
12 |
53 |
Measurements of X-ray diffraction
To measurements of X-ray diffraction, the bioactive glass sample was made into a powder with a grain size of 75 µm. The five bioactive glass samples have been separated into a for X-ray diffraction measurements.
Machine is used for X-ray diffraction
Machine Name – RIGAKU-Miniflex II difractometer
Radiation- (Cu-Kα) radiation (λ=1.5405 Å)
Voltage and current-40kV and current of 35 Mili amperes
Range- between 20degree to 80degree
The step size was set to 0.02degree and the measuring speed was onedegree/minute. JCPDS data cards were used as a position for identifying the peaks in the graph.
Density measurement
To find the density were calculated at normal temperature with a digital weighing scale (Satorius, Model- BP221S, USA) which has an accurateness of ±0.0001 gm after that we cut 1-1 pieces from the five glass samples, the size (1cm*1cm*1cm) of all the bioactive glass sample was kept the same. We measured the weight of the entire glass sample, once put in water take weight of glass sample and once removed from the water take dry weight of bioactive glass sample. Then by using the density formula, we will find the densities of all bioactive glass samples. Using the Archimedes principle Density is determined. Formula is given below.
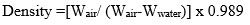
Where, ‘W’air weight in air, ‘Wwater’ weight in water
Mechanical Properties Measurement
Flexural Strength
To measure the mechanical properties, a glass sample was cut into a size of (1cm x 1cm x 1cm) cube, and then polished. Using an Instron Universal Testing Machine (AGS 10kND, SHIMADZU) whose cross- head speed was 0.5 mm/min bearing a full scale load of 2500 kg three point bending test at normal temperature for calculation of flexural strength. Formula is given below.
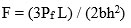
Where ‘Pf’ is the load and ‘L-Length’, ‘b-breadth’, ‘h-height’ of sample respectively.
Compressive Strength
To find out the compressive strength, we used the Kinston Universal Testing Machine (UTM) was used having a cross-speed of 0.05 cm/min and full scale load of 2500 kg. We cut bioactive glass pieces according to the ASTM standard D3171 to perform the test. The size of the bioactive glass sample was kept at 2 cm * 2 cm * 1 cm. We conducted the test at normal temperature.
Hardness
To find out hardness, we used the Hardness Testing Machine have used loads- range of 30mN – 2000mN and velocity of 0.1 cm/sec cut the glass pieces according to the ASTM standard C730-98 to carry out the test. The size of the glass pieces was kept at (1 cm *1 cm * 1 cm). We conducted the test at room temperature. Formula of Hardness of bioactive glass is given below.
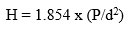
P-Load’ and‘d-diagonal of the indentation
Elastic Properties
The instrument we used to find the Elastic Properties of a glass sample is named Olympus (M-45, USA). According to the instrument, we cut the glass sample, whose size we kept (1 cm * 1 cm * 1 cm) then polished the glass sample with a polishing machine, after that we used the instrument to find two types of velocity. The name is shear and longitudinal wave velocities. To find the value of shear and longitudinal wave velocities we used two types of gel. The first one is sonfech shear gel and second is couplant glycerin. Using the various formulas, find the Elastic properties.
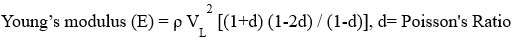
VL= longitudinal velocity, VT= Shear velocity
Shear Modulus (G) = VT2 ρ , ρ= density
Bulk modulus (K) = E/3(1-2d) , E= Young’s modulus
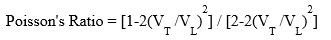
Discussion of Results
Bioactive glasses analysis by X-Ray
The X-RD results of each and every one 1393 and substitution of boron trioxide (B2O3) bioactive glass samples are shown in figure 1. As per the obtained result, it was observed that no crystalline phase was found in the glass sample. This shows that our glass samples are of amorphous nature. The lump for 2 theta values between 25 degree and 35 degree intensifies with the substitution of boron trioxide (B2O3) and is the only change visible in the different graphs. It also shows that boron trioxide (B2O3) is totally dissolved in the glass matrix. Katharina et. al. also show that this type of result in paper title of paper is influence of the replacement of silica by boron trioxide on the in paper properties of bioactive glass scaffolds 13.
 |
Figure 1: X-RD 1393 & SiO2 replace by boron trioxide (B2O3) bioactive glass sample. |
Density Measurements of 1393, G-1, G-2, G-3 and glass samples
Using Archimedes’ principle the variation in density measurement data are show in table-2 and calculation is depicted in figure-2 which obviously show that the SiO2 replace by boron trioxide (B2O3) in 1393 bioactive glass sample small change in density from 2.88 to 2.97 g/cm3. This small change can be ascribed due to the density of SiO2 and boron trioxides (B2O3) near to the same level as each other. Contracting volume type of behavior shows the borate based bioactive glass. Density is dependent on particle size on the basis of particle size density is increased boron trioxide (B2O3) in 1393 bioactive glass samples.
