Synthesis of some Novel Mixed Ligand Complexes of Ni(II) and their Characterization
Department of Chemistry, Govt. P.G. College, Rajouri, Jammu and Kashmir, India.
Corresponding Author E-mail: saleem82mohd@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370520
Article Received on : 05-May-2021
Article Accepted on : 05-Sep-2021
Article Published : 10 Sep 2021
Reviewed by: Dr. Thube Dilip Raosaheb
Second Review by: Dr.P.Thirumavalavan
Final Approval by: Dr. Pounraj Thanasekaran
A new series of metal Complexes were prepared by refluxing nickel chloride with hot methanolic solution mixture of 8-Hydroxyquinoline and Schiff bases(L1-L6). Schiff base ligands were synthesised by the condensation of 4-aminopheno, 4-aminosalicylic acid, 2-aminobenzthiazole with salicylaldehyde and vanillin. The metal complexes have been characterised by gravimetric metal analysis, magnetic moment,conductivity measurement, 1HNMR and IR analysis.
KEYWORDS:Conductivity Measurement; 8- Hydoxyquinoline; IR; NMR; Schiff bases; Transition Metals
Download this article as:| Copy the following to cite this article: Saleem M, Riaz M, Synthesis of some Novel Mixed Ligand Complexes of Ni(II) and their Characterization. Orient J Chem 2021;37(5). |
| Copy the following to cite this URL: Saleem M, Riaz M, Synthesis of some Novel Mixed Ligand Complexes of Ni(II) and their Characterization. Orient J Chem 2021;37(5). Available from: https://bit.ly/3BZ6tBH |
Introduction
Mixed ligand complexes of transition metal ions are of great interest for the researchers who wishes to study their coordination behavior for exploiting their properties in different fields especially in the antibacterial activity 1-3
Schiff bases ligands are prepared by condensation of carbonyl compounds and primary amines. A Schiff base is a compound with the general structure R₁R₂C=NR’. Schiff bases are also considered as a sub-type of imines, being either secondary aldimines or secondary ketimines depending upon their structure. Lone pair of electrons on sp2hybridized orbital of nitrogen atom of the azomethine group is of considerable biological and chemical importance. Schiff bases ligands and their metal complexes are very important since they are used in a variety of homogeneous catalytic reactions 4. Chelating Schiff’s bases/polydentate Schiff base transition metal complexes are important in environmental, chemical and biological fields 5-9. For olefin polymerization Schiff base metal complexes have become more important as highly active catalyst 10. Many Schiff bases complexes are also considered important as theymimic the catalytic activities of metalloenzymes and act as models for running important biological reactions 11.
The presence of C=N bond is a crucial fact for the smooth formation of various structures in natural systems thus contributing to the importance of Schiff bases in building if different structures. In addition to this, in some cases, the condensation was also enabled by coordination of Schiff base components which otherwise could not take place in aqueous media 12. Thus, ligand building could be possible that otherwise do not exist as separate molecules.
Schiff bases have been studied as biologically active, such as, antifungal and antibacterial activities 13-16. Schiff base ligands and their mixed ligand metal complexes are also reported as biologically active in anti-cancer and herbicidal activities 17-18. They also antiviral, antiprotozoal, anti-HIV and anthelmintic activities when studied against the said organisms 19.
8-Hydroxyquinoline is very interesting and important molecule because of its ability to form a vast variety of complexes with metal ion especially transition metal ions. 8-Hydroxyquinoline in presence of Schiff base undergoes complexation with metal ion by deprotonation of its phenolic oxygen and also make bond by utilizing its ring nitrogen 20-24.
Materials and Methods
Chemicals and instruments
All chemical compounds used in this study were supplied by Himedia, and Fluka chemical companies were used as received with 98% purity.Metal was analysed by gravimetric method 25. Molar conductivity was measured at 25-26 0C in DMSO/DMF for the metal complexes by an Elico conductivity bridge of type CM82T. The IR spectra for Schiff base ligands and their mixed ligand metal complexes were recorded inPerkin-Elmer spectrum two,115013 spectrophotometerand 1HMR in DMSO-d6 solvent recorded by Bruker Advance- 111 300 MHz NMR Spectrometer.
