Spectral Description of Cellulose Textile Colored with Date Pits Extract: Study of the Fluorescence Characters
1Chemistry Department, College of Science, IMSIU (Imam Mohammad Ibn Saud Islamic University), Riyadh 11623, kingdom of Saudi Arabia.
2Laboratory of Synthesis Heterocyclic and Natural Substances, Faculty of Sciences of Monastir, Boulevard of Environment, 5000 Monastir, Tunisia.
Corresponding Author E-mail: bh_naoufel@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/370504
Article Received on : 14-Jul-2021
Article Accepted on : 01-Sep-2021
Article Published : 09 Sep 2021
Reviewed by: Dr. S. P. S. Jadon
Second Review by: Dr. Selvaraju K
Final Approval by: Dr. Ioana stanciu
This work purposes to evaluation the fluorescence parameters of cellulose dyed with date pits extract. Fluorescence measurements have been advanced on cellulose models before and later dyeing. The emission spectrum of the nondyed cellulose fibers not demonstrate any bond and, in the opposing, dyed cellulose sample existing important photoluminescence characters and the maximum value was noted at 672 nm.
KEYWORDS:Cellulose fabric; Date pits extract; Dyeing; Fluorescence property
Download this article as:| Copy the following to cite this article: Hamadi N. B, Guesmi A. Spectral Description of Cellulose Textile Colored with Date Pits Extract: Study of the Fluorescence Characters. Orient J Chem 2021;37(5). |
| Copy the following to cite this URL: Hamadi N. B, Guesmi A. Spectral Description of Cellulose Textile Colored with Date Pits Extract: Study of the Fluorescence Characters. Orient J Chem 2021;37(5). Available from: https://bit.ly/3yQL8Ze |
Introduction
Until the end of the last century, natural dyes were the only source of color for all textiles commonly used by humans. These dyes were also used for dyeing wood, feathers, etc., and they provided part of the pigments used in painting and were also of great importance in cosmetics, pharmaceuticals and food. Unlike synthetic dyes, natural dyes usually do not consist of just one coloring molecule. They almost always result from a combination of several coloring molecules, usually from different chemical groups. This gives them a subtlety of unparalleled tones which explains why the artisan dyers of many civilizations have remained attached to the use of natural dyes for their most prestigious textile productions or those most loaded with symbolic and religious meaning. However, the accidental discovery in 1856 of a synthetic dye, mauveine, initiated the end of the traditional art of dyes, especially since they present a difficulty in standardizing and reproducing the results. Therefore, the transition to synthetic dyes was rapid as the new dyes were safe, convenient, and generally less expensive industrially.
The natural dye is combined in the human mind with the notions of environmental and physiological consciousness. This idea alone is sufficient to explain the rebirth of this art, as well as the marked tendency of several researchers to switch from a natural artisan dye to an academic natural dye. In addition to the brilliance and harmony of the colors generated by natural dyes, these are, more than synthetic dyes, renewable sources as they are mostly biodegradable 1-7. For those motives, numerous works have projected natural colorants as a substitute to the prepared ones. Dates represent share of a fashionable existence amongst the population of the Middle Eastern 8. In adding, there are additional possible applications counting oil mining from the date pits or exploitation them as a dietary-fiber benefactor in bakeshop preparations 9. Fluorescence is a natural emission which translates the capacity of certain pigments to re-emit light at certain particular wavelengths by the reverse process of absorption. We’re talking about spontaneous broadcast. Fluorescence is widely used in the biology as a non-invasive analytical tool, particularly since the discovery of fluorescent molecules that can be used as probes in place of radioactive elements. In the case of plants, this technique is all the more interesting than the leaves naturally contain fluorescent molecules called fluorophores 10.
This study was established in order to describe the fluorescence characters of cellulose fibers dyed with date pits extract.
Experimental section
Materials and methods
Date nucleus
Discarded date nucleus were collected and cleaned with tap water. Then, they were dried from the residual water at room temperature. After that, they were frozen at -50 °C for 3 hours and finally freeze-dried. A hammer mill was then used to ground the freeze-dried date-nucleus, and the derived powder was kept at -50 ᵒC for further uses.
Cellulose fabric
Commercially bleached cotton fabric with the following specifications was kept for developing the dyeing experiments (knitted cotton of 150 g/m2).
Methods
Extraction of dye
The freeze-dried date-pits powder (1 g) was extracted with 100 mL of an extraction solvent at temperatures of 25 and 50 °C. The used solvents were (acetone: water) in the ratio (1:1) (Suresh et al. 2013). Conventional extraction was carried out with a magnetic stirrer hot plate. The extraction was carried out for 2 h in a conical flask tailored with a refrigerant to avoid any losses of solvent. Filtrate was evaporated in vacuum until reducing the volume of each extract from 100 to 50 mL.