Table 2: Density of (1393), (G-1), (G-2), (G-3) and (G-4) glass samples
|
Glass Sample |
(1393) |
(G-1) |
(G-2) |
(G-3) |
(G-4) |
|
Density(gm/cm3) |
2.88 |
2.91 |
2.93 |
2.94 |
2.97 |
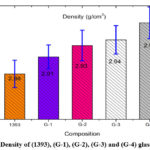 |
Figure 2: Density of (1393), (G-1), (G-2), (G-3) and (G-4) glass samples. |
Mechanical Properties of (1393), (G-1), (G-2), (G-3) and (G-4) glass samples Flexural strength
Flexural strength results data given in table -3 and all 1393 and substitution of boron trioxide (B2O3) in figure-3 shows bioactive glass samples. The outcome of the flexural strength of 1393 is [44.45], G-1[57.24], G-2[58.41], G-3[62.49] and G-4[66.55] M-Pa bioactive glass samples. Increasing tendency shows the results and in flexural strength as the percentage of boron trioxide (B2O3) is increased in 1393 bioactive glass samples to replace SiO2. B3+ ions may act as network intermediate increase results may be due to this region, thus more the solidity of glass structure 14.
Table 3: Flexural strength (MPa) of (1393), (G-1), (G-2), (G-3) and (G-4) glass samples
|
Glass samples |
(1393) |
(G-1) |
(G-2) |
(G-3) |
(G-4) |
|
Flexural strength |
44.45 |
57.24 |
58.41 |
62.49 |
66.55 |
 |
Figure 3: Flexural strength of 1393, G-1, G-2, G-3 and G-4 glass samples. |
Compressive strength
Compressive strength results data given in table-4 and all 1393 and substitution of boron trioxide (B2O3) in figure-4 shows bioactive glass samples. The outcome of the compressive strength of 1393 is [69.82], G-1 [78.63], G-2 [81.35], G-3 [79.15] and G-4[84.13] M-Pa glass samples. An increasing tendency of compressive strength since the % of boron trioxides (B2O3) is increases in 1393 bioactive glass sample replace SiO2. This enhance may be owed to the boron ion may act as network intermediate, thus more stiffens of glass arrangement 14.
Table 4: Compressive strength (MPa) of (1393), (G-1), (G-2), (G-3) and (G-4) glass samples
|
Glass samples |
(1393) |
(G-1) |
(G-2) |
(G-3) |
(G-4) |
|
Compressive strength |
69.82 |
78.63 |
81.35 |
79.15 |
84.13 |
 |
Figure 4: Compressive strength (MPa) of (1393), (G-1),(G-2), (G-3) and (G-4) glass samples. |
Micro Hardness
Data of micro hardness is given table- 5 and in figure-5 shows bioactive glass samples result of the micro hardness of 1393 is [5.45], G-1 [5.58], G-2 [5.61], G-3 [5.87] and G-4 [6.09] M-Pa glass samples. An increasing tendency in Micro Hardness results shown as the % of boron trioxides (B2O3) is increased in 1393 bioactive glass samples to replace SiO2. This enhancement may be due to the B3+ ion may act as network intermediate, thus more the solidity of glass structure 14. We found in the present study that the mechanical properties change in glass samples (SiO2 replaced byB2O3) because the boron ion acts as an intermediate and simultaneously stiffens the structure. This justifies the changing trends in the mechanical properties.
Table 5: Micro Hardness (MPa) of (1393), (G-1), (G-2), (G-3) and (G-4) glass samples
|
Glass samples |
(1393) |
(G-1) |
(G-2) |
(G-3) |
(G-4) |
|
Micro Hardness |
5.45 |
5.58 |
5.61 |
5.87 |
6.09 |
 |
Figure 5: Micro Hardness of (1393), (G-1), (G-2), (G-3) and (G-4) glass samples. |
Elastic Properties
The elastic properties of all bioactive glass samples measured Using the longitudinal and shear ultrasonic wave velocities are shown graphically in figure 6 (a),(b),(c),(d).Young’s, Shear and Bulk modulus of (1393), (G-1), (G-2), (G-3) and (G-4) glass samples are increase but Poisson’s Ratio slight decrease. An increase in boron ion concentration in bioactive glass samples can increase the values of Young’s, shear and bulk modulus but a slightly decreasing trend in values is observed for Poisson’s ratio. Author Gaafar and Kannapan 15, 16 when increase in connectivity in the glass network show the increase tendency in elastic modulus values but type of bonding in the glass structure show the slight reduction in Poisson’s ratio 17.
 |
Figure 6: Elastic properties of (1393), (G-1), (G-2), (G-3) and (G-4) glass samples |
Conclusion
We found in the present investigation that if boron had been taken in place of silica in 1393 glass samples, various types of properties were carried out in the following results obtained. Firstly, the X-RD analysis confirmed that no crystalline phase has been found in glass samples and shows that our glass samples are of amorphous nature and the mechanical properties namely flexural strength, compressive strength, hardness and elastic properties namely Young’s, bulk and shear moduli show an upward trend on increasing the concentration of B2O3 in the bioactive glass. However, the values for Poisson’s ratio reduce slightly on boron ion addition. Therefore on summarizing the results obtained from this investigation it can be concluded that boron trioxides (B2O3) substituted 1393 bioactive glass can be used in biomedical applications as a potential biomaterial.
