Density and Viscosity of LiCl, LiBr, LiI and Kcl in Aqueous Methanol at 313.15K
V. V. Kadam1* , A. B. Nikumbh2
, A. B. Nikumbh2 , T. B. Pawar3
, T. B. Pawar3 and V. A. Adole4
and V. A. Adole4
1Department of Chemistry, Arts, Science and Commerce College Surgana, Dist. Nashik, Maharashtra, Affiliated to SPPU Pune, India.
2Department of Chemistry, S.S.G.M. College, Kopargaon Dist. Ahemadnagar, Affiliated to SPPU Pune, India.
3Department of Chemistry, L.V.H. Arts, Science and Commerce College Panchavati, Dist. Nashik, Maharashtra, Affiliated to SPPU Pune, India.
4Department of Chemistry, Arts, Science and Commerce college, Manmad , Dist. Nashik , Maharashtra , Affiliated to SPPU Pune, India.
Corresponding Author E-mail: kadamvv18@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370510
Article Received on : 02-Apr-2021
Article Accepted on :
Article Published : 05 Oct 2021
Reviewed by: Dr. Mandar Karve

Second Review by: Dr. Soon Min Ho

Final Approval by: Dr. Ayssar Nahle
The densities and viscosities of electrolytes are essential to understand many physicochemical processes that are taking place in the solution. In the present research, the densities and viscosities of lithium halides, LiX (X = Cl, Br, I ) and KCl in (0, 20, 40, 50, 60, 80 and 100) mass % of methanol + water at 313.15K were calculated employing experimental densities (ρ), the apparent molar volumes( ϕv) and limiting apparent molar volumes (0v) of the electrolytes. The (0v) of electrolyte offer insights into solute-solution interactions. In terms of the Jones-Dole equation for strong electrolyte solution, the experimental data of viscosity were explored. Viscosity coefficients A and B have been interpreted and discussed. The B-coefficient values in these systems increase with increase of methanol in the solvents mixtures. This implied that when the dielectric constant of the solvent decreases, so do the solvent-solvent interactions in these systems.
KEYWORDS:Apparent Molar Volume; Density; Viscosity
Download this article as:| Copy the following to cite this article: Kadam V. V, Nikumbh A. B, Pawar T. B, Adole V. A. Density and Viscosity of LiCl, LiBr, LiI and Kcl in Aqueous Methanol at 313.15K. Orient J Chem 2021;37(5). |
| Copy the following to cite this URL: Kadam V. V, Nikumbh A. B, Pawar T. B, Adole V. A. Density and Viscosity of LiCl, LiBr, LiI and Kcl in Aqueous Methanol at 313.15K. Orient J Chem 2021;37(5). Available from: https://bit.ly/2YwT3Pb |
Introduction
The density and viscosity data of electrolyte solutions have been extremely valuable in determining whether or not ion-solvent interactions exist in aqueous and non-aqueous solutions.1 For a better knowledge of many physicochemical processes that occur in the chemical industry and in nature, Crystallization, desalination, waste water treatment, pollution control, oil recovery, heat and mass transfer, fluid flow, mineral transport and deposition, corrosion, and other processes all rely on the transport properties (viscosity and thermal conductivity) of aqueous electrolyte solutions in a wide range of solvent concentrations, solution temperatures, and pressures. In many applications, these processes take place at high temperatures and pressures. For understanding ion-solvent interactions, temperatures and concentration dependencies of the viscosity of aqueous electrolyte solutions are indeed important.2-9
Viscosity is one of the important transport properties of electrolyte solutions and belongs to a dynamic state property, while density is one of the key thermodynamic features of electrolyte solutions and contributes to an equilibrium property.10-12 Researchers have explored the use of density and viscosity of mixtures to derive thermodynamic properties like dynamic viscosity, kinematic viscosity, deviations in dynamic viscosity, excess molar volume, surface tension deviation, apparent molar volumes, etc.13-16. In this paper we report, limiting apparent molar volume (f0v) of some lithium halides LiX (X= Cl Br I) in water + methanol at different temperature using density property. An attempt is to make determine the effect of variation of (f0v) with methanol content for a given electrolyte. The viscosities of Lithium halides solution in (0, 20, 40, 50, 60, 80 and 100) mass % of methanol + water at different temperatures to see how changing the solvent content affects the viscosity B-coefficient.
Experimental
Water was distilled over alkaline KMnO4 in a rapid fit system, and then distilled again over H2SO4. The electric conductance of distilled water varied between 1×10-6 and 9×10-77 Ω-1 cm-1. Methanol with A.R. grade from SD Fine Chemical limited was directly used without further purification. LiCl anhydrous was from S D fine chemicals limited Mumbai product no. 230374, batch no. KO3Y/0703/1910/31 with purity 99%. LiBr anhydrous was from Sigma-Aldrich product no. 44987-3 batch no. MKBF4487V with purity 99.9%. LiI anhydrous was from Sigma-Aldrich product no. 43974-6 batch no. MKBD 2730 with purity 99.9%.KCl was from SD Fine Chemical limited Mumbai product no. 20198, batch no.KO5,H10Y/0710/1408/31 with purity 99.5%.
By mixing known quantities of water and methanol in glass-stoppered flasks, methanol + water mixes of compositions (0, 20, 40, 50, 60, 80, and 100) mass percent methanol were prepared. A bicapillary pycnometer was used to measure the density of the solution, with an accuracy of ± 1×10-4 g/cm3 as described earlier17-19. The pycnometer was placed in a temperature-controlled water bath with thermal stability of ± 0.01K for 15 min to attain thermal equilibrium. An Ubbelohde suspended level viscometer was used to measure viscosities20-22. Water conductivity was used to calibrate the device. For the flow time measurements, an electronic digital stop watch with a readability of ±0.01s was employed.
