Antioxidant and Antidiabetic Activities of Biologically Synthesized Silver Nanoparticles using Linumusitatissimum Extract
Department of Chemistry, Holy Cross College (Autonomous), Affiliated to Bharathidasan University, Tiruchirappalli – 2, Tamilnadu, India..
Corresponding Author E-mail: kanmanivincy@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370531
Article Received on : 17-Aug-2021
Article Accepted on :
Article Published : 15 Sep 2021
Reviewed by: Dr. Tati Suhartati
Second Review by: Dr. Shahla Danesh
Final Approval by: Dr. Ayssar Nahle
This research work was mainly focused to study the anti-oxidant and anti-diabetic activities of biologically synthesis of silver nanoparticles (AgNPs) from the flaxseed extract of Linumusitassimum. Qualitative tests identify the presence of phytochemicals in the flaxseed extract and its results showed the presence of tannins, terpenoids, saponins, flavonoids, steroids, cardiac glycosides, anthraquinones, coumarins, xanthoproteins, alkaloids, emodins, and carbohydrate in it. Preliminarily AgNPs formation is confirmed by the UV spectra and it showed maximum adsorption band at 438nm. FT-IR spectroscopic studies reveal the Phyto-constituents which are involved in the reduction of silver (Ag+1) into silver nanoparticles (Ag0). The spherical shapes of AgNPs are observed with crystalline nature are found in the aid of SEM and XRD analysis. Synthesized AgNPs have the maximum percentage of a silver element which is examined by the EDX analysis. The in-vitro antioxidant and antidiabetic activities of L. usitatissimummediated AgNPs were analyzed by using the DPPH, alpha-amylase, and alpha glycosides assays respectively. The DPPH result shows that the AgNPs possess 59.01% of radical scavenging property and the standard ascorbic acid reveals 48.63% at 100µg/ml concentration. Similarly in anti-diabetic activity, AgNPs shows the maximum inhibition of 79.84% in the alpha-amylase assay, and for alpha-glucosidase, AgNPs showed 58.86% at 100µg/ml concentration.
KEYWORDS:Anti-oxidant activity; Anti-diabetic activity; Linumusitassimum; Silver nanoparticles
Download this article as:| Copy the following to cite this article: Kanmani R, Scleeva P. I. Antioxidant and Antidiabetic Activities of Biologically Synthesized Silver Nanoparticles using LinumUsitatissimum Extract. Orient J Chem 2021;37(5). |
| Copy the following to cite this URL: Kanmani R, Scleeva P. I.. Antioxidant and Antidiabetic Activities of Biologically Synthesized Silver Nanoparticles using LinumUsitatissimum Extract. Orient J Chem 2021;37(5). Available from: https://bit.ly/395vzlY |
Introduction
Nanoscience technology is a multidisciplinary subject and it is widely used in research areas of physics and chemistry. Various physical, chemical, and greener syntheses processes are available in nanoparticle (NPs) production. Chemical and physical methods are more labor-intensive and also possess hazardous than the biological (green method) synthesis. The greener process is non-toxic, reproducible, eco-friendly, one step, easy, and more efficient. In additionally the greener method consumes low energy, it produces harmless and stable products. In the greenway, the plant extract-based nanoparticles synthesize get more attention than the other green methods (fungus, algae, bacteria, etc.). 1-4
The most viable nanomaterial is silver nanoparticles (AgNPs) which are more commercialized nano-materials and nearly 500tons of AgNPs were produced per year. Owing to high sensitivity in the detection of biomolecules, medicine, and catalysis silver nanoparticles were recognized as a strong pharmacophore. A wide range of medical applications are revealed by the silver nanoparticles (AgNPs) and are wound healing, dental material fills, bone repairing, anti-diabetics, vaccine adjuvants, bio-imaging, and biosensors.5,6Flax or Linumusitatissimum is commonly known as the linseed which is the flowering plant, and it belongs to the Linaceae family. Traditionally it is used in the treatment of gastrointestinal infections and diarrhea. Flaxseed has macro and micronutrients along with that it has an excess of omega-3 fatty acids and vitamin B6. It has 54% of omega-3 fatty acids, 18% of oleic acid (omega-9 fatty acids), 6% of linoleic acid (omega-6 fatty acids), 9% of saturated fat, and 5% of palmitic acid. Diabetes mellitus is an endocrine disorder. Due to insulin resistance and deficiency which results from the high level of sugar in the blood for an extended period which is referred to as diabetes mellitus. This research work mainly aimed to study the In-vitro of anti-oxidant and anti-diabetic activities of green synthesized silver nanoparticles mediated from Linumusitatissimum. 7-9
Materials and Methods
Plant collection and Extraction
The fresh flax seeds were collected from the Thiruvarur district, Tamilnadu, India. Disease-free seeds were washed thoroughly with the running water several times. Then again seeds were washed by double distilled water to remove other water-soluble impurities. After that seeds were dried in sunshade then make into a fine powder using the mechanical grinder. 100grams of seed powder was taken in a 500ml dry beaker and to that 300ml of ethanol was added. Then heated on the water bath, the final extract is filtered through the Whatman No.41 filter paper. Filtered extract was stored in the brown bottle which is used for further analysis.
