Antimicrobial Activity and Physicochemical Analysis of Bio- Degradable Films from Cucurbita Pepo and Musa Paradisiaca
J. Morris Princey* , A. Nandhini
, A. Nandhini , G. Poojadevi and S. Nobil Divya
, G. Poojadevi and S. Nobil Divya
Department of Chemistry, Holy Cross College (Autonomous), Tiruchirapalli-620002, Tamil Nadu, India.
Corresponding Author E-mail: princeymorris@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370623
Article Received on : 28-07-2021
Article Accepted on :
Article Published : 10 Dec 2021
Reviewed by: Dr. Saiful Zubairi

Second Review by: Dr. Pawan Kumar

Final Approval by: Dr. Tanay Pramanik
Cucurbita pepo and Musa paradisiaca can be considered as a large source of starch which makes it appropriate to be used for the preparation of bio-plastic material. In this study, biodegradable films from Cucurbita pepo and Musa paradisiaca were developed and investigated for their properties like pH, ash, moisture content, amylose content, biodegradability, and antimicrobial activity. 1,2,3-Propanetriol, gingelly oil, and agarose gel was used to reduce the brittleness of the developed starch- based bioplastic films. The investigation of films was done by Spectroscopic and Surface Analysis techniques. The developed bio- films showed substantial properties like less engorgement and insolubility in water which makes it worth a commercial viable product for food packaging.
KEYWORDS:Anti- microbial Studies; Cucurbita pepo; Liquid food Packaging Material; Musa paradisiaca; Moisture and Amylose content
Download this article as:| Copy the following to cite this article: Princey J. M, Nandhini A, Poojadevi G, Divya S. N. Antimicrobial Activity and Physicochemical Analysis of Bio- Degradable Films from Cucurbita Pepo and Musa Paradisiaca. Orient J Chem 2021;37(6). |
| Copy the following to cite this URL: Princey J. M, Nandhini A, Poojadevi G, Divya S. N. Antimicrobial Activity and Physicochemical Analysis of Bio- Degradable Films from Cucurbita Pepo and Musa Paradisiaca. Orient J Chem 2021;37(6).Available from: https://bit.ly/3pJ7rgV |
Introduction
One of the foremost challenges in dry waste management is the plastic waste, which leaches out harmful chemicals inadvertently into the environment. The durability and increasing usage of plastics creates a major waste management problem which accounts for approximately 10 per cent of the waste generated1 by man kind. Interaction of plastic waste with soil and water affects their quality and uniqueness2, the toxic gases from plastics also pollute the environment and increase the global warming. When plastic waste is discarded in landfills, it is viable for the percolation of the hazardous chemical into the potable water 3 affecting its quality. Due to these environmental issues, some alternative methods are used for the preparation bio- plastics. Bio-plastic is usually developed from a renewable source like starch, sawdust, cellulose, food waste, vegetable fats, and oils which is used for the packing of cutlery, crockery, food items etc.4 The production of bioplastics can significantly reduce the emission of greenhouse gases and it can also decrease the consumption of non-renewable energy sources. The present study aims to prepare and analyze the bio- films obtained from Cucurbita pepo and Musa paradisiaca using 1,2,3-Propanetriol, gingelly oil, and agarose gel as the plasticizing agents. In the last few years, Glycerol (1,2,3- Propanetriol) has been used in commercial applications because of its properties like high tensile strength, solubility in water, and biodegradability. It is also generally used as a plasticizing agent to increase the flexibility of the bio-plastic film. Apart from 1,2,3- Propanetriol, gingelly oil and agarose gel has been used as plasticizers in this study. The bio-films obtained were further analyzed and characterized by different spectral and surface investigation techniques.
Materials and Methods
Abstraction of Starch
The dry pulp of Cucurbita pepo and peels of Musa paradisiaca was ground into a fine powder, soaked in water for about a day, filtered and used for the abstraction of its starch content. Finally, the dried starch from Cucurbita pepo and Musa paradisiaca was used for further studies.