Results and Discussion
The values of experimentally determined density for the pure liquids at are given in Table-1. Experimental densities (ρ) and viscosities (η) of pure liquids were in good agreement with literature values at different temperatures. The densities of LiCl, LiBr, LiI and KCl solutions having concentrations ranging between 0.005 to 0.05 M in 0, 20, 40, 50, 60, 80 and 100 wt % of water + methanol binary mixtures at different temperatures are measured. The observed densities ρ, of the solutions Lithium halides in water, methanol and methanol + water mixtures are used to calculate the apparent malar volumes fv using the equation.
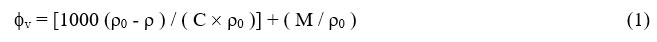
Table 1: Comparison of Experimental Densities (ρ) and Viscosities (η) of Pure Liquids with Literature Values at different temperatures.
|
Temp. K) |
ro (g/ cm3) |
h0 (m Pa s ) |
||
|
Expl. |
Lit. |
Expl. |
Lit. |
|
|
Water |
||||
|
298.15 |
0.99706 |
0.997127 |
0.8944 |
0.894928 |
|
0.9970528 |
0.890329 |
|||
|
303.15 |
0.99570 |
0.995727 |
0.7987 |
0.80030 |
|
308.15 |
0.99405 |
0.99440630 |
0.7195 |
0.72127 |
|
313.15 |
0.99208 |
0.992327 |
0.6538 |
0.65427 |
|
0.992231 |
0.6526332 |
|||
|
Methanol |
||||
|
298.15 |
0.78662 |
0.7866228 |
0.5490 |
0.54433 |
|
303.15 |
0.78139 |
(0.7825- |
0.5126 |
0.50727 |
|
|
0.78181)33 |
|
||
|
308.15 |
0.77640 |
0.77699034 |
0.4787 |
0.47427 |
|
313.15 |
0.77130 |
0.772327 |
0.4477 |
0.45027 |
Where ρ and ρ0 are the densities of solution and solvents respectively, C is the concentration in mol liter-1. The densities ρ and apparent molar volumes fv, for LiCl, LiBr, LiI and KCl in different methanol + water mixtures at 313.15 K are given in the Table-2 and Table-3. The densities and viscosities of LiCl, LiBr, LiI and KCl were found to increase with the increase in concentration of electrolytes. fv varied linearly with C1/2 over the concentration range. The electrolytes’ limiting partial molar volume (f0v) was calculated using computerised least square fitting of the Masson equation-2.

Table 2: Densities ρ, apparent molar volumes fv, and viscosities ɳ, for LiCl and LiBr in different methanol + water mixtures at 313.15K
|
LiCl |
LiBr |
||||||||||
|
C/ mol.dm-3 |
ρ /g.cm-3 |
fv / cm3. mol -1 |
ɳ / mPa s |
C/ mol.dm-3 |
ρ /g.cm-3 |
fv /cm3. mol-1 |
ɳ / mPa s |
||||
|
0 % Methanol |
|||||||||||
|
0.0050 |
0.99228 |
18.99 |
0.6603 |
0.0059 |
0.99252 |
25.39 |
0.6603 |
||||
|
0.0120 |
0.99244 |
19.09 |
0.6612 |
0.0103 |
0.99279 |
25.45 |
0.6608 |
||||
|
0.0160 |
0.99253 |
19.14 |
0.6617 |
0.0160 |
0.99314 |
25.55 |
0.6614 |
||||
|
0.0207 |
0.99264 |
19.19 |
0.6622 |
0.0206 |
0.99343 |
25.59 |
0.6619 |
||||
|
0.0257 |
0.99276 |
19.24 |
0.6628 |
0.0270 |
0.99382 |
25.68 |
0.6625 |
||||
|
0.0310 |
0.99288 |
19.28 |
0.6634 |
0.0303 |
0.99402 |
25.70 |
0.6628 |
||||
|
0.0351 |
0.99298 |
19.31 |
0.6639 |
0.0360 |
0.99437 |
25.75 |
0.6633 |
||||
|
0.0420 |
0.99313 |
19.36 |
0.6646 |
0.0400 |
0.99461 |
25.80 |
0.6637 |
||||
|
0.0454 |
0.99321 |
19.38 |
0.6650 |
0.0462 |
0.99499 |
25.88 |
0.6643 |
||||
|
0.0508 |
0.99334 |
19.42 |
0.6656 |
0.0515 |
0.99531 |
25.93 |
0.6647 |
||||
|
20 % Methanol |
|||||||||||
|
0.0051 |
0.95795 |
13.05 |
0.9663 |
0.0053 |
0.95814 |
24.68 |
0.9680 |
||||
|
0.0105 |
0.95811 |
13.10 |
0.9677 |
0.0123 |
0.95858 |
24.81 |
0.9705 |
||||
|
0.0168 |
0.95830 |
13.15 |
0.9691 |
0.0162 |
0.95882 |
24.88 |
0.9717 |
