Overtone Frequencies of NO2 Using Lie Algebraic Model
Department of Mathematics, GITAM (Deemed to be University), Hyderabad, India.
Corresponding Author E-mail: vijayjaliparthi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370631
Article Received on : 04-Oct-2021
Article Accepted on :
Article Published : 26 Nov 2021
Reviewed by: Dr. Angyang

Second Review by: Dr. Harlem Marpaung

Final Approval by: Dr. Ioana stanciu
In this paper, we used the one dimensional unitatry Lie algebraic model to compute the vibrational frequencies of nitrogen dioxide (NO2) molecule in the gas phase up to the sixth overtone. In this model, the Hamiltonian operator describes the stretching and bending vibrations with algebraic parameters. The calculated fundamental vibrational frequencies are compared with experimental values and results consistent with the reference values.
KEYWORDS:Lie Algebraic Model, Nitrogen Dioxide, Vibrational Frequencies
Download this article as:| Copy the following to cite this article: Vijayasekhar J, Kumar K. A, Srinivas N. Overtone Frequencies of NO2 Using Lie Algebraic Model. Orient J Chem 2021;37(6). |
| Copy the following to cite this URL: Vijayasekhar J, Kumar K. A, Srinivas N. Overtone Frequencies of NO2 Using Lie Algebraic Model. Orient J Chem 2021;37(6). Available from: https://bit.ly/3l6O28k |
Introduction
The subject of the vibrational spectra of molecules is an interesting and innovative work in molecular spectroscopy. Studying the vibrational spectra of small-sized molecules at the fundamental and higher overtones aids in a better understanding of their molecular structure and allows to compare in vivo experimental results. The advent of novel experimental techniques to compute vibrational frequencies (higher overtones) of molecules requires a robust theoretical method for their elucidation of molecular structure. This paper examines vibrational spectra of nitrogen dioxide in higher overtones using a theoretical approach – Lie algebraic model. In this model, Hamiltonian operator describes the vibrational energies of the molecule. In 1991, Iachello and co-researchers applied U(2) Lie algebraic approach for the analysis of ro-vibrational spectra of small size molecules 1 and then this method was improved in subsequent works to study molecular spectra of polyatomic molecules 2-10.
Lie algebraic model
Nitrogen dioxide is a bent molecule with C2v point group symmetry.with symmetry species – A1 (Symmetric Stretch), B2 (Antisymmetric Stretch) and A1 (Bend).
 |
Figure 1: Structure of nitrogen dioxide. |
The general Hamiltonian for n vibrational modes (N-O and N-O)is
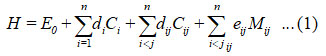
In the equation (1), Ci and Cij are invariant operators of uncoupled and coupled bonds respectively and known as
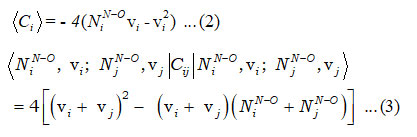
and the Majorana operator Mij is used to express the local mode interactions in pairs and contains diagonal and non-diagonal matrix elements,
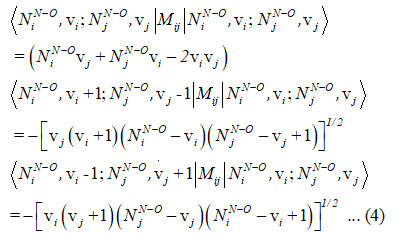
Hamiltonian for two stretching vibrationsof nitrogen dioxidecan be written as
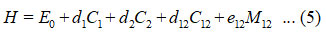
In equation (5), d1, d2, d12 and e12 are algebraic parameters (cm-1), determined from available spectroscopic data. From the structure of nitrogen dioxide, two bonds (N-O) are equivalent; hence we consider as d1= d2,=d, N1N-o = N2N-o = NN-o in equations (2), (3) and (4).Vibrational frequencies of the molecule can be determined from the Hamiltonian matrix.
For the first two local oscillators, the Hamiltonian matrix is
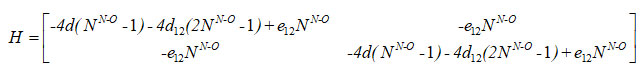
Results and Discussion
The energy equation (6) is used to detrmine the starting value of parameter d,
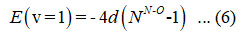
and the value for e12 calculated from the relation,
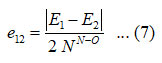
Where, E1, E2 are symmetric and antisymmetric vibrational frequencies of the molecule, respectively. The Lie algebraic method was used to calculate nitrogen dioxide vibrational frequencies, which are presented in table (2) and table (3).