 |
Figure 1: Images of Linumusitatissimum |
Qualitative and Quantitative analysis of Aqueous Extract of LinumUsitatissimum
The various phytochemical tests (qualitative) were performed to establish the profile of the Linumusitatissimum extract. Primary and secondary metabolites presence was tested by the screening tests against the metabolites such as tannins, carbohydrates, terpenoids, phenolics, anthocyanins, and flavonoids, etc., Quantification of these metabolites can be performed by the standard protocols which estimate the total amount of Flavonoids, Tannins, Saponins, Alkaloids, Phenol and Terpenoids in the seed extract.10
Synthesis and Characterisation of AgNPs using Linumusitatissimum Extract
1mM concentration of silver nitrate is prepared in a 50ml standard flask, and the flask was covered with aluminum foil to prevent photochemical reactions with sunlight. 50ml of 1mM silver nitrate solution was taken in a 300ml beaker to this 50ml of seed extract is slowly added then kept on a stirrer for 5hrs. After that, the reaction mixture was kept at room temperature for 24 hours to complete the reaction. Then formed nanoparticles were collected by centrifuging at 6000rpm. Collected AgNPs were washed with double distilled water and followed by ethanol several times to remove impurities from that. Subsequently, AgNPs were dried in a hot air oven for 6 to 12hrs to achieve high purity of obtained AgNPS.
The formation of any nanoparticles is initially confirmed by the UV-Visible spectral studies. The same AgNPs formation is identified by analyzing 2ml of colloidal AgNPs on UV-Visible. A small quantity of dried AgNPs was taken for the FT-IR analysis in the range of 500 cm-1to 4000cm-1 to identify the functional groups present in the extract which involves in the AgNPs synthesis. Similarly, the morphology of synthesized AgNPs was analyzed by SEM (1-10nm), Elemental composition by EDX (1-10KeV), and Crystallinity by XRD (10o<2θ<80o) studies.11,12
In-vitro Antioxidant Activity of AgNPs by DPPH Method
The 20, 40, 60, 80, and 100 µg/ml of AgNPs were taken in the five different test tubes then 1ml of ethanol was added and mixed well. To each test tubes, 3ml of 0.1mM DPPH was added and kept in a dark room for 30mins. After the reaction time interval, the absorbance of each mixture was recorded at 517nm using the spectrophotometer. Blank served as without adding NPs. From the below equation the percentage of inhibition of DPPH scavenged is calculated.

A and B represents the absorbance value for the test samples (NPs added DPPH) and blank sample. The percent inhibition versus concentration curve and 50% inhibition was determined from the concentration verse inhibition percentage graph.13
In-Vitro Antidiabetic activity of AgNPs by Alpha-Amylase Inhibition Assay:
The alpha-amylase inhibitory protocol was given by McCue and Shetty. Five different concentration of AgNPs (20, 40, 60, 80 and 100µg/ml) was taken in a test tube and 1ml of ethanol added to each tube. To this 250μL of sodium phosphate buffer (pH 6.9) containing α-amylase solution (0.5mg/mL) is added and incubated for 10mins at room temperature. Over 250μL of 1% starch in 0.02M sodium phosphate buffer (pH 6.9) was added again incubated for 10mins. To that 500μL of dinitrosalicylic acid (DNS) reagent is added slowly and heated on a water bath for 5mins and their absorbance was measure at 540nm in a spectrophotometer by diluting and cooling the samples. The α-amylase inhibition percentage was calculated by the below equations.