||||
|
0.0201 |
0.95840 |
13.17 |
0.9698 |
0.0203 |
0.95908 |
24.95 |
0.9728 |
||||
|
0.0256 |
0.95856 |
13.21 |
0.9710 |
0.0267 |
0.95948 |
25.02 |
0.9745 |
||||
|
0.0305 |
0.95871 |
13.24 |
0.9720 |
0.0294 |
0.95965 |
25.05 |
0.9752 |
||||
|
0.0365 |
0.95888 |
13.27 |
0.9732 |
0.0347 |
0.95998 |
25.11 |
0.9765 |
||||
|
0.0413 |
0.95903 |
13.29 |
0.9741 |
0.0408 |
0.96036 |
25.17 |
0.9779 |
||||
|
0.0474 |
0.95921 |
13.32 |
0.9753 |
0.0457 |
0.96067 |
25.20 |
0.9791 |
||||
|
0.0510 |
0.95931 |
13.33 |
0.9760 |
0.0528 |
0.96111 |
25.26 |
|
||||
|
40 % Methanol |
|||||||||||
|
0.0058 |
0.92147 |
9.74 |
1.4296 |
0.0051 |
0.92162 |
22.35 |
1.1280 |
||||
|
0.0127 |
0.92170 |
9.75 |
1.4368 |
0.0111 |
0.92202 |
22.30 |
1.1318 |
||||
|
0.0155 |
0.92180 |
9.75 |
1.4393 |
0.0158 |
0.92233 |
22.27 |
1.1344 |
||||
|
0.0209 |
0.92198 |
9.76 |
1.4438 |
0.0220 |
0.92274 |
22.24 |
1.1375 |
||||
|
0.0268 |
0.92218 |
9.77 |
1.4483 |
0.0247 |
0.92292 |
22.23 |
1.1388 |
||||
|
0.0336 |
0.92240 |
9.78 |
1.4532 |
0.0314 |
0.92337 |
22.20 |
1.1419 |
||||
|
0.0355 |
0.92247 |
9.78 |
1.4545 |
0.0350 |
0.9236 |
22.18 |
1.1435 |
||||
|
0.0400 |
0.92262 |
9.78 |
1.4575 |
0.0428 |
0.92412 |
22.15 |
1.1468 |
||||
|
0.0467 |
0.92284 |
9.79 |
1.4619 |
0.0448 |
0.92426 |
22.14 |
1.1476 |
||||
|
0.0525 |
0.92303 |
9.80 |
1.4656 |
0.0513 |
0.92469 |
22.12 |
|
||||
|
50 % Methanol |
|||||||||||
|
0.0052 |
0.90038 |
7.05 |
1.1174 |
0.0065 |
0.90065 |
17.83 |
1.1086 |
||||
|
0.0103 |
0.90056 |
7.00 |
1.1263 |
0.0104 |
0.90093 |
17.77 |
1.1122 |
||||
|
0.0168 |
0.90080 |
6.96 |
1.1354 |
0.0153 |
0.90128 |
17.70 |
1.1162 |
||||
|
0.0200 |
0.90091 |
6.94 |
1.1394 |
0.0211 |
0.90169 |
17.63 |
1.1205 |
||||
|
0.0260 |
0.90113 |
6.91 |
1.1463 |
0.0263 |
0.90206 |
17.58 |
1.1241 |
||||
|
0.0296 |
0.90126 |
6.89 |
1.1502 |
0.0319 |
0.90246 |
17.53 |
1.1279 |
||||
|
0.0362 |
0.90150 |
6.86 |
1.1570 |
0.0340 |
0.90261 |
17.51 |
1.1292 |
||||
|
0.0418 |
0.90171 |
6.84 |
1.1625 |
0.0416 |
0.90315 |
17.46 |
1.1340 |
||||
|
0.0456 |
0.90184 |
6.83 |
1.1660 |
0.0445 |
0.90336 |
17.44 |
1.1358 |
||||
|
0.0513 |
0.90205 |
6.81 |
1.1712 |
0.0502 |
0.90376 |
17.41 |
|
||||
|
60 % Methanol |
|||||||||||
|
0.00497 |
0.86746 |
2.96 |
1.0232 |
0.0055 |
0.86767 |
14.63 |
1.0103 |
||||
|
0.0111 |
0.86770 |
2.98 |
1.0369 |
0.0105 |
0.86804 |
14.64 |
1.0160 |
||||
|
0.0158 |
0.86789 |
2.99 |
1.0456 |
0.0164 |
0.86848 |
14.65 |
1.0220 |
||||
|
0.0205 |
0.86808 |
3.00 |
1.0534 |
0.0216 |
0.86886 |
14.66 |
1.0268 |
||||
|
0.0251 |
0.86826 |
3.01 |
1.0604 |
0.0263 |
0.86921 |
14.67 |
1.0310 |
||||
|
0.0314 |
0.86851 |
3.02 |
1.0695 |
0.0313 |
0.86958 |
14.68 |
1.0353 |
||||
|
0.0356 |
0.86868 |
3.03 |
1.0752 |
0.0362 |
0.86994 |
14.69 |
1.0395 |
||||
|
0.0409 |
0.86889 |
3.04 |
1.0821 |
0.0392 |
0.87017 |
14.69 |
1.0419 |
||||
|
0.0452 |
0.86906 |
3.05 |
1.0875 |
0.0462 |
0.87068 |
14.70 |
1.0475 |
||||
|
0.0505 |
0.86927 |
3.06 |
|
0.0509 |
0.87103 |
14.71 |
|
||||
|
80 % Methanol |
|||||||||||
|
0.0050 |
0.83033 |
-1.20 |
0.7993 |
0.0056 |
0.83055 |
10.85 |
0.7873 |
||||
|
0.0100 |
0.83054 |
-1.16 |
0.8098 |
0.0102 |
0.8309 |
10.98 |
0.7921 |
||||
|
0.0165 |
0.83083 |
-1.12 |
0.8209 |
0.0153 |
0.8313 |
11.09 |
0.7968 |
||||
|
0.0196 |
0.83096 |
-1.10 |
0.8256 |
0.0214 |
0.83177 |
11.21 |
0.8020 |
||||
|
0.0249 |
0.83119 |
-1.08 |
0.8332 |
0.0253 |
0.83207 |
11.30 |
0.8051 |
||||
|
0.0306 |
0.83143 |
-1.05 |
