Density Study of (D (+) Mannose + Water), (D (+) Mannose + Water + Sodium Cyclamate), (D(+)Maltose Monohydrate + Water) and (D (+) Maltose Monohydrate + Water + Sodium Cyclamate) Systems at T = 298.15 K
1Department of Chemistry, RNC Arts, JDB Commerce and NSC Science College, Nashik Road-422101, (Maharashtra) India.
2Department of Chemistry, HPT Arts and RYK Science College, Nashik- 422005, (Maharashtra) India.
Corresponding Author E-mail: sanjeevan.kharat@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370607
Article Received on : 01-Oct-2021
Article Accepted on :
Article Published : 20 Dec 2021
Reviewed by: Dr. Mervette El-Batouti

Second Review by: Dr. N. Mohondas Singh

Final Approval by: Dr. Bal Krishna
Effect of different concentrations of aqueous solutions of sodium cyclamate on sugars (mono and disaccharides) are observed by measuring the densities of (sugar + water) and (sugar + water + sodium cyclamate) systems. Densities of aqueous solutions of D (+) mannose (monosaccharide) and D (+) maltose monohydrate (disaccharide) in (0.05, 0.15, 0.3) mol.kg-1 of sodium cyclamate (Na-Cyclamate) at T = 298.15 K have been measured. From experimental values of densities , Vɸ0( partial molar volumes) ΔtrsVɸ0 (partial molar volumes of transfer) ASV (apparent specific volumes) interaction parameters (𝑉𝐴𝐵) and (𝑉𝐴𝐵𝐵 ) have been determined. The calculated values of various parameters have been used to interpret the results in terms of (D (+) mannose – water), (D (+) mannose –water– Na-cyclamate), (D (+) maltose monohydrate – water) and (D (+) maltose monohydrate – water – Na-cyclamate) interactions in sugar– water – Na-cyclamate and quality of taste sense of solutions.
KEYWORDS:Apparent Specific; Density; D (+) Mannose; D (+) Maltose Monohydrate
Download this article as:| Copy the following to cite this article: Kharat S. J, Patil M. D. Density Study of (D (+) Mannose + Water), (D (+) Mannose + Water + Sodium Cyclamate), (D(+)Maltose Monohydrate + Water) and (D (+) Maltose Monohydrate + Water + Sodium Cyclamate) Systems at T = 298.15 K. Orient J Chem 2021;37(6). |
| Copy the following to cite this URL: Kharat S. J, Patil M. D. Density Study of (D (+) Mannose + Water), (D (+) Mannose + Water + Sodium Cyclamate), (D(+)Maltose Monohydrate + Water) and (D (+) Maltose Monohydrate + Water + Sodium Cyclamate) Systems at T = 298.15 K. Orient J Chem 2021;37(6).Available from: https://bit.ly/32mDfA0 |
Introduction
Carbohydrate gain importance and attention of researchers because of their multifunctional and significant roles in different areas like biological reaction, industrial field, pharmaceuticals, organic synthesis, structural determination and separation. The stabilities of such compounds and their position are verbalized by thermodynamic consideration 1. Sugars are the energetic biomolecule of living being. Sugar solutions are of considerable interest in various aspects of basic researches and application. Interactions of sugars with sweetener in aqueous media and its hydration properties are of significant biological and thermodynamic importance. Combinations of Non-caloric sweetener are mostly used to formulate pharmaceutical doses and food products.
Combination of two sweeteners results in synergistic effect which means sweetness of mixture or combination is high as compare with the sweetness of individual sugar. This was reported true in some blends of such sweetener 2. Also by combining with other sugars reduces the bitterness of one of the sugar and enhances the taste quality. Application of non -nutritive sugars or artificial sugars are less caloric and thus provides choices for caloric sugars, reduces weight, assistance in the management of diabetes, provides cost effectiveness 3, also have many applications in food and pharmaceutical industry.
For present work one monosaccharide (D (+) mannose) and one disaccharide (D (+) maltose monohydrate) taken. Mannose is a monosaccharide consists of one sugar unit and belongs to aldohexoses. It is nutritional and healthy food supplement. It is a natural bioactive. Maltose monohydrate also known as malt sugar, compose of two glucose unit join to each other by α 1-4-glycosidic bond. It plays important role in energy metabolism.
