Synthesis, Characterization Study of Schiff Base Complexes Derived from Ampicillin and 4 Hydroxy3-Methoxy Benzaldehyde
S.Subitha1* , V.Gnana Glory Kanmoni1
, V.Gnana Glory Kanmoni1 , C.Isac Sobana Raj2
, C.Isac Sobana Raj2 , J.Jona 3
, J.Jona 3 and V.Vibi4
and V.Vibi4
1Department of Chemistry and Research, Women’s ChristianCollege , Nagercoil,Tamil Nadu-629001, India.
2Department of Chemistry and Research, Nesamony Memorial Christian College, Marthandam,Tamil Nadu- 629165,India.
3Department of Chemistry , Francis Xavier College of Engineering, Vannarpet, Tirunelveli-627003, India .
4Department of Chemistry, White memorial of arts and science for women-695505,India.
Corresponding Author E-mail: buvana_subi@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/370407
Article Received on : 28-Jun-2021
Article Accepted on : 30 Jul 2021
Article Published : 26 Jul 2021
Reviewed by: Dr. Emaime Uwanta
Second Review by: Dr. Archana Saxena
Final Approval by: Dr. Julia Ubaskina
Ampicillin is a derived form of pencillin which is emi synthetic -lactum antibiotic used to treat bladder infections, pneumonia, respiratory infection. schiff’s bases are the most widely studied chelating ligands in coordination chemistry. Recently metal complexes of schiff bases particularly derived from carbonyl compounds base on hetero-cyclic rings have been the centre of attraction in many areas. Present paper brief the synthesis of AHMBL and its transition metal (II) complexes and also the coordination / characterization studies such as magnetic susceptibility, molar conductance, Electronic spectra, Thermal study, FT-IR, H-NMR, XRD, and SEM .
KEYWORDS:Ampicillin; 4-hydroxy; 3-methoxy benzaldehyde; Metal complexes; Schiffbase
Download this article as:| Copy the following to cite this article: Subitha S, Kanmoni V. G. G, Raj C. I. Jona J, Vibi V. Synthesis, Characterization Study of Schiff Base Complexes Derived from Ampicillin and 4 Hydroxy3-Methoxy Benzaldehyde . Orient J Chem 2021;37(4). |
| Copy the following to cite this URL: Subitha S, Kanmoni V. G. G, Raj C. I. Jona J, Vibi V. Synthesis, Characterization Study of Schiff Base Complexes Derived from Ampicillin and 4 Hydroxy3-Methoxy Benzaldehyde . Orient J Chem 2021;37(4). Available from: https://bit.ly/3BRBWqm |
Introduction
Schiff bases are compounds that are derived by condensation reaction of primary amines with carbonyl groups (Abbas H Abdulasada et al., 2018). Schiff base ligand are ‘privileged ligands’ and are able to stabilize different metals in various oxidation states. It are extensively studied due to synthetic flexibility, selectivity and sensitivity towards variety of metal ions. It have application towards, degradation of organic compounds, radiopharmaceuticals and as corrosion inhibitors in especially acidic environments for various alloys and metals like steel, aluminium and copper Complexes of transition and non-transition metals with schiff base ligands are promising materials for opto electronic applications due to their outstanding photo and electroluminescent properties and the case of synthesis that readily allows structural modification for optimization of material properties. Various transition and inner transition metals complexes with bi, tri-and tetradentate schiff bases containing nitrogen and oxygen donor atoms play important role in biological systems and represent interesting models for metalloenzymes, which efficiently catalyze the reduction of dinitrogen and dioxygen (Mohammed Shakir, 2010). The schiff base derivatives are infer in the field of optical chemistry and biochemistry. It have been utilized as synthons in the preparation of a number of industrial and biologically active compounds like formazans, 4-thiazolidinines, benzoxazines and so forth, via ring closure, cycloaddition and replacement reaction (Anu Kajal et al., 2013), (Jarrahpur.A et al., 2007).