0.8407 |
0.0327 |
0.83264 |
11.42 |
0.8108 |
||||
|
0.0364 |
0.83168 |
-1.01 |
0.8480 |
0.0345 |
0.83278 |
11.46 |
0.8122 |
||||
|
0.0407 |
0.83187 |
-0.99 |
0.8531 |
0.0388 |
0.83311 |
11.54 |
0.8154 |
||||
|
0.0454 |
0.83207 |
-0.97 |
0.8586 |
0.0464 |
0.83369 |
11.63 |
0.8209 |
||||
|
0.0520 |
0.83236 |
-0.95 |
|
0.0517 |
0.8341 |
11.69 |
|
||||
|
100 % Methanol |
|||||||||||
|
0.0053 |
0.77248 |
-1.75 |
0.0053 |
0.0059 |
0.77273 |
7.55 |
0.4597 |
||||
|
0.0100 |
0.77269 |
-1.68 |
0.0100 |
0.0103 |
0.77308 |
8.12 |
0.4598 |
||||
|
0.0155 |
0.77293 |
-1.65 |
0.0155 |
0.0167 |
0.77359 |
8.56 |
0.4605 |
||||
|
0.0206 |
0.77315 |
-1.61 |
0.0206 |
0.0214 |
0.77397 |
8.69 |
0.4612 |
||||
|
0.0269 |
0.77342 |
-1.56 |
0.0269 |
0.0259 |
0.77432 |
8.97 |
0.4620 |
||||
|
0.0290 |
0.77351 |
-1.54 |
0.0290 |
0.0326 |
0.77485 |
9.19 |
0.4633 |
||||
|
0.0365 |
0.77384 |
-1.50 |
0.0365 |
0.0371 |
0.7752 |
9.50 |
0.4642 |
||||
|
0.0403 |
0.77401 |
-1.49 |
0.0403 |
0.0413 |
0.77553 |
9.62 |
0.4651 |
||||
|
0.0450 |
0.77421 |
-1.47 |
0.0450 |
0.0447 |
0.7758 |
9.62 |
0.4659 |
||||
|
0.0492 |
0.77439 |
-1.45 |
0.0492 |
0.0498 |
0.7762 |
9.88 |
|
||||
Table 3: Densities ρ, apparent molar volumes fv, and viscosities ɳ, for LiI and KCl in different methanol + water mixtures at 313.15K
|
LiI |
KCl |
||||||||||||
|
C/mol. dm-3 |
ρ /g.cm-3 |
fv/cm3 .mol -1 |
ɳ/mPa s |
C/mol. dm-3 |
ρ /g.cm-3 |
fv/cm3 .mol -1 |
ɳ/mPa s |
||||||
|
0 % Methanol |
|||||||||||||
|
0.0051 |
0.99265 |
37.23 |
0.6599 |
0.0054 |
0.99241 |
29.26 |
0.6599 |
||||||
|
0.0101 |
0.99314 |
37.27 |
0.6603 |
0.0099 |
0.99261 |
29.48 |
0.6601 |
||||||
|
0.0156 |
0.99367 |
37.30 |
0.6607 |
0.0158 |
0.99287 |
29.78 |
0.6604 |
||||||
|
0.0201 |
0.99411 |
37.32 |
0.6610 |
0.0203 |
0.99307 |
29.96 |
0.6605 |
||||||
|
0.0251 |
0.99459 |
37.34 |
0.6613 |
0.0253 |
0.99329 |
30.08 |
0.6607 |
||||||
|
0.0299 |
0.99505 |
37.36 |
0.6616 |
0.0296 |
0.99348 |
30.19 |
0.6609 |
||||||
|
0.0344 |
0.99549 |
37.38 |
0.6619 |
0.0352 |
0.99373 |
30.24 |
0.6610 |
||||||
|
0.0400 |
0.99603 |
37.40 |
0.6623 |
0.0402 |
0.99395 |
30.31 |
0.6612 |
||||||
|
0.0500 |
0.99699 |
37.43 |
0.6629 |
0.0450 |
0.99416 |
30.39 |
0.6613 |
||||||
|
|
|
|
|
0.0501 |
0.99438 |
30.48 |
0.6615 |
||||||
|
20 % Methanol |
|||||||||||||
|
0.0053 |
0.95833 |
36.12 |
0.9749 |
0.0152 |
0.95854 |
27.01 |
0.9663 |
||||||
|
0.0109 |
0.95888 |
36.25 |
0.9799 |
0.0208 |
0.95882 |
26.64 |
0.9666 |
||||||
|
0.0201 |
0.95979 |
36.38 |
0.9860 |
0.0254 |
0.95904 |
26.86 |
0.9669 |
||||||
|
0.0251 |
0.96028 |
36.45 |
0.9888 |
0.0304 |
0.95928 |
27.01 |
0.9672 |
||||||
|
0.0313 |
0.96089 |
36.51 |
0.9920 |
0.0352 |
0.95950 |
27.41 |
0.9674 |
||||||
|
0.0349 |
0.96125 |
36.55 |
0.9938 |
0.0401 |
0.95974 |
27.32 |
0.9677 |
||||||
|
0.0398 |
0.96173 |
36.60 |
0.9961 |
0.0463 |
0.96003 |
27.55 |
0.9679 |
||||||
|
0.0523 |
0.96296 |
36.71 |
1.0014 |
0.0504 |
0.96022 |
27.70 |
0.9681 |
||||||
|
0.0554 |
0.96326 |
36.74 |
1.0027 |
0.0554 |
0.96046 |
27.70 |
0.9683 |
||||||
|
40 % Methanol |
|||||||||||||
|
0.0056 |
0.92187 |
30.81 |
1.1244 |
0.0152 |
0.92211 |
22.01 |
1.1223 |
||||||
|
0.0098 |
0.92231 |
30.77 |
1.1258 |
0.0202 |
0.92237 |
22.24 |
1.1223 |
||||||
|
0.0199 |
0.92338 |
30.70 |
1.1287 |
0.0254 |
0.92265 |
22.42 |
1.1223 |
||||||
|
0.0261 |
0.92403 |
30.66 |
1.1305 |
0.0305 |
0.92292 |
22.59 |
1.1223 |
||||||
|
0.0306 |
0.92451 |
30.64 |
1.1317 |