Schiff base Synthesis
Schiff base ligands were synthesised by reacting various amines (4-aminophenol, 4-aminosalicylic acid, 2-aminobenzthiazole) with aldehydes (Salicylaldehyde, Vanillin). Equimolar ratio of aldehyde and amines were mixed and taken in a round bottom flask and then refluxed for 2 hours. Reaction mixture was left undisturbed for overnight and solid product was obtained. The solid product so obtained was filtered and then recrystallized in ethanol, finally dried in desiccator (scheme 1). General reaction scheme for the preparation of ligands is shown in scheme 1.
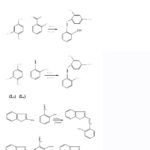 |
Scheme 1 |
Synthesis of Metal complexes
Complexes were prepared by known method [26]. Add hot Methanolic Solution of 8-Hydroxyquinoline (0.05 mmol)to the methanolic solution of Schiff bases ligands (L1-L6)(0.05 mmol) in a round bottom flask. To the solution of metal chloride in methanol add the above reaction mixture drop wise with constant stirring. Reflux the resulting reaction mixture for 6 hours on magnetic stirrer with constant stirring and then place undisturbed for overnight. Check the progress of reaction by TLC. Filter the precipitates thus formed and dry in desiccator. Recrystallize the complex so obtained in ethanol. Chemical reaction for the representative schiff base ligand (L1) is shown in scheme 2.
 |
Scheme 2 Click here to View scheme |
Thin layer Chromatography
The progress of the reaction was checked from time to time by TLC method. Polar solvent system Pet-ether and ethyl acetate (20%) was used and iodine vapours was used as a spraying reagent. A single spot appeared for all the complexes at the end of the reaction and starting material disappeared thus confirming the completion of reaction.
Result and Discussion
The physico-chemical and analytical studiesdepict that all metal complexes are octahedral and mononuclear with general formula [Ni(L)2QN].Where L is bidentate Schiff base ligands (L1-L6)and Qn is 8-Hydroxyquinoline.All complexes are stable in air, coloured and soluble in DMF/DMSO and partly soluble in EtOH and MeOH.Metal was analysed by gravimetric method and percentage of metal as revealed experimentally is shown in Table 1.
Table 1: physical properties and metal analysis data for the complexes
|
Ligands |
Metal Complex |
color |
M.P |
solubility |
% Of Ni |
|
L1 |
C35H26NiN3O5 |
Brown |
195-197 |
PS in ethanol Soluble in DMSO |
09.10% |
|
L2 |
C35H26NiN3O5 |
yellow |
211-212 |
DMSO |
09.07% |
|
L3 |
C37H24NiN3O9 |
Light yellow |
188 |
DMSO & DMF |
08.11% |
|
L4 |
C37H24NiN3O9 |
Yellow |
189 |
DMSO & DMF |
08.09% |
|
L5 |
C37H24NiN3O3S2 |
Reddish brown |
137-139 |
PS in ethanol Soluble in DMSO |
08.33% |
|
L6 |
C37H24NiN3O3S2 |
Brown |
135-137 |
DMSO |
08.45% |
Molar conductivity
Molar conductivity was measured at 25-26 0C in DMSO/DMF for the prepared metal complexes by an Elico conductivity bridge of type CM82Tusing 10-3mol L-1 solution of Ni(II) complexes.
The value of molar conductivity for all the six metal complexes was obtained between 16.33 and 11.90 mho cm2mol– which are less than the value of 70-160 mho cm2 mol-1 obtained for 1:1 electrolyte inthe solvent. Thus, it confirms the non-electrolytic character of all synthesised mixed ligand metal complexes.
Magnetic Moment
For Metal complexes, the magnetic moment value obtained between 3.1 to the number of unpaired electrons. The variation in magnetic moment is due & 2.8 corresponding to delocalisation. As Ni(II) has 3d8 outer configuration and has high value for magnetic moment as expected for two unpaired electrons in octahedral complexes. This shows that Ni(II) complexes have octahedral geometry.