Literature Review
The articles related to the present study were reviewed for more clearance of the study
Materials And Methods
Synthesis Of Schiffbase Ligand (Ahmb)
The synthesis of ligand AHMB held as follows. To an ethanolic solution ofAmpicillin (0.05 mol) in alkali medium and 4-hydroxy3-methoxy benzaldehyde (0.05 mol) in 10 ml ethanolic solution was droped with constant mixing and heated under reflux for 2 hours on a heating mentle at 60°C(Isac Sobana Raj, et al., 2015). Then, the reaction mixture was cooled by pouring it into cool water Fine shinning yellow precipitate of (AHMB) formed was filtered off, washed thoroughly with ethanol-water and stored in a vacuum decicator to dry.
Synthesis of [M(AHMB)2 (NO3)2] complexes
The Schiff basetransition metal complexes were synthesized as per the following literature procedure (Issac Sobana Raj et al., 2015). A 0.1 mol of ligand (AHMB) was dissolved in 10 ml of ethanol. To this a solution of 0.05 mol M(NO3)2.XH2O (where M = Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) in 10 ml water, ratio of 2:1 was added drop by drop with constant stirring under relux for 10-12 hours in a heating mantle at 60-70°C. The product was cooled by pouring it into cool water. A fine precipitate complexes formed was filtered off and dried.
Findings and Analysis
Molar conductivity of complexes
Molar conductivity of the transition metal complexes synthesised were measured using a systronic conductivity bridge type 305. Ethanol was the solvent, the observed values in the range 47-63 ohm-1cm2mol-1 are tabulated in the table given below.
Table 1: Molar conductance of AHMBL ligand and their metal complexes
|
S. No. |
Denotion of compounds |
Molar conductance (ohm-1 cm2mol-1) |
|
1 |
AHMBL |
– |
|
2 |
Mn(AHMBL)2(NO3)2 |
52 |
|
3 |
Co(AHMBL)2(NO3)2 |
47 |
|
4 |
Ni(AHMBL)2(NO3)2 |
61 |
|
5 |
Cu(AHMBL)2(NO3)2 |
56 |
|
6 |
Zn(AHMBL)2(NO3)2 |
63 |
Molar conductivity values suggests that those are non-electrolytes.
FT-IR spectra
FT-IR spectroscopy technique was utilized for find the bonding nature of AHMBL and their transition metal complexes. The FT-IR spectrum of (AHMBL) and their metal complexes are shown in figure.1 – 6. The stretching representation was given in the table 2.
The FT-IR range at 1666 cm-1 schiffbase ligand indicates the presence of (C – N) group. In following metal complexes this value varies from 15 to 20 cm-1 indicates a strong double bond character of the imine band and the azomethine nitrogen atom has coordination with transition metal ion. This is also confirmed by the intensity band around 462 cm-1 assigned to (M – N) vibration.
The broad band at 3332 cm-1 of FT-IR shows the stretching frequency of hydroxyl compound. The band ranges from 3340 cm-1 – 3325 cm-1 exhibit the non-coordination of –H2O molecules in metal complexes to the central metal ion. This also proved by the M – O vibrational bands from 598 cm-1 – 586 cm-1.
Table 2 : FT-IR data for ligand (AHMBL) and their transition metal complexes.