This paper reports densities of D (+) mannose – water, D (+) mannose – water – Na- cyclamate, D (+) maltose monohydrate – water, and D (+) maltose monohydrate – water – Na-cyclamate systems at 298.15 K. Furthermore, the parameter calculated from densities of aqueous solutions are reported to obtain the information regarding interactions present in D (+) mannose – water, D (+) mannose – water – Na-cyclamate, D (+) maltose monohydrate – water, and D (+) maltose monohydrate – water – Na-cyclamate systems.
Material and Methods
D (+) Mannose, D (+) Maltose monohydrate and Sodium cyclamate were bought from suppliers Sigma Aldrich used for this study. Table 1. includes the details of chemicals
Table 1: Chemicals details
|
Chemical name |
CAS Number |
Molar mass |
% Purity |
|
D (+) Mannose |
M8574 |
180.36 |
≥ 99.0% |
|
D (+) Maltose monohydrate |
M5885 |
360.31 |
≥ 99.0% |
|
Sodium Cyclamate |
71440 |
201.22 |
≥ 99.0% |
Aqueous solutions of D (+) mannose and D (+) maltose monohydrate were freshly prepared using triply distilled water. In dry airtight stoppered glass bottle solutions were prepared using weight/weight method. Dhona balance accurate to 0.0001gm was used to undertake weight of solute and solvent. Densities were measured in glass-walled thermostat at 298.15 K using Bi-capillary pycnometer 4-7 with bulb size of 15cm3 volume. Triply distilled water and pure organic solvents were used to calibrate pycnometer. Observed uncertainties in temperature and measured density were 0.005 K and 5.4 ×10−2 kg m−3, respectively.
Result and Disscusion
In this present work, aqueous solutions of sugars (D (+) mannose and D (+) maltose monohydrate) in (0.05, 0.15, 0.3) mol.kg-1 of Na-Cyclamate have been studied at T = 298.15 K. Density data of sugars (mono and disaccharides) of concentration ranges from (0.04 to 0.2) mol.kg-1 in water and in (0.05, 0.15, 0.3) mol.kg-1 Na-Cyclamate at T= 298.15K have been generated. From the experimentally measured density values, apparent molar volume ( Vɸ) values are calculated using equation no.1.8-9.

where, M is molar mass of the solute, and m molality of the solution, ρo density of solvent, and ρ density of the aqueous solution. Literature [10] value for density of water (ρo = 997.07 kg.m-3) at T = 298.15 K has been considered for the calculation of Vɸ . Calculated values of (ρ ) and ( Vɸ ) for aqueous solutions of (D (+) mannose and D (+) maltose monohydrate) in (0.05, 0.15, 0.3) mol.kg-1 of Na-Cyclamate are given in Table 2 and 3.
Table 2: Density (ρ) and apparent molar volume ( Vɸ) values of D (+) mannose in water and (0.05, 0.15, 0.3) mol.kg-1 of Na-Cyclamate at T = 298.15 K.
|
m (mol.kg-1) |
P(kg.m-3) |
106Vɸ(m3.mol-1) |
m (mol.kg-1) |
P/(kg.m-3) |
106Vɸ(m3.mol-1) |
|
D (+) mannose + Water |
D (+) mannose + (0.05) m Na-cyclamate |
||||
|
0.0402 |
999.81 |
111.99 |
0.0403 |
1003.82 |
112.31 |
|
0.0803 |
1002.48 |
112.31 |
0.0806 |
1006.50 |
112.51 |
|
0.1203 |
1005.11 |
112.56 |
0.1196 |
1009.05 |
112.74 |
|
0.1604 |
1007.70 |
112.82 |
0.1602 |
1011.66 |
113.00 |
|
0.2005 |
1010.24 |
113.12 |
0.1990 |
1014.11 |
113.26 |
|
D (+) mannose + (0.15) m Na-cyclamate |
D (+) mannose + (0.3) m Na-cyclamate |
||||
|
0.0402 |
1011.11 |
112.55 |
0.0401 |
1022.74 |
112.81 |
|
0.0803 |
1013.75 |
112.79 |
0.0800 |
1025.33 |
113.16 |
|
0.1197 |
1016.30 |
113.04 |
0.1200 |
1027.89 |
113.44 |
|
0.1604 |
1018.89 |
113.29 |
0.1603 |
1030.40 |
113.69 |
|
0.1998 |
1021.35 |
113.56 |
0.2000 |
1032.84 |
113.95 |
Table 3: Density (ρ) and apparent molar volume ( Vɸ ) values of D (+) maltose monohydrate in water and (0.05, 0.15, 0.3) mol.kg-1 of Na-Cyclamate at T = 298.15 K.