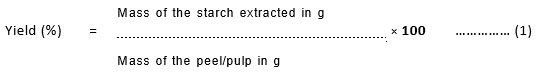
The Moisture content of Starch
About 3g of the starch samples were taken in the pre-heated, cooled Petri- dishes and kept in the oven at a temperature of 1250C5. After an hour it was cooled to room temperature and the moisture content of the sample was found out using the following equation (2)

Ash content
About 3g of the starch samples were incinerated and completely carbonized in the pre-heated and cooled crucibles for about an hour at 1000 C. After cooling, the percentage of ash content 6 was calculated using equation (3).

Starch pH
About 2g of the starch sample obtained from Musa paradisiaca and Cucurbita pepo was shaken in distilled water for 25 minutes, the starch was let on to clear and the pH was measured using a Systronics, Digital pH Meter 335 with 7% buffer solution.
Titratable acidity
0.1M of Sodium Hydroxide was used as the titrant for 3g of the starch sample dissolved in 30 ml of deionized water using phenolphthalein as the indicator.
Test for Amylose and Amylopectin content
About 1ml of ethanol and 9ml of NaOH were added to 0.1g of the starch sample in a test tube covered by an aluminum foil, after which it was completely mixed and heated for about 10minutes7. The cooled dispersion was diluted to10 times it’s volume from which 0.5ml of the extract was used for the analysis. To the extract, 0.1 ml of CH3COOH and 0.2ml of I2 in CCl4 were added and made up to 10 ml with distilled water. The Amylose content was determined using Aer Infra Digi, Digital Photo Colorimeter. The Amylose and Amylopectin content (%) was determined using equations 4 & 5.
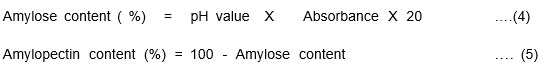
Preparation of the Bio-film
The bio-films were made using the starch of Cucurbita pepo and Musa paradisiaca. The bio-plastic from Cucurbita pepo was developed by taking 3g of its starch to which a mixture of 2g of agarose gel and 2ml of gingelly oil was added and heated in a low flame to get a colloidal gel. The gel was spread on a flat aluminum foil and dried8 under the sunlight for two days to obtain the bio-film. About 3g of Musa paradisiaca peel was soaked with sodium metabisulphite (Na2SO3) for about 45 minutes. Then, the peel was boiled for half an hour, filtered, dried, and ground into a paste. 2.5g of Musa paradisiaca paste was taken to which, 3ml of sodium hydroxide, 3ml of hydrochloric acid, and 2ml of 1,2,3-Propanetriolwere added and mixed well. The mixture was poured into a petri dish, and heated for about half an hour at 110 0C in an oven. After cooling a brown-colored bio-plastic film was formed.
Water Holding Capacity of the film
The bio-film was cut into 2cm x 2cm size and placed into a beaker containing 100ml of water9 for an hour. The initial and final weight of the films were noted from which the water holding capacity of the films was calculated using Equation (6),

Biodegradability test
About 2g of the bio-film was taken and buried under the soil in a beaker at the depth of 5cm from the ground surface for about 15 days. The weight of the film was taken in a cycle of three days and results were recorded accordingly10. The weight of the sample before (W0) and after burial (Wf) in the compost soil was noted and the weight loss of the samples was calculated using Equation (7),
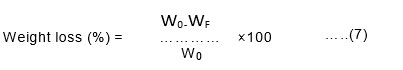
Bio-films as Liquid food Packaging Material
Both the bio-films of Cucurbita pepo and Musa paradisiaca were tested as a packaging material for liquid food. The pre-weighed bioplastic (W1) was cut into 2×2 cm and immersed in normal water and coconut oil at room temperature for three hours after which it was re-weighed(W2). The weight change (%) of the bio-film was calculated using Equation.8. If the weight change of bio-film after being plunged into the liquid food products is less than 15% then the bio-film is to be compatible with the liquid food product and can be used as a packaging material for the same.
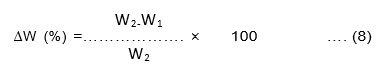
Characterization of the Bio-films
The investigation of the bio-films was carried out by different Spectral and Surface Analyzing techniques.
FT-IR analysis
The functional groups present in the films were determined through an Attenuated Total Reflectance (ATR) accessory with 8 scans at the range of 4000-400cm-1(Perkin Elmer FT-IR Spectrometer Frontier). The Bio-film was mixed KBr in a ratio of 1:511. The peaks were obtained in the range of 4000 to 400 cm-1.