|
Ligand / Complexes |
VC–H |
VC=C |
VC=N |
VS=O |
VO–H VH2O |
VN–H |
VNO2 |
Vm–N |
Vm–O |
|
AHMBL |
3062.96 |
1519.91 |
1666.50 |
1381 |
3332.99 |
3471 |
1519 |
|
|
|
Mn(AHMBL)2(NO3)2 |
3093.82 |
1504.48 |
1650.79 |
1357 |
3341.44 |
3595 |
1504 |
462 |
586 |
|
Co(AHMBL 2(NO3)2 |
3032.96 |
1509.81 |
1648.50 |
1364 |
3345.12 |
3524 |
1509 |
455 |
596 |
|
Ni(AHMBL)2(NO3)2 |
3011.42 |
1491.71 |
1652.50 |
1329 |
3330.70 |
3499 |
1521 |
431 |
594 |
|
Cu(AHMBL)2(NO3)2 |
3071.21 |
1456.91 |
1653.50 |
1391 |
3325.24 |
3506 |
1532 |
462 |
598 |
|
Zn(AHMBL)2(NO3)2 |
3044.01 |
1479.12 |
1647.27 |
1346 |
3339.70 |
3571 |
1501 |
432 |
586 |
 |
Figure 1: FT-IR spectrum of the schiff base ligand (AHMBL). |
 |
Figure 2: FT-IR spectrum of the [Mn(AHMBL)2 (NO3)2] complex. |
 |
Figure 3: FT-IR spectrum of the [Co(AHMBL)2 (NO3)2] complex. |
 |
Figure 4: FT-IR spectrum of the [Ni(AHMBL)2 (NO3)2] complex. |
 |
Figure 5: FT-IR spectrum of the [Cu(AHMBL)2 (NO3)2] complex |
 |
Figure 6: FT-IR spectrum of the [Zn(AHMBL)2 (NO3)2]complex |
Electronic spectra
To find information regarding the coordination geometry, electronic spectra of the synthesised complex were determined with DMF at normal temperature notices and compared with magnetic moment values and parameters. The observed data was given in table 3. Low intensity absorption bands and low molar extinction belongs to d-d-electron transition.
The colour of synthesised ligand (AHMBL) is yellow and the metal complexes synthesised from the schiff base ligand (AHMBL) are different and also it differ from the corresponding metal ions, it seems the properties of the metal complexes are different from its corresponding metal ions.
The schiff base ligand (AHMBL) showed two different absorption bands at 251nm and 330 nm assigned (p-p*) electronic transition and (n – p*) electronic transition.
In the observation ligand has two absorption bands assigned to p-p* and n-p* transitions. The spectra of metal complexes also has the same transitions, but they shifted lower and higher frequencies confirming the coordination of ligand with transition metal ions.
Metal complex [Mn(AHMBL)2(NO3)2], showed four absorption bands. Two are of low wavelength shows the presence of (p-p*) and (n-p*) transition. The third and fourth are at low intensity and high wavelength region that is 414 nm and 502 nm are attributed to charge transfer which is (6A1g – 4A1g, 6A1g®4Eg) electronic transitions assumed the complex is high spin octahedral geometry.
The metal complex [Co(AHMBL)2 (NO3)2], showed five absorption bands. Two bands are low wavelength which are in UV-region that is 312 nm, 347 nm shows (p-p*), (n-p*) transitions. The other are in visible region are 417 nm, 556 nm, 639 nm can be assigned the charge transfer of 4T1g®4T2g(F), 4T1g®4A2g(F), 4T1g®4T1g(P). This showed it is octahedral geometry.
The electronic spectrum of [Ni(AHMBL)2(NO3)2] also showed five bands. Two were in UV-region and the other three in visible region appeared at 417 nm, 584 nm, 761 nm) which showed the charge transfer 3A2g(F) ®3T2g(F), 3A2g(F) ®3T1g(F), 3A2g(F) ®3T1g(P) (d-d) electronic transition. These showed Ni(II) complex showed octahedral geometry.
The Cu(II) complex of synthesised ligand has three electronic spectrum, one with low intensity at 771 nm to charge transfer 2Eg®2T2g electronic transition, suggested octahedral geometry.
Electronic spectrum of Zn(II) complex produced only two bands in UV region and there is no absorption in visible region because of completely filled d-orbitals. The geometry of Zn(II) complex is octahedral.