|
m (mol.kg-1) |
p(kg.m-3) |
106Vɸ(m3.mol-1) |
m (mol.kg-1) |
p/(kg.m-3) |
106Vɸ(m3.mol-1) |
|
D (+) maltose monohydrate + Water |
D (+) maltose monohydrate + (0.05) m Na-cyclamate |
||||
|
0.0400 |
1002.31 |
228.34 |
0.0401 |
1006.32 |
228.83 |
|
0.0801 |
1007.44 |
228.76 |
0.0801 |
1011.41 |
229.12 |
|
0.1199 |
1012.42 |
229.07 |
0.1202 |
1016.39 |
229.48 |
|
0.1599 |
1017.31 |
229.39 |
0.1600 |
1021.22 |
229.82 |
|
0.1999 |
1022.10 |
229.65 |
0.1999 |
1025.96 |
230.06 |
|
D (+) maltose monohydrate + (0.05) m Na-cyclamate |
D (+) maltose monohydrate + (0.05) m Na-cyclamate |
||||
|
0.0399 |
1013.57 |
229.21 |
0.0400 |
1024.59 |
229.43 |
|
0.0800 |
1018.63 |
229.48 |
0.0801 |
1029.59 |
229.74 |
|
0.1199 |
1023.55 |
229.76 |
0.1201 |
1034.46 |
229.96 |
|
0.1601 |
1028.39 |
230.09 |
0.1601 |
1039.22 |
230.27 |
|
0.2002 |
1033.11 |
230.39 |
0.2000 |
1043.88 |
230.48 |
From the measured density data, it is understood that density of solutions of (D (+) mannose and D (+) maltose monohydrate) in water and in Na-Cyclamate varies linearly with molalities.
Figure 1 and Figure 2 show 3-D plots of ( Vɸ) vs ( m ) of (D (+) mannose and D (+) maltose monohydrate) in (water) and in (0.05, 0.15, 0.3) mol.kg-1 of Na-Cyclamate, respectively at T = 298.15K.
 |
Figure 1: 3-D Plots of ( ) vs ( m ) of (D (+) mannose in (water) and in (0.05, 0.15, 0.3) mol.kg-1 of Na-Cyclamate at T = 298.15 K. |
 |
Figure 2: 3-D Plots of ( ) vs ( m ) of (D (+) maltose monohydrate in (water) and in (0.05, 0.15, 0.3) mol.kg-1 of Na-Cyclamate at T = 298.15 K. |
Figure 1 and figure 2 show the effect of concentration of Na-cyclamate on the Vf values for D (+) mannose and D (+) maltose monohydrate. Vf varies linearly with the concentration of (D(+) mannose and D(+) maltose monohydrate) in water and in (0.05, 0.15, 0.3) mol.kg-1 of Na-Cyclamate at T = 298.15 K. Vf of (D (+) mannose and D (+) maltose monohydrate) increases with increase in molality of Na-cyclamate.
Equation 2 shows the correlation between calculated Vf and m [11].

Where Vɸ0 is the partial molar volume, Vɸ m and Sv are apparent molar volume, (solute-solute) interaction parameter, and m molality, respectively. Vɸ0 and Vɸ were calculated using least square method .The Vɸ values throw light on valuable information about strength of the solute-solvent interactions12-13. Table 4 and 5 summarise the values of Vɸ0 and Sv .