IR spectra
The IR spectra for free ligands (Schiff bases and8-Hydroxyquinoline) and their mixed ligand metal complexes were recorded in Perkin-Elmer spectrophotometer within the IR range 4000-400 nm. For aromatic C-H a band was observed between 3066-3012cm-1 and that between 1488-1461cm-1 was assigned to C=C bond. A sharp band observed in the region of 3250cm-1to 3350cm-1 for the free ligands is absent in the spectra of Ni(II) complexes is because of the co-ordination of ligand with the metals ion through the O atom of the phenolic group of 8-Hydroxyquinoline. Spectral peaks for C=N and C-O of 8-Hydroxyquinoline showed a shift to lower frequencies indicating bonding of ligands to metal.A sharp IR band at ca3412 appeared in the Schiff base ligands (L1, L2, L3 and L4) as well as their complexes assigned toⱽ(OH) not involved in bonding [27-31].The Schiff base spectra contained strong bands in the range 1655-1610 assigned to C=N bond of the free ligand(L1-L6) was shifted towards lower frequencies due to the lower C=N bond order and the formation of metal nitrogen bond in the metal complexes.The value of IR spectra for M-O and M-N are observed in the range 575-560cm-1 and 519-490cm-1indicates Schiff base coordination with metal as demonstrated table 2 and Spectra of Ni(II)(L1)(QN) is shown in fig 1.
Table 2: Showing value of IR Spectra Ni(II) complexes.
|
Ligand |
Metal Complex |
ⱽ(C-H)Ar |
ⱽ(C=C) |
ⱽ(C=N) |
ⱽ(C=N) |
ⱽ(C-O) |
ⱽ(M-O) |
ⱽ(M-N) |
|
Form 8-Hydroxyquinoline |
||||||||
|
L1 |
C35H26NiN3O5 |
3048 |
1471 |
1593 (1610) |
1508 (1540) |
1094 (1110) |
575 |
519 |
|
L2 |
C35H26NiN3O5 |
3012 |
1461 |
1610 (1640) |
1504 (1534) |
1102 (1115) |
560 |
510 |
|
L3 |
C37H24NiN3O9 |
3045 |
1463 |
1601 (1635) |
1494 (1514) |
1098 (1110) |
565 |
498 |
|
L4 |
C37H24NiN3O9 |
3030 |
1488 |
1630 (1655) |
1501 (1520) |
1105 (1115) |
560 |
495 |
|
L5 |
C37H24NiN3O3S2 |
3066 |
1487 |
!625 (1653) |
1489 (1505) |
1102 (1120) |
565 |
490 |
|
L6 |
C37H24NiN3O3S2 |
3050 |
1472 |
1618 (1640) |
1497 (1514) |
1100 (1118) |
560 |
512 |
 |
Figure 1: IR Spectra of Ni(II)(L1)2QN] Complex. |
1HNMR Spectra
1HNMR of the complexes were determined in DMSO with TMS as reference compound. The 1HNMR spectra of Schiff bases ligands(L1-L6) and their metal complexes are compared. The 1HNMR spectra of the Schiff bases showed a signal at 10.26 and 9.0 ppm because of the – CH=N- group. A multiplet in the range 6.8-7.8 ppm due to aromatic protons is observed in the spectra of ligands as well as metal complexes. In case of ligands having salicylic acid the OH group was observed at 9.0 -8.8.A singlet in off-set at high δ values is always given by the phenolic protons of ligands, thus confirms its involvement in an intramolecular hydrogen bond between the neighbouring nitrogen atoms [32] that is absent in complexes indicating bonding through O of OH group. A singlet is observed for free NH2 in a region of 4-5 ppm [33]. The 1HNMR spectra for Ni(II)(L1)(QN) is shown in fig 2.
 |
Figure 2: 1HNMR of Ni(II)(L1)2QN] complex |
Conclusion
The Ni(II) complex synthesized by using Schiff base ligand and 8-Hydroxyquinoline. The mixed ligand metal complexes synthesized are soluble in DMF/DMSO,partly soluble in EtOH and MeOH, coloured and are characterised by IR and 1HNMR spectra. They show low molar conductance value and hence are non-electrolytic in nature.
 |
Scheme 3: Structure of the Ni(II)(L1)2QN] Complex. |
References
- S. Patil, Sunil.; A. Thakur, Ganesh.; M. Shaikh, Manzoor.; International Scholarly Research Network, 2011, 168539, 1-6.
CrossRef - H AL-Noor, Taghreed.;J Jarad, Amer.; Obaid, Abaas.; Research Journal of Pharmaceutical, Biological and Chemical Sciences,2017, 8(3), 132-139.