Table 3: Electronic spectral data of ligand (AHMBL) and their complexes.
|
Ligand / complexes |
lmax (nm) |
Electronic transition |
Charge transfer |
Geometry |
|
AHMBL |
251 330 |
p-p* n-p* |
|
|
|
Mn(AHMBL)2(NO3)2 |
272, 314 414. 502 |
p-p* n-p* |
6A1g®4TAg 6A1g ®4Eg |
Octahedral |
|
Co(AHMBL)2(NO3)2 |
312, 347 471, 556, 639 |
p-p* n-p* |
4T1g®4T2g(F) 4T1g®4A2g(F) 4T1g®4T1g(P) |
Octahedral |
|
Ni(AHMBL)2(NO3)2 |
269, 352 471, 584, 761 |
p-p* n-p* |
3A2g(F) ®3T2g(F) 3A2g(F) ®3T1g(F) 3A2g(F) ®3T1g(P) |
Octahedral |
|
Cu(AHMBL)2(NO3)2 |
287, 366 771 |
p-p* n-p* |
2Eg®2T2g |
Octahedral |
|
Zn(AHMBL)2(NO3)2 |
264 363 |
p-p* n-p* |
No d-d transition |
Octahedral |
Magnetic Susceptibility Measurements
The magnetic susceptibility value of metal complexes synthesised from schiff base ligand (AHMBL) are given below.
Table 4: Magnetic susceptibility values of metal complexes.
|
Ligand / complexes |
meff(BM) calculated |
meff(BM) observed |
|
Mn(AHMBL)2(NO3)2 |
5.41 |
5.33 |
|
Co(AHMBL)2(NO3)2 |
4.29 |
4.64 |
|
Ni(AHMBL)2(NO3)2 |
3.06 |
3.73 |
|
Cu(AHMBL)2(NO3)2 |
1.83 |
2.11 |
|
Zn(AHMBL)2(NO3)2 |
Diamagnetic |
Diamagnetic |
The magnetic moment of Mn(II), Co(II) and Ni(II) complexes of ligand shows high spin octahedral geometry. But Cu(II) complex shows low magnetic moment indicates low spin distorted octahedral geometry Zn(II) complex shows zero magnetic moment because of d10 system of electronic configuration.
1H NMR spectra
1H NMR spectrum of synthesised ligand (AHMBL) and its Zn(II) metal complex were recorded in chloroform solution (CDCl3). Tetramethyl silane (TMS) calibrate the chemical shits of each analyte proton which act as internal standard.
The figure 4.8 and 4.9 showed the recorded signals of 1H NMR spectrum of ligand and metal complexes. In ligand a singlet at d = 12.12 ppm was attributed to hydrogen of Ar(OH) group. A signal at d = 8.127 ppm showed azomethine proton (N = CH). A strong signal appeared between d(3-4 ppm, 2H) was due to excess phenyl amine group.
The Zn(II) complex was examined and compared with ligand showed Zn(II) complex was shifted downfield compared ligand.
 |
Figure 7: 1H NMR spectrum of schiff base ligand (AHMBL). |
 |
Figure 8: 1H NMR spectrum of [Zn(AHMBL)2 (NO3)2] complex. |
From the data, the structure of coordinated metal complex compounds prepared from ligand (AHMBL) is shown in figure.
 |
Figure 9: Structure of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) complex of schiff base ligand (AHMBL). |
XRD Analysis
X-ray diffraction gives information about crystallographic structure, chemical composition and physical properties of materials. Examined value of powder XRD for the ligand (AHMBL) and its metal complex [Cu(AHMBL)2(NO3)2] data are listed in table 5 and 6. The graphical representation showed feeble peaks for ligand which explains the microcrystalline nature of the sample and also the strong peak indicates the complex formation. Scherrer’s equation is used to determine the size of crystals in the form of powder.
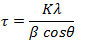
where t – particle size, K = 0.9 (shape factor), l – X-ray wavelength, b – line broadening at half the maximum intensity, q – Bragg angle.