Table 4: Vɸ0 , Sv , ASV, ΔtrsVɸ0 of D (+) mannose in water and (0.05, 0.15, 0.3) mol.kg-1 Na-cyclamate at T = 298.15 K.
|
|
Water |
0.05 m |
0.15 m |
0.3 m |
|
106 Vɸ0 |
111.73 |
112.05 |
112.29 |
112.57 |
|
106 Sv |
6.918 |
5.965 |
6.289 |
7.013 |
|
106 ASV |
0.620 |
0.622 |
0.623 |
0.625 |
|
106 ΔtrsVɸ0 |
– |
0.320 |
0.560 |
0.840 |
Table 5: Vɸ0 , Sv ,ASV, ΔtrsVɸ0 of D(+)maltose monohydrate in water and (0.05, 0.15, 0.3) mol.kg-1 Na-cyclamate at T = 298.15 K.
|
|
Water |
0.05 m |
0.15 m |
0.3 m |
|
106 Vɸ0 |
228.07 |
228.51 |
228.89 |
229.19 |
|
106 Sv |
8.133 |
7.910 |
7.423 |
6.572 |
|
106 ASV |
0.633 |
0.634 |
0.635 |
0.636 |
|
106 ΔtrsVɸ0 |
– |
0.440 |
0.820 |
1.120 |
It is observed that with increasing concentration of solute and cosolute , Vɸ0 values go on increasing. This observed behaviour of Vɸ0 is due to the strong [(D (+) mannose and D(+) maltose monohydrate) – cosolute (Na-cyclamate)] interactions. Reported and experimental Vɸ0 values for D (+) mannose and D (+) maltose monohydrate in water at T = 298.15 K were compared. The reported Vɸ0 values for D (+) mannose in water are ( 111.72 14, 111.70 15, 111.70 23 ) × 10-6 m3.mol-1 at T = 298.15 K while experimental Vɸ0 value is 111.73 × 10-6 m3.mol-1 at T = 298.15 K. The reported values of Vɸ0 D (+) maltose monohydrate in water are (228.12 15, 228.14 16, 227.78 17) × 10-6 m3.mol-1 at 298.15K and experimental Vɸ0 value is 228.07× 10-6 m3.mol-1 at T = 298.15 K. The reported Vɸ0 values and experimental Vɸ0 values for studied mono and disaccharides are very close. Experimentally observed Sv values are positive and smaller than Vɸ0 values interprets strong solute –solvent interaction than solute –solute interaction.
The values of standard transfer partial volume ( ΔtrsVɸ0 ) are estimated to focus on the nature and extent of solute cosolute interactions. ΔtrsVɸ0 at infinite dilution of sugar solutions from water to Na- cyclamate have been calculated using Equation (3)
ΔtrsVɸ0 = Vɸ0 (in aqueous sodium cyclamate) — Vɸ0 (in water) …………. (3)
ΔtrsVɸ0 values for (D (+) mannose and D (+) maltose monohydrate in (0.05, 0.15, 0.3) mol.kg-1 of Na-Cyclamate at T = 298.15 K are reported in table 4 and table 5, respectively. Positive ΔtrsVɸ0 values are observed for (D (+) mannose and D (+) maltose monohydrate) in water and in presence of Na- cyclamate ΔtrsVɸ0. values go on increasing with increase in concentration of Na-cyclamate. Dey P. C et al18 show the same trend in results , Banipal et al19 reported ΔtrsVɸ0 values for all D (+) mannose and D (+) maltose monohydrate) studied as positive and increase with increase in concentration of guanidine hydrochloride. Authors 20 also reported same trend in results for ΔtrsVɸ0 values of sugars in presence of cosolute. The concentration effect of co-solute (Na- cyclamate) on ΔtrsVɸ0 values of (D (+) mannose and D (+) maltose monohydrate) shown in Figure 3.