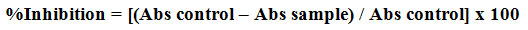
Where Abs control and Abs of the sample represent the absorbance value for the test and blank sample respectively.
In-Vitro Antidiabetic activity of AgNPs by Alpha Glucosidase Inhibition Assay
The α-glucosidase protocol was initiated by Saccharomyces cerevisiae. Five different concentration of AgNPs (20, 40, 60, 80 and 100µg/ml) was taken in a test tube and 1ml of ethanol added to each tube. 100μL of α- glucosidase is added to each test tube and incubated for 10mins. To that 50μL of 3mM P-nitro phenyl glucopyranoside was added followed by incubation for 20mins at 37oC. Two milliliters of 0.1M sodium carbonate were added then the absorbance of each mixture is spectroscopically measured at 405nm by spectrophotometer. Blank was performed similarly except for the addition of samples. The inhibition percentage of α-glucosidase was calculated by the below equation.
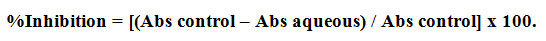
Where A and B represent the absorbance value for the test and blank sample respectively.14,15
Results and Discussion
Qualitative results of Linumusitatissimum seed extract
The results of phytochemical screening of Linumusitatissimumseed extract on different qualitative tests are represented in Table 1 and figure-2. The screening results revealed that the seed extract shows the presence of tannins, saponins, flavonoids, steroids, terpenoids, cardiac glycosides, anthraquinones, coumarins, xanthoproteins, alkaloids, emodin, carbohydrates which are responsible for medicinal activities while leucoanthocyanin, phlobatannin, and phenol were absent.
 |
Figure 2: Images of Phytochemical Screening Results of Linumusitatissimum |
Table 1: Qualitative analysis of seed of Linumusitatissimum
|
S.No |
Phytocompounds |
Observation |
Results |
|
1 |
Terpenoids |
No precipitate |
Absent |
|
2 |
Flavanoid |
Yellow color |
++ |
|
3 |
Saponin |
Forth form |
+++ |
|
4 |
Tannin |
Brownish green |
+++ |
|
5 |
Alkaloid |
Yellow color |
+++ |
|
6 |
Steroids |
No precipitate |
Absent |
|
7 |
Glycosides |
No precipitate |
Absent |
|
8 |
Phlobatannins |
No precipitate |
Absent |
|
9 |
Proteins |
White precipitate |
++ |
|
10 |
Coumarin |
Yellow color |
+++ |
|
11 |
Emodin |
Red color |
+++ |
|
12 |
Anthraquinone |
Red |
+++ |
|
13 |
Carbohydrates |
Reddish violet color |
+++ |
|
14 |
Leucoanthocyanin |
No precipitate |
Absent |
|
15 |
Cardiac glycosides |
Brown ring |
++ |
|
16 |
Anthocyanin |
Bluish violet |
+++ |
|
17 |
Xanthoproteins |
Reddish orange precipitate |
+++ |
|
18 |
Phenol |
Blue-black |
+++ |
Quantitative results of Linumusitatissimumseed extract
From the quantitative analysis results, the phytoconstituents composition in the Linumusitatissimumseed extract has been reported in different amounts. It reveals that alkaloids (0.193mg/g) are present in higher percentage in the seed extract followed by saponins (0.089mg/g), phenols (0.011mg/g), tannin (0.008 mg/g), flavonoids (0.006mg/g), and terpenoids (0.003mg/g) which was given in the table-2 and figure-3.
 |
Figure 3: Quantitative Analysis Results of Linumusitatissimumseed extract; FL- Flavonoid, AL- Alkaloids, TR- Terpenoids, PH- Phenolics, SA- Saponins and TA- Tannins quantification images |
Table 2: Quantitative analysis of Linumusitatissimumseed extract
|
S.No |
Phytochemical Constituents |
Samples(mg/g) |
|
1 |
Flavonoids |
0.006mg/g |
|
2 |
Tannin |
0.008mg/g |
|
3 |
Saponins |
0.089mg/g |
|
4 |
Alkaloids |
0.193mg/g |
|
5 |
Phenol |
0.011mg/g |
|
6 |
Terpenoids |
0.003mg/g |
Spectral Characterization of AgNPs Using Linumusitatissimum seed extract
The color changes are the main identification step in nanoparticles synthesis. For AgNPs formation after adding ethanol extract the colorless solution of silver nitrate turns into brown and it extended to dark brown. Similarly, the UV-Visible results of synthesized AgNPs showed maximum adsorption of 445nm which denotes the formation of silver nanoparticles in the resulted mixture i.e. the plasma resonance was observed in the range of 445 nm which related AgNPs.