Acknowledgement
The authors gratefully acknowledge the HOD Department of Mechanical Engineering, National Institute of Technology Uttarakhand -246174, India the honorable Director of National Institute of Technology Uttarakhand, India for providing necessary facilities for the present work and also thankful to HOD Department of Ceramic Engineering, Indian Institute of Technology (Banaras Hindu University) Varanasi -221005, India.
Conflict of interest
My name is Neeraj Gupta. I have taken my M. Tech degree from Department of Ceramic Engineering Indian Institute of Technology (Banaras Hindu University) Varanasi -221005, India. I am currently working as assistant professor through (Technical Education Quality Improvement Programme) in Uttarakhand. I get some fixed salary by the government of India during this I have joined P.hd part time Department of Mechanical Engineering National Institute of Technology Uttarakhand -246174, India. My research interest is bioactive material and bioactive glasses like 45S5, 1393 and Borate glass.
Funding Sources
There are no funding source.
References
- Hench, L. L., Journal of Material Science and Material Medicine, 2006, 17, 967–978.
CrossRef - Ylänen H.O., Bioactive glasses Materials, properties and applications. 2011 Woodhead Publishing Limited, 2, 189-208.
CrossRef - Hench, L. L,.Bioceramics: From concept to clinic. J. Am. Ceram.Soc., 1991, 74 (7), 1487−1510.
CrossRef - Rahaman, M. N.; Day, D. E; Bal B. S.;. Fu. Q., Jung,; Bonewald; L. F. and Tomsia A. P, Bioactive glass in tissue engineering.ActaBiomater., 2011,7 (6), 2355−2373.
CrossRef - Yun Y.; Bray P.; Nuclear magnetic resonance studies of the glasses in the system Na2O–B2O3–SiO2, J. Non-Cryst. Solids, 1978, 27 (3), 363–380.
CrossRef - Chen, Q.Z.; Thompson, I.D. and Boccaccini, A.R.; 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering, Biomaterials, 2006, 27 (11) 2414–2425.
CrossRef - Kokubo, T.; Takadama, H.; How useful is SBF in predicting in vivo bone bioactivity? Biomaterials, 2006, 27 (15), 2907–2915.
CrossRef - Cao W.; and Hench, L.L;. Bioactive materials, Ceram. Int. , 1996, 22 (6) 493–507.
CrossRef - Fu H.; Fu, Q.; Zhou, N.; Huang W.; Rahaman, M.N.; Wang D.; and Liu, X.; In vitro evaluation of borate-based bioactive glass scaffolds prepared by a polymer foam replication method, Mater. Sci. Eng., C, 2009, 29 (7) 2275–2281.
CrossRef - Furukawa, T.; and White, W.B.; Structure and crystallization of glasses in the Li2 Si2O5–TiO2 system determined by Raman spectroscopy, Phys. Chem. Glasses, 1979, 20 (4), 69–80.
- Hench, L. L.; Genetic design of bioactive glass. J. Eur. Ceram. Soc., 2009, 29 (7), 1257−1265.
CrossRef - Emsley J. Nature’s Building Blocks: An AZ Guide to the Elements. Oxford University Press; Oxford, UK: 2011. [Google Scholar]
- Katharina, S.; Usanee, P.; Kristin Enge, Piotr Jele, Zbigniew Olejniczak, Leena Hupa, Maciej Sitarz, Aldo R. Boccaccini, Influence of the replacement of silica by boron trioxide on the properties of bioactive glass scaffolds, Accepted: 21 January 2021. DOI: 10.1111/ijag.15894.
CrossRef - Srivastava, A.K.; Journal of Materials Science Research, April 2012,Vol.No.2.
- Gaafar,M. S.; Marzouk, S. Y.; Zayed, H. A.; Soliman, L. I.; and Serag, A. H.; El-Deen, Curr. Appl. Phys.,2013, 13, 152-158.
CrossRef - Kannappan, A. N.; Thirumaran, S.; and Palani, R.; ARPN J. Eng. Appl. Sci., 2009, 4, 27-31.
- Gaafar, M. S.; ElBatal,F. H.; ElGazery, M.; and Mansour, S. A.; Acta Phys. Polonica A, 2009, 115, 671-678.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