 |
Figure 3: Plot of standard transfer partial volume( vs molality m of aqueous solution of (0.05, 0.15, 0.3) mol.kg-1 Na-cyclamate for D (+) mannose and D (+) maltose monohydrate at T=298.15 K. |
From figure 3, we observed that ΔtrsVɸ0 increases with increase in the molecular complexity from mono to disaccharides. ΔtrsVɸ0 values for D (+) maltose monohydrate is high as compared with ΔtrsVɸ0 values of D (+) mannose. This is due to addition of one sugar unit in disaccharide as compared to monosaccharide. Additional (-OH) group and (-O-) glycosidic bond in maltose monohydrate responsible for this effect as compared with mannose. ΔtrsVɸ0 values for D (+) mannose and D (+) maltose monohydrate in presence of Na- cyclamate increase with the molality of cosolute (Na-cyclamate) suggests stronger interaction between polar groups (–C=O, –OH and–O–) group of D (+) mannose and D (+) maltose monohydrate) and ions (Na+)/ (cyclamate–) of Na-cyclamate. Prevalence of interaction between hydrophilic groups of sugars and ions of cosolute leads to dehydration of sugars.
At an infinite dilution when the of sugar is equal to can be expressed using Shahidi’s equation 21 as given below:
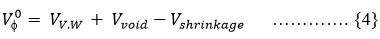
Where VV.W denotes the van der Waals volume, Vvoid represents empty volume and Vshrinkage is the shrinkage volume due to solute-water interactions. Vshrinkage arise due to hydrogen bonding between water molecule and hydroxyl group of sugars. The magnitude of hydrogen bonding between water molecule and hydroxyl group of sugars. The magnitude of VV.W and Vvoid are same in presence of water and aqueous electrolyte solutions. Thus the positive ΔtrsVɸ0 is because of decrease in shrinkage volume which is further due to the reduced electrostriction of water20 in aqueous solutions of Na- cyclamate. Thus the positive ΔtrsVɸ0 values depict the sugar dehydration in aqueous solutions of Na-cyclamate .
Equation (5) has been used for calculation of interaction parameters (VAB (doublet) and VABB (triplet)) using ΔtrsVɸ0 based on McMillan-Mayer theory of solutions.22-23.
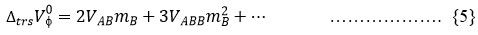
where A symbolizes sugars (D (+) mannose and D (+) maltose monohydrate) and B symbolizes Na-cyclamate. By using least square method values of 𝑉𝐴𝐵 and 𝑉𝐴𝐵𝐵 were calculated. The observed values of (𝑉𝐴𝐵) and (𝑉𝐴𝐵𝐵) interaction parameters are reported in Table 6.
Table 6 : 𝑉𝐴𝐵 and 𝑉𝐴𝐵𝐵 for D (+) mannose and D (+) maltose monohydrate at T = 298.15 K.
|
(𝑉𝐴𝐵) / (𝑉𝐴𝐵𝐵 ) |
D (+) mannose |
D (+) maltose monohydrate |
|
𝑉𝐴𝐵 × 106 (m3mol-2 kg) |
2.634 |
3.921 |
|
𝑉𝐴𝐵𝐵 × 106 (m3mol-2 kg2) |
-2.775 |
-4.598 |
The sign of magnitude of 𝑉𝐴𝐵 and 𝑉𝐴𝐵𝐵 values predicts which kind of interactions occurring between sugars (D (+) mannose and D (+) maltose monohydrate) and Na- cyclamate. For all studied solutions positive 𝑉𝐴𝐵 values and negative 𝑉𝐴𝐵𝐵 values suggest pairwise interactions. The positive values of 𝑉𝐴𝐵have been interpreted by group additivity model 24-26. This model suggest four types of interaction occurs between the polar groups of sugars and ions of electrolyte as (Hydrophilic–ionic, hydrophobic –ionic, hydrophilic-hydrophilic interaction between polar group, and hydrophobic-hydrophobic interaction between non-polar groups).
According to structural interaction and hydration models [27-28], the positive contribution to 𝑉𝐴𝐵 is due to sugar-cation interaction. Thus positive values of 𝑉𝐴𝐵 for studied systems are contributed by interaction between polar (hydrophilic) groups of D (+) mannose and D (+) maltose monohydrate) and ions of Na- cyclamate.