HPLC analysis
HPLC analysis of phenolic acids in date nucleus was established affording to the technique presented by Chang Lee and Mohamed Al-Farsi with modification (Al-Farsi et al. 2008). 20 milliliters of (methanol: 15% acetic acid) was additional to 0.5 g of freeze-dried date-pits powder. The mixture was stirred for 5 minutes, then sonicated for 30 minutes. Later that, 5 milliliter of purified water and 5 milliliter of sodium hydroxide (5 M) were poured. The all has been effervesced with nitrogen, and strongly stirred for 4 h at room temperature. The pH of the presented solution has been adjusted to 2, then the released phenolic acids were extracted three times with 25 milliliters of (diethyl ether: ethyl acetate) (50:50). The resulted mixture was evaporated, and then liquified in 2 milliliters of MeOH. The solution was then filtered through a micro filter (porosity of 0.45 μm). Then 20 μL of the solution was inserted into a high-performance liquid chromatography apparatus (Merck) equipped with Prodigy ODS-2 column. HPLC technique were as follows: Solvent A was 50 mM phosphoric acid, pH 2.5, and solvent B was acetonitrile. The elution gradient was as shadows: 0–5 min, isocratic elution 95% A and 5% B; 5– 55 min, linear gradient 80% A and 20% B; 55–60 min, linear gradient 95% A and 5% B. Flow rate of the mobile phase was 0.9 ml/min. The wavelengths of the diode array detector were set at 254, 270, 280 and 329 nm for monitoring of phenolic acids.
Determination of total phenolic amount Spectrophotometric
Determination of the total phenolic amount (TPC) was complete with the Folin–Ciocalteu technique using gallic acid as a standard (Yoo et al. 2004): 1 milliliter of extract dye was poured in 1 milliliter of Folin–Ciocalteu’s reactive and allowable to react for 10 minutes. Then, 15 milliliter of 10% NaCO3 solution were poured. Afterward that, the ending volume was complete up to 25 milliliter with deionized water. After keeping the whole reacting within 1 hour at room temperature, the absorbance of the solution was measured at 750 nm. The total phenolic concentration was expressed as milligrams of gallic acid equivalents per gram of date pits powder.
Dyeing procedure
Dyeing experiments were developed in a research laboratory using Ahiba Datacolour machine with the prepared solutions at a liquor to good ratio of 40:1. For all experiments, pH value was fixed to 4, temperature to 90 °C and for a constant duration of 90 minutes. The colored samples were lastly washed with cold water and dehydrated at moderate temperature.
Determination of color intensities quantities
The color produce of the colored samples was estimated by bright reflectance quantities using SF 300 spectrophotometer apparatus. Comparative color intensities (K/S values) were resolute using the Kubelka-Munk equation.
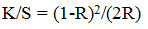
Spectral characterization of cellulose fabric
The spectra of the fluorescence of dyed wool fibers were attained exploitation with fluorescence spectrophotometer (Perkine-Elmer LS-3).
Results and discussion
Extraction of dye from date pits
The UV spectrum of the dye pits extract illustrations a attitude of absorption in the visible range amongst 465 to 515 nm, this demonstrations that the aqueous solution offerings a combination of dyed molecules 4.
Determination of phenolic compounds
Figure 1 demonstrations the phenolic acid compounds of pits date extract. Shahidi and Naczk demonstrated that the phenolic acid molecules checked in fruits are frequently current in certain formulae, and they projected a action of hydrolysis with strong acid to liber them in order to agree their quantification. The retention times as well as the documentation of the phenolic molecules are showed in Table 1. A nine phenolic acids compounds were identified. Caffeic and protocatechuic were the focal phenolic molecules noticed. The overhead consequences were in agreement with the results described in the works. The total phenol contents and the total flavonoid compounds were calculated for the conventional technique. The phenol compounds were 77 (mg GA / g date stone powder) at 50 ° C. In totaling, the flavonoid compounds was 56 (mg catechin / g date stone powder) at 50 ° C.
 |
Figure 1: HPLC chromatogram of phenolic acids. |
 |
Table 1: HPLC retention time |
Dyeing of cellulose with date pits extract
Cellulose fabric was dyed with date pits extract; consequences are showed in table 2. It is exposed from the taking pictures of the colored textile and from the positive values of a* and b* that the type of all colored examples lies in the red-yellow zone 11,12.
Table 2: CIE L*a*b*, K/S values of cellulose textiles colored with natural dye.
|
CIE a*,b*,L* and K/S values |
|||
|
a* |
b* |
L* |
K/S |
|
8.57 |
13.81 |
55.81 |
14.82 |
Spectral propriety of dyed cellulose fibers
This part of the work was therefore advanced with a view to verifying whether the extract of date pits communicates its fluorescence property to the textile support on which it is fixed.