 |
Figure 4: UV-Vis spectrum of synthesized silver nanoparticles. |
FT-IR spectrum of ethanolic extract of L. usitatissimum, which gives information about the functional groups of phytochemicals present in the raw extract. Observed peaks were shown in the figure-5 and their data was given in the table 3.
Table 3: FTIR band values of synthesized silver nanoparticles.
|
The observed frequency of the synthesized AgNPs (cm-1) |
Assignment of functional group |
|
Strong band at 3331 |
O-H Stretching and H-bonded |
|
Strong band 2358 |
C-N in aliphatic amine |
|
Strong band at 1639 |
C-O stretching in aliphatic ether |
|
Strong band at 1462 |
C-F stretching in fluoro compound |
|
Broadband at 659 |
-C=C- in alkenes. |
|
A medium band at 599 |
Medium C=C stretching conjugated alkenes |
|
Strong band at 557 |
C-Br stretching in Halo compound |
 |
Figure 5: FT-IR spectrum of synthesized silver nanoparticles |
The SEM images of synthesized AgNPs were given in the figure-6. The average size of obtained particles was found to be 82.34 nm, and it was in needle shapes with well dispersed and almost uniform in nature. Mostly in the greener method, spherical shapes of NPs were observed but for the first time, needle shapes of AgNPs were reported in the green method.
 |
Figure 6: SEM image for synthesized silver nanoparticles |
Through Energy dispersive X-ray (EDX) analysis the elemental composition of AgNPs was studied and their signals (from table-4 and figure-7) confirms the presence of elemental silver in it. In this 59% of silver was present in the formed AgNPS and similarly, it has 25% of oxygen, 12% of copper, and 3% of sulfur.
Table 4: Elemental composition of synthesized AgNPs
|
Element |
Wt% |
At% |
|
O |
24.82 |
25.29 |
|
Cu |
18.14 |
12.25 |
|
S |
11.23 |
3.44 |
|
Ag |
45.81 |
59.02 |
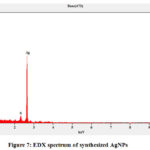 |
Figure 7: EDX spectrum of synthesized AgNPs |
In X-ray crystallography analysis of AgNPs was studied and its resulted spectra are shown in the figure-8. The results exhibit that AgNPs are in crystalline form with the intense diffraction peaks at 2Ɵ values of 38.35, 45.63, 67.14, and 78.28 which are correspond to the plane of (111), (200), (220), and (311) respectively. The observed AgNPs patterns were in face-centered cubic which has a similar pattern of JCPDS, File No. 04-0783. Few unassigned peaks were found in the XRD results which may be from ethanolic extract of L. usitatissimum (because of the presence of phytochemicals).
 |
Figure 8: XRD patterns of silver nanoparticles synthesized using a SAMPLE |
Antioxidant Activity of Synthesized AgNPs by DPPH Assay
The result depicts that the synthesized AgNPs showed maximum potent antioxidant activities at high concentrations as compared with standard ascorbic acid Table 5. The synthesized silver nanoparticles showed 59.01% of inhibition at the concentration of 100µg/ml while ascorbic acid has 48.63% at the same concentration which was shown in figure-9. AgNPs reduces more DPPH than the ascorbic acid which showed higher anti-oxidant activity than the standard.
 |
Figure 9: Antioxidant activity of synthesized silver nanoparticles using Linumusitatissimum seed by DPPH assay |
Table 5: Antioxidant activity of synthesized silver nanoparticles using Linumusitatissimum seed by DPPH assay.
|
S.No |
Concentrations |
Scavenging Effect (%) |
|
|
Silver Nanoparticles |
Ascorbic Acid |
||
|
1 |
20(µg/ml) |
37.81±0.91 |
12.78±0.34 |
|
2 |
40(µg/ml) |
40.57±0.41 |
16.56±0.21 |
|
3 |
60(µg/ml) |
48.13±0.78 |
23.08±0.45 |
|
4 |
80(µg/ml) |
57.23±0.45 |
30.12±0.28 |
|
5 |
100(µg/ml) |
59.01±0.12 |
48.63±0.34 |
In vitro alpha-amylase inhibitory assay
In this study, in vitro alpha-amylase, inhibitory activities of L. usitatissimumseed extract were investigated and their results are given in figure-10. The results showed that a dose-dependent activity occurs which increases the percentage of inhibitory activity against the alpha-amylase enzyme.