The valuable information about quality of taste for studied solutions of D (+) mannose and D (+) maltose monohydrate in absence/presence of Na-cyclamate can be obtained from apparent specific volume (ASV). Values of ASV were calculated using equation

Apparent specific volume values estimate about the taste quality of sweeteners and distinguish them as sweet, salty, sour and bitter, 29. The complete variety of human taste responsiveness is more or less narrowed to ASV 30 between (0.1-0.9) m3. kg-1. ASV value range from (0.51- 0.71) × 10-6 m3. kg-1 fit nicely for sweet taste molecules reported by Shamil et al. 31. ASV value 32 for perfect sweet taste obtained at middle of the range 0.618 × 10-6 m3. kg-1.
Table no. 4 and table no.5 report observed values of ASV for (D (+) mannose and D (+) maltose monohydrate) in water (0.05, 0.15, 0.3) mol.kg-1 of Na-Cyclamate at T = 298.15 K, respectively. Experimentally observed ASV values for studied systems lies in the range from (0.620-0.636) × 10-6 m3. kg-1. Thus, studied all solutions show sweet taste. ASV values for D (+) mannose and D (+) maltose monohydrate increases with increase in concentration of Na-cyclamate. Figure 4 shows the variations of ASV values for D (+) mannose and D (+) maltose monohydrate with molality of Na-cyclamate.
 |
Figure 4: Plot of ASV (D (+) mannose and D (+) maltose monohydrate) vs (m) of sodium cyclamate at T = 298.15 K. |
Conclusion
From measured density values volumetric parameters Vɸ0 , Sv, ASV, ΔtrsVɸ0 and interaction parameters (𝑉𝐴𝐵 and 𝑉𝐴𝐵𝐵) have been calculated. The observed values reveal the following conclusions.
Observed positive values of Vɸ0 specify strong sugar-water interactions in absence and presence of Na- cyclamate
Prevalence of interactions between polar groups of D (+) mannose and D (+) maltose monohydrate and ions of Na-cyclamate suggested by positive values of ΔtrsVɸ0 and 𝑉𝐴𝐵.
ASV values for (D (+) mannose and D (+) maltose monohydrate) in water and in (0.05, 0.15, 0.3) mol.kg-1 of Na-Cyclamate lies in range from (0.620 – 0.636) × 10-6 m3.kg-1. Hence studied combinations of aqueous solutions of (D (+) mannose and D (+) maltose monohydrate and Na-cyclamate under investigation show sweet taste.
Acknowledgement
MDP sincerely thanks Principal (HPT /RYK College, Nashik) for providing facilities to perform this research
Conflict of interest
There is no conflict of interest.
Funding Sources
There is no funding Source.
References
- Robert N. Goldberg and Yadu B. Tewari, J. Phys. Chem. Ref. Data, 1989, 18.
CrossRef - Schiffman, S. S., Booth B. J., Brain Research Bulletin, 1995, 38(2), 105. https://doi.org/10.1016/0361-9230(95)00062-J
CrossRef - Alternative Sweeteners 3rd edition edited by Lyn O’Brien Nabors.
- Kharat, S. J., J. Mol. Liqs, 2008, 140, 10-14. https://doi.org/10.1016/j.molliq.2007.12.006
CrossRef - Kharat, S. J., Int. J. Thermophys., 2010, 31, 585-594. https://doi.org/10.1007/s10765-010-0736-6
CrossRef - Kharat, S.J., Thermochimica Acta, 2013,566,124-129. https://doi.org/10.1016/j.tca.2013.05.030
CrossRef - Kharat, S. J., Phys. Chem. Liqs, 2014 ,52 (1),7-16.
- Harned H.S., Owen B.B., The physical Chemistry of electrolytic solutions, ACS monograph No. 137 third ed., Reinhold publishing Corp., New York, (1958).
- Kupke D.W., Physical principles and techniques of physical chemistry, Part C, Academic press, New York, (1973).
- Herington E.F.G., Pure Appl. Chem.,1976, 45, 1-9.