SEM analysis
The surface morphology of a biopolymeric film can be visualized using scanning electronic microscope12 under normal atmospheric conditions. The SEM analysis utilizes a focused beam of high-energy electrons to produce an assortment of signals at the surface of the specimens.
Antimicrobial Activity Test
The antimicrobial activity of the bio- film for micro-organisms was done by the Disc-diffusion method13. The Petri dish was prepared with Muller Hinton Agar and immunized with test organisms. Sterile disc of 6- millimeter width was infused with the bioplastic film at different concentrations of 20-100 microgram per milliliter respectively. The impregnated disc was placed on the top layer of agar plates for 30 minutes at room temperature for compound diffusion. The dishes were incubated for 24 hours at 370C and the zone of inhibition was recorded in millimeters and the experiment was repeated twice.
Results and Discussion
Yield of Starch
The percentage yield of starch from Cucurbita pepo and Musa paradisiaca is given in Table.1 From the values it can found that bio-films prepared from the starches can be used as a packaging material for food products.
Table 1: Results of Physical, Chemical parameters and Proximate analysis of Cucurbita pepo and Musa paradisiaca
|
No |
Parameters |
Curcubita pepo |
Musa paradisiaca |
|
1. |
Appearance |
Yellow |
Brown |
|
2. |
Odor |
Odorless |
Odorless |
|
3. |
% yield |
76.25 |
82.9 |
|
4. |
pH |
5.30 |
6.64 |
|
5. |
Titrable acidity |
3.8 |
3.5 |
|
6. |
Moisture content |
11.99 |
11.9 |
|
7. |
Ash content |
5.86 |
3.9 |
|
8. |
Amylose Content |
20.27 |
34 |
|
9. |
Water Holding Capacity |
63.68 |
89 |
Moisture content
The moisture content for Cucurbita pepo starch and Musa paradisiaca starch was found to be in the range of 11-12%(Table.1). The result shows that the moisture content value for both starches is low when compared to other starches14 which may be due to reasons like handling problems and level of ripping. High moisture content in food can lead to microbial spoilage and short shelf life to the food items, leading to its deterioration. Less percentage of moisture content in food items is acceptable and good.
Ash content
The mineral content of the bio-film15is identified by the ash test. The ash content of both Cucurbita pepo and Musa paradisiaca starches was found in the range of 3-5%. The mineral and organic salt in the tubers is expressed as the ash content which is the food residue after the combustion process. The low value of ash indicates a low mineral content in the fruit or the vegetable under investigation.
pH of the Starch
The pH value of the Cucurbita pepo and Musa paradisiaca starches were found to be around 5-7. If the pH of the starch is in the range of 4-716 it can be used as a packaging material in food industry.
Amylose content
The Amylose content test will help to detect the Amylose and Amylopectin percentage in the starch. The Amylose content present in the Cucurbita pepo (20.27%)and Musa paradisiacal (34%) is given in Table.1. The Amylose content leads to chain formation in the bioplastic preparation.
Water Holding Capacity of the bio- film
The water holding capacity is an important study to determine the suitable source for bio-polymers.17 The water uptake value of the bio-plastic sheet from Musa had 89% percentage followed by Cucurbita pepo bio-plastic sheet with 63.68% of absorption capacity(Table.2). Due to the hydrophilic nature of the films the water upholding capacity was found to be more than 50% and this may be due to the plasticizing agent1,2,3,- propanetriol. It is a low molecular carbohydrate that tends to absorb water according to the molecular weight of its structure18 and the number of hydroxyl groups present in it.
Application of bio-films as a Packaging Material for Liquids
Table 2: The weight change of the bio-plastics after being immersed in test samples for one hour.
|
No |
Test samples |
Curcubita pepo |
Musa paradisiaca |
|
1. |
Distilled water |
63.68 |
89 |
|
2. |
Vegetable oil |
2.9 |
4.87 |
From Table. 2 it can be seen that the penetration of vegetable oil is low compared to the penetration of water in the synthesized bio-films.. The weight of the biofilm increased to a maximum than its original weight. This indicates that the bio-plastic of Cucurbita pepo and Musa paradisiaca can be used as a packaging material for vegetable oils.