The synthesized AgNPs showed the inhibitory activity from 61.03% to 79.84% at concentrations of 20-100µg/ml (Table 6 and figure-10). Acarbose is a standard drug for α-amylase inhibitor which showed α-amylase inhibitory activity from 40.12 to 57.01% at the same concentrations. While comparing the results synthesized AgNPs reveal a greater inhibitory effect than the standard.
Table 6: In vitro alpha-amylase activity of synthesized silver nanoparticles vs standard acarbose.
|
S.No |
Concentrations |
Alpha Amalyse |
|
|
Synthesized Silver Nanoparticles |
Acarbose |
||
|
1 |
20(μg/ml) |
61.03±0.03 |
40.12±0.98 |
|
2 |
40(μg/ml) |
66.08±0.17 |
43.56±0.95 |
|
3 |
60(μg/ml) |
68.01±0.23 |
48.21±0.55 |
|
4 |
80(μg/ml) |
74.09±0.11 |
52.32±0.67 |
|
5 |
100(μg/ml) |
79.84±0.14 |
57.01±0.33 |
 |
Figure 10: Antidiabetic activity of synthesized silver nanoparticles using Linumusitalissium plant extracts by an alpha-amylase method. |
In vitro α-glucosidase inhibitory assay
The results of antidiabetic activity using α- glucosidase inhibitory assay of synthesized AgNPs using a Linumusitatissimumseeds extract are shown in Table 7 and figure-11. The percentage inhibition at 20-100µg/ml concentration of AgNPs showed a dose-dependent activity with the inhibition varied from 43.34% to 58.86% from highest concentration to the lowest concentration. While comparing the activity of AgNPs with the acarbose at higher concentration (100μg/ml) AgNPS showed 58.86% of inhibition but the standard reveals 44.06% of inhibition against the α- glucosidase inhibitory assay. Thus, the inhibitory of α-glucosidase by AgNPs would delay the degradation of carbohydrates, which causes a decrease in the absorption of glucose, as a result, the reduction of postprandial blood glucose level elevation.
Table 7: In vitro alpha-glucosidase activity of synthesized silver nanoparticles vs standard acarbose.
|
S.No |
Concentrations |
Alpha Glucosidase (%) |
|
|
|
|
Synthesized Silver Nanoparticles |
Acarbose |
|
1 |
20 (μg/ml) |
43.34±0.45 |
31.12±0.44 |
|
2 |
40 (μg/ml) |
47.78±0.18 |
36.34±0.56 |
|
3 |
60 (μg/ml) |
49.90±0.11 |
38.67±0.74 |
|
4 |
80 (μg/ml) |
54.01±0.45 |
41.02±0.09 |
|
5 |
100 (μg/ml) |
58.86±0.90 |
44.06±0.05 |
 |
Figure 11: Antidiabetic activity of synthesized silver nanoparticles using Linumusitalissimium plant extracts by an alpha-glucosidase method. |
Conclusion
In this study, silver nanoparticles were successfully performed by the green method using silver nitrate and L. usitatissimumextract used as the reducing agent. The aqueous extract of L. usitatissimumshowed the great capability to synthesize the silver nanoparticles. The present study for the first time reports the needle shapes of silver nanoparticles in a greener way. The synthesized nanoparticle has 59% of elemental silver with noticeable activities of anti-oxidant and anti-diabetic. This study suggests that L. usitatissimum mediated silver nanoparticles can be alternate antioxidants and antidiabetic agents for the treatments which is less toxic and more effective than the existing one.
Acknowledgement
The authors thank the Holy Cross College (Autonomous), Trichy for permitting us to do this research work in their laboratory.
Conflicts of Interest
The authors have declared no conflicts of interest.
Reference
- Ju-Nam, Y.; Lead, J. R. Manufactured nanoparticles: an overview of their chemistry, interactions, and potential environmental implications. Sci. Total Environ.,2008, 400 (1-3), 396–414.