The Vibron number N1 (dimensionless) for stretching bonds of nitrogen dioxide using
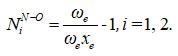
Where, ue (= 1906.52), ue xe (= 14.504) are harmonic vibrational frequency and vibrational anharmonicity (spectroscopic) constants, respectively for the bond N-O 12.
Table 1: Fitted algebraic parameters
| NN-o (stretch) |
130 |
| No-N-o (bend) |
72 |
| d(stretch) |
-2.7221 |
| d(bend) |
-2.5213 |
| d12(stretch) |
0.1447 |
| d12(bend) |
-0.0033 |
|
e12(stretch) |
1.1538 |
|
e12(bend) |
3.3641 |
Table 2: The fundamental vibrational frequencies (in cm-1) of NO2
|
Vibrational Mode |
Experimental# |
Lie algebraic method |
|
A1 (Symmetric Stretch) |
1318 |
1318.0976 |
|
B2(Antisymmetric Stretch) |
1618 |
1618.0856 |
|
A1 (Bend) |
750 |
751.656 |
Table 3: Vibrational frequencies (in cm-1) of NO2 in higher overtones (Lie algebraic method).
|
Overtone |
Vibrational mode |
||
|
A1 (Symmetric Stretch) |
B2 (Antisymmetric Stretch) |
A1 (Bend) |
|
|
I |
2566.2043 |
3212.8301 |
1456.0532 |
|
II |
3912.0712 |
4787.0381 |
2186.8400 |
|
III |
5297.0095 |
6446.8082 |
2954.4269 |
|
IV |
6575.9632 |
7975.3307 |
3687.4820 |
|
V |
7893.7510 |
9679.4237 |
4476.7362 |
|
VI |
9184.0196 |
10877.3129 |
5175.6613 |
 |
Figure 2: Graphical representation of higher overtone vibrational frequencies |
Conclusion
In the table (2), the estimated vibrational frequencies in fundamental mode were compared with the experimental data available and in table (3), vibrational frequencies upto the fifth harmonic by Lie algebraic approach are reported. The calculated results can be helpful for experimentalists and theorists to extend the vibrational spectra of NO2 in higher overtones.
References
- F. Iachello, S. Oss, R. Lemus, J. Mol. Spectrosc., 1991, 149 (1), 132-151. doi: 10.1016/00222852(91)90148-4
CrossRef - F. Iachello, S. Oss, J. Mol. Spectrosc., 1992, 153 (1–2), 225-239. doi: 10.1016/0022- 2852(92)90471-Y
CrossRef - A. Frank, R. Lemus, R. Bijker, F. Péilez Bernal, J.M. Arias, Ann. Physics, 1996, 252, 211-238.
CrossRef - Zhaochi Feng, Guang Xiong, Qihua Yang, Qin Xin & Can Li, Chin Sci Bull, 1999, 44, 1961–1964. doi:10.1007/BF02887118
CrossRef - F. Iachello, S. Oss, Chem. Phys. Lett., 1991, 187 (5), 500-505.
CrossRef - F. Iachello, S. Oss, Chem. Phys. Lett., 1993, 205 (2-3), 285-289.
CrossRef - M. R. Balla, V. S. Jaliparthi, Mol. Phys., 2021, 115 (5), e1828634.
CrossRef - M. R. Balla, V. S. Jaliparthi, Polycyclic Aromatic Compounds , 2021.doi: 10.1080/10406638.2021.1901126.
CrossRef - M. R. Balla, S. Venigalla, V. Jaliparthi,Acta Phys. Pol. A , 2021, 140 (2), 138-140. doi: 10.12693/APhysPolA.140.138
CrossRef - V. Jaliparthi, M. Rao Balla, Spectrochim. Acta A, 2022, 264, 120289(1-8). doi:10.1016/j.saa.2021.120289
CrossRef - J. Vijayasekhar, Orient J Chem, 2018, 34(4), 2208-2210. doi:10.13005/ojc/3404065
CrossRef - Irikura, K.K. J. Phys. Chem. Ref. Data., 2007, 36(2), 389-398.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