Biodegradability test
The degradation tests of bio-films was conducted using the soil burial technique. The biodegradability of bioplastics was determined by allowing them to degrade in the soil for a month. The degradation of Cucurbita pepo and Musa paradisiaca bio-film was monitored regularly. Figs.1 & 2 show the percentage weight loss of the samples within one month for Cucurbita pepo (87%) and Musa paradisiaca (93%) respectively. Generally, the results show the increase in the percentage of degradation of the bio-film gradually after five days. The weight loss was monitored periodically for every five days and after 30 days it was found to be fully degraded. Visually the texture of the bio-plastics showed literal damage on the surface of the bio- plastics19.
 |
Figure 1: Degradation Percentage of bio-film from Curcubita Pepo. |
 |
Figure 2: Degradation Percentage of bio-film from Musa paradisiaca |
Spectral Analysis of the Bio-films
 |
Figure 3: FT-IR Spectra of the bio-film from Cucurbita pepo. |
 |
Figure 4: FT-IR spectra of the bio-film from Musa paradisiaca. |
Figs. 3 and 4 show the IR spectra of Cucurbita pepo and Musa paradisiaca. In the IR spectrum of Cucurbita pepo bio-film the characteristic peak at 3605.99cm-1shows the presence of O-H stretching bond20, the peak at 2326.18 cm-1shows O=C=O stretching bond21, the absorption bands at 2155.99 cm-1and1743.92 cm-1 shows the presence of N=N=N and C=O stretching and the band at 1472.50cm-1 shows C=C ring stretching aromatic bond. The characteristics peaks at 3205.90 cm-1,2957.66cm-1,2132 cm- 1,1421 cm-1and at 857cm-1of the Musa paradisiacal biofilm were attributed to N-H stretching ( secondary amine) hydrogen-bonded nitriles, strong C-H stretching (alkanes)22, C≡Calkynes, C-H stretching (alkene) and aromatic C- H stretching respectively in the FT-IR Spectrum.
Surface Analysis Technique
The Scanning Electron Microscopy studies showed that Musa paradisiaca starch granules23 are rectangular (Fig.5) with a smooth surface. The starch granules of Cucurbita pepo were found to be spherical with a smooth surface (Fig.6).
 |
Figure 5: Sem image of the developed bio-film from Musa paradisiaca |
 |
Figure 6: Semimage of the developed bio-film from Cucurbita pepo |
Anti-microbial Activity
The anti-fungal and anti-bacterial results of Cucurbitapepo and Musa paradisiaca bio-films reveal that both the starches are effective against Aspergillus niger24 and Staphylococcus Aureus25 compared to Escherichia coli and Candida albicans. The results are given in Tables 3 and 4.
Table 3: Antifungal activity of the plant starches
|
Starch |
Concentration (µl/disc) |
Organisms /Zone of Inhibition |
|
|
Candida albicans |
Aspergillusniger |
||
|
Curcurbito pepo |
100 |
5 |
6 |
|
Musa paradisiaca |
100 |
3 |
4 |
Table 4: Anti-bacterial activity of the plant starches
|
Extract |
Concentration (µl/disc) |
Organisms /Zone of Inhibition |
|
|
Escherichia coli |
Staphylococcus aureus |
||
|
Curcurbita pepo |
100 |
7 |
6 |
|
Musa paradisiaca |
100 |
5 |
8 |
Conclusion
The development of packaging material for food products involves sustainability and environmental responsibility. In this study, Cucurbita pepo and Musa paradisiaca starch have been used for the preparation of bio-films. The bio-film also investigated by spectral and surface morphological analysis. Anti – microbial studies showed that the bio-films were effective against Aspergillus niger and Staphylococcus aureus. The different physical and chemical properties like percentage yield of starch, pH, Titrable acidity , proximate analysis like amylose content and moisture content of the biofilm showed that it can be used as an alternative food packaging material for vegetable oils.
Acknowlegdement
The authors wish to accord their sincere thanks to the Principal and Management of Holy Cross College (Autonomous), Tirichirapalli .
Conflict of Interest
There is no conflict of interest
Funding Sources
There are no funding source.
References
- Richard Thompson, C.; Shanna Swan, H.; Charles Moore, J.; and Fredrick Vom Saal, S. Phil. Trans. R. Soc. B, 2009,364,1973-1976.