0.0351 |
0.92316 |
22.78 |
1.1223 |
||||||
|
0.0354 |
0.92502 |
30.62 |
1.1330 |
0.0401 |
0.92342 |
22.99 |
1.1223 |
||||||
|
0.0412 |
0.92563 |
30.59 |
1.1346 |
0.0451 |
0.92368 |
23.16 |
1.1223 |
||||||
|
0.0496 |
0.92652 |
30.56 |
1.1368 |
0.0503 |
0.92395 |
23.30 |
1.1223 |
||||||
|
0.0551 |
0.92710 |
30.54 |
1.1382 |
0.0551 |
0.92420 |
23.40 |
1.1223 |
||||||
|
50 % Methanol |
|||||||||||||
|
0.0047 |
0.90071 |
25.78 |
1.1029 |
0.0051 |
0.90048 |
19.65 |
1.0990 |
||||||
|
0.0107 |
0.90137 |
25.65 |
1.1064 |
0.0108 |
0.90080 |
20.17 |
1.1000 |
||||||
|
0.0203 |
0.90244 |
25.50 |
1.1114 |
0.0201 |
0.90131 |
20.92 |
1.1016 |
||||||
|
0.0249 |
0.90295 |
25.44 |
1.1136 |
0.0266 |
0.90167 |
21.22 |
1.1027 |
||||||
|
0.0319 |
0.90373 |
25.36 |
1.1169 |
0.0318 |
0.90195 |
21.51 |
1.1037 |
||||||
|
0.0350 |
0.90408 |
25.33 |
1.1184 |
0.0351 |
0.90212 |
21.73 |
1.1043 |
||||||
|
0.0399 |
0.90462 |
25.27 |
1.1206 |
0.0416 |
0.90247 |
21.93 |
1.1054 |
||||||
|
0.0504 |
0.90579 |
25.18 |
1.1252 |
0.0497 |
0.90291 |
22.13 |
1.1069 |
||||||
|
0.0551 |
0.90632 |
25.14 |
1.1273 |
0.0551 |
0.90319 |
22.27 |
1.1079 |
||||||
|
60 % Methanol |
|||||||||||||
|
0.0049 |
0.86782 |
22.27 |
1.0070 |
0.0054 |
0.86759 |
14.86 |
1.0007 |
||||||
|
0.0109 |
0.86851 |
22.23 |
1.0125 |
0.0094 |
0.86784 |
15.18 |
1.0019 |
||||||
|
0.0225 |
0.86984 |
22.18 |
1.0217 |
0.0230 |
0.86866 |
15.77 |
1.0058 |
||||||
|
0.0254 |
0.87017 |
22.17 |
1.0238 |
0.0267 |
0.86888 |
16.00 |
1.0069 |
||||||
|
0.0301 |
0.87071 |
22.16 |
1.0272 |
0.0305 |
0.86911 |
16.21 |
1.0080 |
||||||
|
0.0352 |
0.87129 |
22.15 |
1.0307 |
0.0362 |
0.86944 |
16.52 |
1.0097 |
||||||
|
0.0410 |
0.87196 |
22.13 |
1.0347 |
0.0430 |
0.86984 |
16.78 |
1.0117 |
||||||
|
0.0498 |
0.87297 |
22.11 |
1.0406 |
0.0528 |
0.87041 |
17.17 |
1.0146 |
||||||
|
0.0551 |
0.87358 |
22.10 |
1.0441 |
0.0551 |
0.87055 |
17.22 |
1.0153 |
||||||
|
80 % Methanol |
|||||||||||||
|
0.0061 |
0.83083 |
19.39 |
0.7858 |
0.0058 |
0.83049 |
11.92 |
0.7788 |
||||||
|
0.0110 |
0.83140 |
19.56 |
0.7900 |
0.0106 |
0.83079 |
12.07 |
0.7804 |
||||||
|
0.0199 |
0.83245 |
19.77 |
0.7967 |
0.0203 |
0.83141 |
12.72 |
0.7837 |
||||||
|
0.0242 |
0.83295 |
19.86 |
0.7998 |
0.0255 |
0.83174 |
12.90 |
0.7856 |
||||||
|
0.0319 |
0.83385 |
19.99 |
0.8049 |
0.0317 |
0.83213 |
13.23 |
0.7877 |
||||||
|
0.0354 |
0.83426 |
20.06 |
0.8072 |
0.0365 |
0.83243 |
13.37 |
0.7894 |
||||||
|
0.0413 |
0.83495 |
20.15 |
0.8110 |
0.0401 |
0.83265 |
13.50 |
0.7907 |
||||||
|
0.0508 |
0.83605 |
20.30 |
0.8169 |
0.0512 |
0.83334 |
13.83 |
0.7946 |
||||||
|
0.0551 |
0.83655 |
20.36 |
0.8195 |
0.0551 |
0.83358 |
13.94 |
0.7960 |
||||||
|
100 % Methanol |
|||||||||||||
|
0.0067 |
0.77307 |
15.13 |
0.4574 |
0.0057 |
0.77266 |
2.60 |
0.4638 |
||||||
|
0.0103 |
0.77351 |
15.46 |
0.4569 |
0.0107 |
0.77302 |
2.99 |
0.4653 |
||||||
|
0.0197 |
0.77464 |
16.10 |
0.4567 |
0.0206 |
0.77373 |
3.50 |
0.4686 |
||||||
|
0.0253 |
0.77531 |
16.44 |
0.4570 |
0.0261 |
0.77412 |
3.76 |
0.4705 |
||||||
|
0.0312 |
0.77602 |
16.71 |
0.4574 |
0.0309 |
0.77446 |
3.92 |
0.4721 |
||||||
|
0.0357 |
0.77656 |
16.93 |
0.4579 |
0.0358 |
0.77481 |
4.08 |
0.4738 |
||||||
|
0.0410 |
0.77719 |
17.18 |
0.4585 |
0.0411 |
0.77518 |
4.29 |
0.4756 |
||||||
|
0.0520 |
0.77850 |
17.60 |
0.4600 |
0.0508 |
0.77586 |
4.62 |
0.4790 |
||||||
|
0.0551 |
0.77887 |
17.71 |
0.4604 |
0.0556 |
0.77619 |
4.77 |
0.4806 |
||||||