Principle and experimental device for measuring optical properties
This optical characterization involves three steps on a very short time scale: the absorption of photons, the thermalization of the carriers created and the return to equilibrium by radiative recombination. The experimental bench used for measuring the fluorescence of cellulose examples dyed with date pits is shown diagrammatically in Figure 2.
 |
Figure 2: Fluorescence test bench for dyed samples |
Study of optical properties
This part study was advanced in directive to examine the chromatic properties owed to the connections of date pits extract with cellulose fiber. The photography of the colored cellulose textile is exposed in figure 3.
 |
Figure 3: Photograph of a cellulose sample dyed |
It would be renowned that the fluorescence characters of the colored cellulose fibers was evidently quantifiable at naked eye. Afterward, fluorescence quantities have been established on models previously and after coloring cellulose models. Samples were animated using 450 nm light, which is together absorbed by the undyed fiber and the dye fiber. Consequences are showing in figure 4[10].
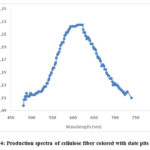 |
Figure 4: Production spectra of cellulose fiber colored with date pits extract. |
The production spectra of the non-dyed cellulose fiber do not demonstrate any band in the visible section. In the opposing, colored cellulose textile existing motivating photoluminescence characters and the maximum value was noted at 672 nm. The divergence may be a importance of connections established between the cellulose and the colorant. Similarly, evaluation of the emission bands from colored and non-colored samples permitted assigning the ipsochromic emission to cellulose textile and the bathochromic one to colorant 10.
Conclusion
The purpose of this work is to estimate the fluorescence characters of cellulose textile colored with date pits extract, natural colorant. It was creating that colored samples existing motivating photoluminescence characters.
Acknowledgement
This research was supported by the Deanship of Scientific Research, Imam Mohammad Ibn Saud Islamic University, Saudi Arabia, Grant No. (19-12-12-005).
Conflict of Interest
There are no conflict of interest
Funding Sources
None
References
- Guesmi A, Ladhari N, Ben hamadi N, Sakli F. Isolation, identification and dyeing studies of betanin on modified acrylic fabrics. Ind Crop Prod. 2012, 37, 342–346.
CrossRef - Guesmi, A., Ben hamadi, N., Ladhari, N., Sakli, F., 2012. Dyeing properties and colour fastness of wool dyed with indicaxanthin natural dye. Ind. Crop. Prod. 37, 493–499.
CrossRef - Guesmi, A., Ben hamadi, N., Ladhari, N., Sakli, F., 2013. Sonicator dyeing of modified acrylic fabrics with indicaxanthin natural dye. Ind. Crop. Prod. 42, 63–69.
CrossRef - Guesmi, A., Ladhari, N., Ben hamadi, N., Msaddek, M., Sakli, F., 2013. First application of chlorophyll-a as biomordant: sonicator dyeing of wool with betanin dye. J. Clean. Prod. 39, 97–104.
CrossRef - Guesmi A, Ladhari N, Sakli F. Ultrasonic preparation of cationic cotton and its application in ultrasonic natural dyeing. Ultrasonics Sonochemistry 2013; 20: 571–579.
CrossRef - Souissi, M. Guesmi, A. Moussa, A. Valorization of natural dye extracted from date palm pits (Phoenix dactylifera) for dyeing of cotton fabric. Part 2: Optimization of dyeing process and improvement of colorfastness with biological mordants. Journal of Cleaner Production 2018, 204, 1143-1153
CrossRef - Souissi, M. Guesmi, A. Moussa, Valorization of natural dye extracted from date palm pits (Phoenix dactylifera) for dyeing of cotton fabric. Part 1: Optimization of extraction process using Taguchi design. Journal of Cleaner Production 2018, 202, 1045-1055.
CrossRef - Aldhaheri, A., Alhadrami, G., Aboalnaga, N., Wasfi, I., Elridi, M., 2004. Chemical composition of date pits and reproductive hormonal status of rats fed date pits. Food Chemistry 86, 93-97.
CrossRef - Nehdi, I., Omri, S., Khalil, M.I., Al-Resayes, S.I. 2010. Characteristics and chemical composition of date palm (Phoenix canariensis) seeds and seed oil. Industrial Crops and Products 32, 360-365.
CrossRef - Guesmi, A. Ben hamadi, N. Ladhari, N. Saidi, F. Maaref, H. Sakli, F. Spectral characterization of wool fabric dyed with indicaxanthin natural dye: Study of the fluorescence property. Industrial Crops and Products 46, 2013, 264-267.
CrossRef - Guesmi A. ben hamadi N. Study on optimizing dyeing of cotton using date pits extract as a combined source of coloring matter and bio-mordant Nat Prod Res. 2019, 32, 810–814.
CrossRef - Dhahri, H., Guesmi, A., Ben Hamadi, N. Application of phenolic compounds as natural dye extracted from date-pits: dyeing studies of modified acrylic fibres. Nat Prod Res. 2018, 32, 1329–1333.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