- Camellia, F. K.; Kader,Abdul.;AshrafulAlam,Md.;Kudrat-E- ZahanMd. and Islam M. S.; Synthesis, Characterization and Antimicrobial Investigation of Three New Mo (VI) Mixed Ligand Complexes”,2018; 4(4): pp.1-5.
CrossRef - Wilkinson, G.;Comprehensive Coordination Chemistry,(Pergamon Press, New York), 1987, 2.
- Zeng,w.; Li, J.; Qin, S.; InorgChemCommun, 2006,9, 10.
- Dyers, L.; JrQue,S. W.; Van Derveer, D.; Bu,X. R.; InorgChim Acta,2006, 359, 197.
CrossRef - Luo, Y.; Lin, J.; MicroporMesopor Mater,2005 86,23.
- Liu, J. Y.; Li, Y. S.; Liu, J.Y.; Li,Z. S.; J MolCatal A:Chem,2005, 244, 99.
- Vaidyanath, V. G.; Weyhermuller, T.; Nair, B. U.; Subramanian, J.; J InorgBiochem,2005, 99,2248.
CrossRef - Ittel, S. D.; Johnson, L. K.; M Brookhart, M.; Chem Rev, 2000, 100,1169.
CrossRef - Bedioui, F.; CoordChem Rev, 1995, 144, 39.
CrossRef - Holm, R. H.; Everett jr, G. W.; Chakravorty, A.; ProgInorgChem, 1966, 7, 83.
- Williams, D. R.; Chem. Rev.,1972, 72, 203.
CrossRef - Campos, A.; Anacona, J. R.; Campos-Vallette, M. M.; Mian group Metal chem.,1999, 22, 283.
CrossRef - Sari, N.; Arslan, S.; Logoglu, E.; Sakiyan, I.; G.U.J. Sci, 2003, 16, 283.
- Verma, M.; Pandeya, S. N.; Singh, K. N.; J P.Stables and Acta Pharm., 2004, 54, 49.
- Cozzi, P. G.; Chem. Soc. Rev.,2004, 410.
CrossRef - Chandra, S.; Sangeetika, J.; J. Indian Chem. Soc, 2004, 81, 203.
- Pandeya, S. N.; Yogecswari, P.; Sriram, D.; Chemotherapy,1999, 45, 192.
CrossRef - Alazawi, S. A. S.; Alhamadani, A. S. S.; Um-Salama Science Journal, 2007, 4, 102.
- Dubey, R. K.; Dubey, U. K.; Mishra, C.; Indian J Chem., 2008, 47A, 1208.
- Halli, M. B.; Patil, V. B.; Sumathi R. B.; Mallikarjun, K.; Der PharmaChemica, 2012, 4 2360.
- Usharani, M.; Akilaand, E.; Rajavel, R.; J Chem Pharm Res., 2012, 4, 726.
- Mohamed, G. G.; and Z H Abd El-Wahab,SpectrochimActa Part A:MoleBiomolecular Spectroscopy, 2005, 61, 1059.
CrossRef - Vogel, A. I.; Text book of Quantitave Inorganic Analysis., Longman,London,1961.
- Wankhede D. N.; et.al.,Pelagia research library, Der ChemicaSinica, 2013, 4, 79.
- Popovic, Z.; Pavlovic, G.; Matkovic-Calogovis, D.; Roje, V.; Leban, I. J. Mol. Struct. 2002, 615, 23.
CrossRef - Schilf, W.; Szady-Chelmieniecka, A.; Grech, E.; Przybylski, P.; Brzezinski, B. J. Mol. Struct. 2002, 643, 115.
CrossRef - Bui, H.-H.; Lien, E.J.; Ren, S. J. Food Drug Anal. 1998, 6, 679.
- Mart, H.; Sacak, M.; Yürük, H.; Sahmetlioglu, E.; Vilayetoglu, A.R. J. Polym. Sci. Part A2004, 42, 1120.
CrossRef - Monfared, H.H.; Pouralimardan, O.; Janiak, C. Z. Naturforsch. 2007, 62b, 717.
CrossRef - Roman, G.; Andree, M.; Bulletin of the Chemists and Technologists of Macedonia,2001, 20, 131.
- Pavia, D. L.; Lampman, G.M.; Kriz, G. S.;Harcourt Brace College Publishers, America ,1996.

This work is licensed under a Creative Commons Attribution 4.0 International License.