Where ‘f0v’ is the limiting partial malar volume at infinite dilution and Sv is the experimental slope. The f0v and Sv values are presented in Table-4. The f0v is regarded as a measure of solute-solvent interactions. The f0v for LiCl, LiBr and LiI is increases regularly as the size of Lithium halide increases. As the solvent composition varies, the values of f0v vary as well. Various researchers have seen a shift in f0v with different concentrations of other solvents in water in the case of electrolytes.23-25 The Sv values of LiCl are negative in water and methanol mixtures at 50 % methanol indicating some ion-ion interactions shown in Table-4. Similarly, the Sv values of LiBr are negative in water and methanol mixtures at 40 % and 50 % methanol and LiI shows it negative at 40, 50, 60 % methanol indicating ion-ion interactions. The negative slope indicating the ionic dissociation of the electrolytes26.
Table 4: Apparent molar volumes, (f0v, cm3 mol-1) and experimental slopes (Sv cm3 L1/2 mol-3/2), along with correlation coefficient, γ, of Lithium halides in different water + methanol at 313.15K.
|
Electrolyte |
Mass % methanol |
f0v, /cm3 mol-1 |
Sv /cm3 L1/2 mol-3/2 |
γ |
|
LiCl |
|
|
|
|
|
|
0 |
18.79 |
2.78 |
0.9998 |
|
|
20 |
12.91 |
1.86 |
0.9993 |
|
|
40 |
9.17 |
0.39 |
0.9868 |
|
|
50 |
7.16 |
-1.56 |
-0.9996 |
|
|
60 |
2.91 |
0.64 |
0.9951 |
|
|
80 |
-1.33 |
1.63 |
0.9960 |
|
|
100 |
-1.89 |
2.01 |
0.9975 |
|
LiBr |
|
|
|
|
|
|
0 |
25.09 |
3.58 |
0.9955 |
|
|
20 |
24.41 |
3.75 |
0.9993 |
|
|
40 |
22.46 |
-1.48 |
-0.9991 |
|
|
50 |
18.07 |
-2.99 |
-0.9992 |
|
|
60 |
14.59 |
0.54 |
0.9933 |
|
|
80 |
10.41 |
5.65 |
0.9988 |
|
|
100 |
6.50 |
15.19 |
0.9951 |
|
LiI |
|
|
|
|
|
|
0 |
37.14 |
1.31 |
0.9995 |
|
|
20 |
35.85 |
3.77 |
0.9998 |
|
|
40 |
30.94 |
-1.70 |
-0.9998 |
|
|
50 |
26.05 |
-3.87 |
-0.9999 |
|
|
60 |
22.34 |
-1.02 |
-0.9983 |
|
|
80 |
18.91 |
6.15 |
0.9997 |
|
|
100 |
13.74 |
16.91 |
0.9999 |
|
KCl |
|
|
|
|
|
|
0 |
28.74 |
8.00 |
0.9891 |
|
|
20 |
25.58 |
9.00 |
0.8923 |
|
|
40 |
20.40 |
12.90 |
0.9981 |
|
|
50 |
18.52 |
16.50 |
0.9962 |
|
|
60 |
13.68 |
14.83 |
0.9943 |
|
|
80 |
10.81 |
13.35 |
0.9968 |
|
|
100 |
1.59 |
13.39 |
0.9996 |
The f0v values for lithium halides in water and water + methanol are plotted against molecular weight of corresponding halide ions using an equation of the form
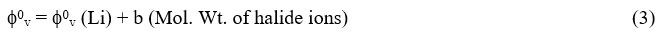
Where ‘b’ is constant and f0v (Li) is the limiting ionic partial molar volume of Li+ ions. The plot of f0v of electrolytes versus molecular weight of corresponding halide ions is given in the Fig.-1. An excellent linear relationship was observed for all lithium halides solutions in all solvents with γ greater than 0.9999. The extrapolation of graphs of f0v versus molecular weight of halide ion to zero ionic formula weight gives the partial molar volume of Li+ ion. The Table-5 represents the value of ionic partial malar volumes of all the ions in all solvents at 313.15K. It demonstrates that the ionic partial molar volumes of halides ions (X-) fluctuate with the solvent composition over time, peaking at 20% methanol. There are no more holes in the binary solvents at 20% methanol, and the dissolving of the third component, the solute, necessitates the solvent’s maximum expansion to accommodate the solute. As a result, there should be a peak in the partial molar volume of the halides ions (X-).