- Masson D O. Solute molecular volumes in relation to solvation and ionization. Philos Mag., 1929, 8, 218-235.https://doi.org/10.1080/ 14786440808564880
CrossRef - Hedwig G. R., J. Solution Chem. 1988,17, 383-397. https:// doi.org/ 10.1039/cs9881700383
CrossRef - Zielenkiewicz W., Perlovich, G.L., Nikitina G.E., and Semeykin A.S., J. Solution Chem. 1996, 25, 135-153.https://doi.org/10.1007/BF00972685
CrossRef - A. F. Fucaloro · Y. Pu · K. Cha · A. Williams ·K. Conrad, J Solution Chem 2007, 36, 61-80. Doi: 10.1007/s10953-006-9100-7.
CrossRef - P.K. Banipal, T.S. Banipal, B.S. Lark, J.C. Ahluwalia, J. Chem. Soc., Faraday Trans. ,1997,93, 81-87.https://doi.org/10.1039/a604656h
CrossRef - P.K. Banipal, A.K. Chahal, T.S. Banipal, J. Chem. Thermodyn. 2009, 41, 452-483. https://doi.org/10.1016/j.jct.2008.11.009
CrossRef - R.V. Jasra, J.C. Ahluwalia, J. Chem. Thermodyn. 1984, 16, 583–590. https://doi.org/10.1016/0021-9614(84)90010-7
CrossRef - ., Motin M.A., Biswas T.K., and Huque E. M., Monatshefte fur Chemie 2003, 134, 797-809.https://doi.org/10.1007/s00706-002-0530-7
CrossRef - T.S. Banipal, Damanjit Kaur, Gagandeep Singh, B.S. Lark, P.K. Banipal Indian Journal of Chemistry, 2002, 41A, 1131-1138.
CrossRef - Teychene J. Balmann H. Roux-De, Galier S., Carbohydrate research, 2017, 448, 117-127.https://doi.org/10.1016/j.carres.2017.06.006
CrossRef - F. Shahidi, P.G. Ferrell, J.T. Edwards, J. Solut. Chem. 1976, 5, 807-816. https://doi.org/10.1007/BF01167236
CrossRef - Kozak J.J., Knight W., Kauzman W., Solute-solute interactions in aqueous solutions, J. Chem. Phys. 1968,48, 675-690. https://doi.org/10.1063/1.1668700
CrossRef - McMillan W.G., Mayer J.E, J.E. Mayer, J. Chem. Phys. 1945, 13, 276-305. https://doi.org/10.1063/1.1724036
CrossRef - Franks F., Pedley M., Michael, D.S. Reid, J. Chem. Soc. Faraday Trans. I. 1976, 72, 359-367. https://doi.org/10.1039/f19767200359
CrossRef - Friedman H.L., Krishanan C.V., Franks F.(Ed), Water: A Comprehensive Treatise, Plenum, New York, ,1993, Vol.3, Chapter 1
- Savage.J.J. , Wood, R., J. Sol. Chem., 1976, 5, 733-750.https://doi.org/10.1007/BF00643457
CrossRef - Desnoyers J. E., Joly M., Perron G., and Jolicoeur C, J. Phys. Chem. 1969 ,73, 3346. https://doi.org/10.1021/j100844a032
CrossRef - Conway B. E., Ionic hydration in Chemistry and Physics, Elsevier, Amsterdam, The Netherlands, 1980.
CrossRef - Parke S.A., Birch G.G., Portman M. O., David K., Food Chemistry, 1999, 67, 247-259. https://doi.org/10.1016/S0308-8146(99)00125-9
CrossRef - Birch G., Parke S., Siertsema R., Westwell J.M., Pure Appl. Chem. 1997, 69, 685-692. https://doi.org/10.1351/pac199769040685
CrossRef - Shamil, S., Birch, G. G., Mathlouthi, M. and Clifford, M.N. Chem senses 1987, 12, 397-409.
CrossRef - Birch G.G., Journal of pure and applied Chemistry, 2002, 74, 1103-1108. https://doi.org/10.1351/pac200274071103
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