CrossRef - Sharma, V. K.; Yngard, R. A.; Lin, Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci., 2009, 145 (1-2), 83–96.
CrossRef - Fabrega, J.; Luoma, S. N.; Tyler, C. R.; Galloway, T. S.; Lead, J. R. Silver nanoparticles: behavior and effects in the aquatic environment. Environ. Int., 2011, 37 (2), 517–531.
CrosssRef - Jagpreet Singh.; TanushreeDutta.; Ki-Hyun Kim.; MohitRawat.; PallabiSamddar.; Pawan Kumar. Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J. Nanobiotechnology., 2018, 16 (84).
CrossRef - Perk, A. A.; Shatynska-Mytsyk, I.; Gerçek, IrynaShatynska-Mytsyk, Yusuf Can Gerçek.;KadirBoztaş.; MevzuleYazgan.; SundasFayyaz.; Ammad Ahmad Farooqi. Rutin mediated targeting of signaling machinery in cancer cells. Cancer Cell. International.,2014, 14 (124).
CrossRef - Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids,Free Radic. Biol. Med., 1994, 16 (6), 845–850.
CrossRef - Badole, S.L.; Zanwar, A.A.; Bodhankar, S.L. Antihyperglycemic Potential of SecoisolaricinolDiglucoside. Bioactive Food as Dietary Interventions for Diabetes. 2013, 5, 53-57. https://doi.org/10.1016/B978-0-12-397153-1.00005-6.
CrossRef - Camille Goudenhooft.; Alain Bourmaud.; Christophe Baley. Flax (Linumusitatissimum L.) Fibers for Composite Reinforcement: Exploring the Link Between Plant Growth, Cell Walls Development, and Fiber Properties. Frontiers Plant Sci., 2019, 10 (411).
CrossRef - Ricky W. Fedeniuk.; Costas G. Biliaderis. Composition and Physicochemical Properties of Linseed (Linumusitatissimum L.) Mucilage. J. Agric. Food Chem., 1994, 42(2), 240-247.
CrossRef - Rosaline Vimala, J.; Margret Sheela, S.; DayanaJeyaleela, G.; IrudayaMonisha, S. Biosynthesis of silver nanoparticles by Meliadubia leaf aqueous extract and its antimicrobial activity, World J. Pharm. Res., 2017, 5(3), 973-981.
- Krishnapriya Madhu Varier.; Mounika Gudeppu.; Arulvasu Chinnasamy.; Sumathi Thangarajan.; Jesudas Balasubramanian.; Yanmei Li.; Babu Gajendran. Nanoparticles: Antimicrobial Applications and Its Prospects, Adv. Nanostructured Mat. Environ. Remedi.,2019,12, 321-355.
CrossRef - Ali, M.S.; Amin, M.R.; Kamal, C.M.; Hossain, M.A. In-vitro antioxidant, cytotoxic, thrombolytic activities and phytochemical evaluation of methanol extract of the A. philippense L. leaves. Asian Pac. J. Trop Biomed., 2013, 3(6), 464–469.
CrossRef - DayanaJeyaleela, G.; Rosaline Vimala, J.; Anthony Diwakarchandran, T.; BennoSusaiVijayakumar, A.Preliminary Phytochemical Analysis, Antioxidant activity and Identification of Bioactive Compound in Stem and Leaf of CombretumOvalifolium, Int. J. Sci. Techno. Res., 2020, 9(3), 3125-3132.
- DilaveezRehana.; Mahendiran, D.; Senthilkumar,R.; Kalilurrahiman, A.In vitro antioxidant and antidiabetic activities of zinc oxide nanoparticles synthesized using different plant extracts.BioprocessBiosyst Eng.2017, 40 (6), 943-957.
CrossRef - ViswanathanVinotha.; BaskaralingamVaseeharan.; ViswanathanVinotha.; ArokiadhasIswarya.; RajagopalanThaya.; MarimuthuGovindarajan.; Naiyf S. Alharbi.; ShineKadaikunnan.; Jamal M. Khaled.; Mohammed N. Al-Anbr.; BaskaralingamVaseeharan. Synthesis of ZnO nanoparticles using insulin-rich leaf extract: Anti-diabetic, antibiofilm, and anti-oxidant properties. J. Photochem. Photobiol. B.2019, 197, 111541.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