 |
Figure 1: Plots of φ0v versus Molecular weights of Cl–, Br–, I– in different methanol + water mixtures at 313.15 K. |
Table 5: Ionic partial molar volumes, ϕ0v of ions in various methanol + water mixtures at 313.15K
|
%Methanol |
K+ |
Cl– |
Br– |
I– |
Li+ |
|
0 |
20.71 |
8.03 |
14.33 |
26.38 |
10.76 |
|
20 |
16.81 |
8.77 |
20.27 |
31.71 |
4.14 |
|
40 |
13.56 |
6.84 |
20.13 |
28.61 |
2.33 |
|
50 |
11.80 |
6.72 |
17.63 |
25.61 |
0.44 |
|
60 |
6.93 |
6.75 |
18.43 |
26.18 |
-3.84 |
|
80 |
3.63 |
7.18 |
18.92 |
27.42 |
-8.51 |
|
100 |
-4.19 |
5.78 |
14.17 |
24.41 |
-7.67 |
The viscosities of solutions of the LiCl, LiBr, LiI and KCl in water methanol and methanol-water mixtures are measured at 313.15 K . The observed viscosities η, of Lithium halides in water, methanol and methanol + water mixtures at 313.15K are analysed with the help of Jones-Dole equation22.

Where η is the viscosity of the solution and ηo is the viscosity of the solvent, and C is the molar concentration. A is the measure of long range Columbic forces between ions, while B reflects the effect of ion- solvent interactions. Plots of (ηr – 1) /C1/2 versus C1/2 for the electrolytes are straight lines with intercept equal to A, and the slope give the values of the viscosity B-coefficients. The A and B coefficients obtained with a computerised least square method are listed in Table-6. Fig.-2 depicts plots of (ɳr – 1)/C1/2 versus C1/2 of LiCl in different methanol + water mixtures at 313.15 K.
Table 6: Parameters of Jones – Dole Equation A and B along with correlation coefficient, γ, for Lithium halides in different water + methanol at 313.15K
|
Electrolyte |
Mass % methanol |
A / dm3/2.mol-1/2 |
B / dm3.mol-1 |
γ |
|
LiCl |
|
|
|
|
|
|
0 |
0.0085 |
0.1471 |
0.9995 |
|
|
20 |
0.0162 |
0.1645 |
0.9997 |
|
|
40 |
0.0883 |
0.2053 |
0.9998 |
|
|
50 |
0.2171 |
0.3354 |
1.0000 |
|
|
60 |
0.3009 |
0.538 |
1.0000 |
|
|
80 |
0.3584 |
0.6271 |
1.0000 |
|
|
100 |
-0.0276 |
0.8181 |
0.9999 |
|
LiBr |
|
|
|
|
|
|
0 |
0.0090 |
0.1180 |
0.9991 |
|
|
20 |
0.0414 |
0.1392 |
0.9995 |
|
|
40 |
0.0596 |
0.2303 |
0.9999 |
|
|
50 |
0.0866 |
0.3471 |
0.9999 |
|
|
60 |
0.1077 |
0.5428 |
1.0000 |
|
|
80 |
0.1292 |
0.6135 |
1.0000 |
|
|
100 |
-0.1322 |
0.8034 |
1.0000 |
|
LiI |
|
|
|
|
|
|
0 |
0.0045 |
0.0859 |
0.9987 |
|
|
20 |
0.1415 |
0.1159 |
0.9995 |
|
|
40 |
0.0150 |
0.2003 |
0.9999 |
|
|
50 |
0.0395 |
0.3106 |
0.9999 |
|
|
60 |
0.0755 |
0.4915 |
1.0000 |
|
|
80 |
0.0985 |
0.5708 |
1.0000 |
|
|
100 |
-0.1862 |
0.7240 |
1.0000 |
|
KCl |
|
|
|
|
|
|
0 |
0.0089 |
0.0230 |
09724 |
|
|
20 |
0.0143 |
0 .0125 |
0.9417 |
|
|
40 |
0.0045 |
-0.0141 |
-0.9906 |
|
|
50 |
-0.0032 |
0.1721 |
0.9998 |
|
|
60 |
-0.0029 |
0.3026 |
0.9943 |
|
|
80 |
-0.0074 |
0.4728 |
1.0000 |
|
|
100 |
-0.0152 |
0.7809 |
0.9999 |
 |
Figure 2: Plots of (ɳr – 1)/C1/2 versus C1/2 of LiCl in different methanol + water mixtures at 313.15 K. |
There is a slow variety in ionic B esteems as the methanol content in the blended dissolvable increments. Both methanol and water are solvents which have intermolecular hydrogen holding. The expansion of methanol to water, first reinforces the three dimensional construction of the water, then, at that point further expansion of methanol causes depolymerisation of water structure, however intermolecular communication among methanol and water lead to arrangement of methanol-water edifices with a greater hydrodynamic element.
The B values of all lithium halides electrolytes (LiCl, LiBr, and LiI) are positive in all solvent compositions and continuously increases with increase of methanol. These positive B-parameters indicate structure making tendency of lithium halides in these solvent mixture. The B-coefficient of the KCl solutions in 50 to 100 wt% methanol are positive and continuously increases with increases of methanol. These positive B-parameters indicate structure making tendency of KCl in these solvents mixtures. The B- coefficient of KCl falls from solution in water to those in 20 to 40 wt % methanol but then rises again in the subsequent solvent mixtures. A-coefficients either positive or negative are very low in magnitudes indicating weak solute- solute interaction.