CrossRef - Rizwana Beevi, K.; Sameeera Fathima, A.R.; Thahira Fathima, A.I.; Thameemunisa, N.,Noorjahan, C.M.; Deepika, T. Int. J. Sci. Technol. Res., 2020, 9,22-29.
- Marichelvam, M.K.; Mohammad Jawaid ; Mohammad Asim, Fibers, 2019 7,32
- Noor Fathimah; Kader Sulfan;Wan Lutti Wan Tohari; BSTR, 2017, 5,12-17.
- Indrianingsih, A.W.; Apriyana, W.; Nisa, K,;Rosyida, T.; Hayati, N.; Darsih, C.; Kusumaningrum, Food Res., 2019, 3(5), 484-490.
CrossRef - Workiye Getnet Abera, Int. J. Biol. Macromol. ,2019, 1, 1-130.
- ArifaShafqa; Nabil Al-Zaqri; Arifa Tahir; Ali Alsame; Saudi J. Biol. Sci., 2021, 28, 1739-1749.
CrossRef - Vikas Mishra; Akash Patel; Darshan Rana; Sanjay Nakum; and Bhupendra Singh; IJSRD, 2015, 452-455.
- Jose Igor Hleap- Zapata ; Therlyn Cruz- Rosero; Laely Tafiana Duran- Rojas; Daniela Hernandez –Trujillo; Luis David Reina- Aguirre; Natalia Tilano-Pemberthy, J. of faculty of Agr. Sci.,2020, 52(2), 395-404.
- Jayachardra Yarododai; Vinay Patil; Sharanabasava Gabachari and Nagaraj, IJPRAS, 2016,5, 56-66.
- Aline Machado Pereira; Fernanda Doring Ramos; Ana Cristina Richte Kroow; Roberta Bascle Santos; Marcia Aricha Gularta, Food Sci. Technol.,2020,40, 352-487.
- Htun Htun Naing; Htay Htay Shwe ; Ni Ni Pe; Yazar Tun,Res. J. Chem. Environ., 2020,3(4), 1353-1361.
- Augustin,Y.E.; and Padmawjiya, K.S.; Mater. Sci. Technol., 2017, 1-7.
- Abubakar,U.S.;Yusuf, K.M.;Safiyan, I.; Abdilahi, S.; Saidu, S.R.Int.J. Food. Sci. Nutr.,2016,1, 25-27
- Raden Cecep; Erwan Andriansyah; Taufik Rahman; Ainia Herminiati; Nurhaidar Rahman; Rohmah Luthfiyanti, IOP Conf. Ser.: Earth Environ. Sci.,2017, 101, 1-10.
CrossRef - Rowe, R.C.; Sheskey, P.; Quin, M.E.;J.Pharm.Pharmacol.,2017, 8, 506-509.
CrossRef - Noor Fatimah Kader Sultan ; Wan Lutfi Wan Johari, BSTR, 2017, 5, 12-17.
- Mathew,A.P.; Dufresne, A.;Biol.Macromolecules,2002,3(5),1101-1108.
CrossRef - Oluwasina,O.O.; Olaleye, F.K.; Olsegun, S.J.; Mohallem, N.D.S. Int.J.Biol.Macromol.,2019, 135, 282-293.
- Ling Yin; Changlin Wang, J.Hortic.,2016, 3, 187.
- Hasan, M.; Zulfadli Nazar, M; Rahmayani, R.F.I.;Fajri, G.; Fansuri. H. Rasayan J. Chem., 2019, 12, 1390-1398.
CrossRef - Asep Bayu Dani Nandiyanto; Rosi Oktiani; Risti Ragadhita; IJoST., 2019, 4(1), 97-118.
- Heloisa Tibolla; Franciele Maria Pelissari; Florencia; Cecillia Menagalli; Food Sci. Technol.,2014, 59, 1311-1318.
CrossRef - Ali Nasse; Saddiq Amna, Afr.J.Microbial. Res., 2012, 6(41),6941-6947.
CrossRef - Amal, A.; Aljuraifani; Asian J. Biol. Sci., 2017, 26(2), 229-235.

This work is licensed under a Creative Commons Attribution 4.0 International License.