In summary, density and viscosity of four alkali chlorides and iodides namely LiCl, LiBr, LiI and KCl in aqueous methanol at 313.15 K were calculated using the electrolytes’ experimental densities, apparent molar volumes, and limiting apparent molar volumes. The experimental data on viscosity of these electrolytes were interpreted using the Jones-Dole equation for strong electrolyte solutions. The densities and viscosities of LiCl, LiBr, LiI and KCl were found to increase with the increase in concentration of electrolytes. The apparent molar volumes varied linearly with C1/2 over the concentration range. Our investigation on density and viscosity of the electrolytic solution studied in the revealed that when the dielectric constant of the solvent decreases, so also the solvent-solvent interactions in these systems. Furthermore, the experimental densities and viscosities of pure liquids were found to be in acceptable concurrence with reported values at various temperatures. In all solvent compositions, the B values of all lithium halide electrolytes are positive and increase with increasing methanol concentration. These positive B-parameters imply that lithium halides in these solvent mixtures have a tendency to form structures.
Acknowledgement
Authors would like to thank principals of SSGM College, Kopargaon and Arts, Science and Commerce College, Surgana for permission and providing necessary research facilities. Dr. Aapoorva Hiray, Coordinator, MG Vidyamandir institute, is gratefully acknowledged for continuous support. NCL, Pune is acknowledged for literature survey.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest
Funding Source
There are no funding Source
References
- Millero, F., Water and Aqueous Solution, 1972. 519-564.
- Anderko, A.; Wang, P.; Rafal, M., Fluid Ph. Equilibria, 2002, 194, 123-142.
CrossRef - Chandra, A.; Bagchi, B., J. Phys. Chem. B, 2000, 104, 9067–9080
CrossRef - Chandra, A.; Bagchi, B., J. Phys. Chem. B, 2000, 113, 3226-3232.
CrossRef - Ling, G.N.; Horne, R.A., New York: Wiley-Interscience,1972, 663-699.
- Horvath, A.L., Halsted Press, 1985.
- Jiang, J.; Sandler, S.I., Ind. Eng. Chem. Res., 2003, 42(25), 6267-6272.
CrossRef - Stokes, R.H.; Mills, R.; Armstrong, H.L., Am. J. Phys., 1966, 34(3), 280-281.
CrossRef - Esteves, M.J.C.; JE de, M.; Cardoso, OE Barcia., Ind. Eng. Chem. Res, 2001, 40, 5021-5028.
CrossRef - Li, X.X.; Liu, Y.X.; Wei, X.H., J. Chem. Eng. Data., 49(4), 2004, 1043-1045.
CrossRef - Wang, L.C.; Xu, H.S.; Zhao, J.H.; Song, C.Y.; Wang, F.A., J. Chem. Eng. Data., 2005, 50(1), 254-257.
CrossRef - Zhang, P.; Wang, F.A.; Wang, J.Y.; Li, C.W.; Ren, B.Z., J. Mol. Liq., 142(1-3), 22-28.
CrossRef - Gahlyan, S.; Bhagat, P.; Maken, S.; Park, S.J., J. Mol. Liq., 2020, 306, 112859.
CrossRef - Alam, M.S.; Siddiq, A.M., J. Mol. Liq., 2017, 242, 1075-1084.
CrossRef - Zhang, S.; Zhao, L.; Yue, X.; Li, B.; Zhang, J., J. Mol. Liq., 2018, 264, 451-457.
CrossRef - Srinivasa Murthy, T., Int. J. Chem. Technol., 2020, 4(2), 109-120
- Nikam, P.S.; Pawar, T.B.; Sawant, A.B.; Hasan M., J. Mol. Liq., 2006, 126, 19-22.
CrossRef - Nikam, P.S.; Hasan, M.; Shewale, R.P.; Sawant, A.B., J. Solution Chem., 2003, 32(11), 987-995.
CrossRef - Bhalodia, J.; Sharma, S., J. Solution Chem., 2013, 42(9), 1794-1815.
CrossRef - Shekaari, H.; Bezaatpour, A.; Elhami, R., J. Solution chem., 2012, 41(3), 516-524.
CrossRef - Ouerfelli, N.; Barhoumi, Z.; Iulian, O., J. Solution chem., 2012, 41(3), pp.458-474.
CrossRef - Li, Y.; Li, Y.H.; Wang, F.A.; Ren, B.Z., J. Chem. Thermodyn., 2013, 66, 14-21.
CrossRef - Nikam, P.S.; Sawant, A.B., J. Chem. Eng. Data, 1997, 42(3), 585-589.
CrossRef - Conway, B.E.; Verrall, R.E. Desnoyers, J.E., J. Chem. Soc. Faraday Trans., 1966, 62, pp.2738-2749.
CrossRef - Macdonald, D.D.; Hyne, J.B., Can. J. Chem. 1970, 48(15), 2416-2422.
CrossRef - Hazra, D.K.; Das, B., J. Chem. Eng. Data, 1991, 36, 403-405.
CrossRef - Mikhail, S.Z.; Kimel, W.R., J. Chem. Eng. Data, 1961, 6(4), pp.533-537.
CrossRef - Zhang, S.; Li, H.; Dai, S.; Wang, T.; Han, S.; J. Chem. Eng. Data, 1997, 42(4), 651-654.
CrossRef - Dean, J.A., McGraw Hill, Bombay, 13th ed., 1987.
- Grant-Taylor, D.F.; Macdonald, D.D., Can. J. Chem., 1976, 54(17), 2813-2819.
CrossRef - Riddick, J.A., Techniques of chemistry, Organic solvents, Wiley, 1970.
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K., Willey Inter Science, 1970.
- Timmermans, J., Physico-Chemical Constants of Pure Organic Compounds, Elsevier, New York, 1965.
- Sakurai, M.; Nakagawa, T., J. Chem. Thermodyn., 1984, 16(2), pp.171-174.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