Table 5: XRD data of ligand (AHMBL).
|
Angle |
Net Intensity |
d – value (Å) |
Gross intensity |
Rel. Intensity % |
|
60.353 |
84.319 |
1.5324 |
876.31 |
7.2 |
|
40.952 |
153.554 |
2.202 |
1899.26 |
13.2 |
|
35.838 |
195.479 |
2.5036 |
2112.34 |
16.7 |
|
34.101 |
283.079 |
2.6271 |
2237.80 |
24.2 |
|
32.171 |
387.908 |
2.7801 |
2348.19 |
33.2 |
|
31.535 |
413.233 |
2.8348 |
2366.93 |
35.4 |
|
29.685 |
484.775 |
3.0071 |
2395.60 |
41.5 |
|
28.471 |
443.607 |
3.1325 |
2307.12 |
38 |
|
26.443 |
248.274 |
3.3679 |
1998.81 |
21.3 |
|
25.791 |
143.680 |
3.4516 |
1848.90 |
12.3 |
|
22.611 |
268.710 |
3.9293 |
1732.02 |
23 |
|
21.482 |
423.427 |
4.1332 |
1793.91 |
36.3 |
|
20.413 |
330.275 |
4.3472 |
1600.78 |
28.3 |
|
20.216 |
291.133 |
4.3890 |
1541.95 |
24.9 |
|
19.365 |
249.550 |
4.5799 |
1410.62 |
21.4 |
|
17.122 |
133.259 |
5.1745 |
1021.97 |
11.4 |
|
16.20 |
1167.51 |
5.4656 |
1929.72 |
100 |
|
15.532 |
141.180 |
5.7005 |
805.318 |
12.1 |
|
7.931 |
19.5284 |
11.1387 |
65.027 |
1.7 |
Table 6: XRD data of metal complex
|
Angle |
Net Intensity |
d – value (Å) |
Gross intensity |
Rel. Intensity % |
|
42.410 |
116.485 |
2.1296 |
1545.00 |
23.4 |
|
28.335 |
496.990 |
3.1472 |
2074.92 |
100 |
|
19.010 |
209.704 |
4.6646 |
1093.29 |
42.2 |
 |
Figure 10: Diffractogram of schiff base ligand (AHMBL). |
 |
Figure 11: Diffractogram of [Cu(AHMB)2(NO3)2] complex |
SEM Analysis
SEM (Scanning Electron Microscopy) investigate a wide range of materials for surface fractures, flaws, corrosion or contaminants. It gives magnified images of the sample’s surface topography. The SEM images of ligand (AHMBL) and Ni(II) complex are shown in figure 12 and 13.
The figure showed the ligand look like cheese structure and metal complex looks graval like structure.
 |
Figure 12: SEM pattern of ligand (AHMBL). |
 |
Figure 13: SEM pattern of metal complex |
Recommentations and Conclusions
The schiff base ligand AHMBsynthesised from Ampicillin and 4-hydroxy,3-methoxy benzaldehyde. And Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) metal complexes were derived from AHMBL. They were characterized by following physic-chemical methods. FTIR, UV-Vis, H1NMR spectroscopy, molar conductivity, magnetic moment data. The XRD and SEM analysis support to find the size and morphology of ligand and metal complexes. They were also recommended to study their biological activity for the pharmacological appliances.
Acknowledgement
We are grateful and thankful to the following organizations to carry out the analysis work. STIC Trivandram and SEM Instrumentation Centre, Alagappa University, Karaikudi.
Conflict to interest
The authors declare that they have no conflict of interest.
References
- Abbas, H.A; Noor, H.N; Ahmed, K.H,Acta chemical and pharmaceutica Indica.2018, .8(1),123.
- Ahmed, M.A; Laila, H. A; Nabawia, M.I,synthesis and reactivity in inorganic metal-organic and nano metal chemistry. 2015,47(3),
- Jarrahpur, A; Khalili, D; DeClercq, E; Salmi, C; Brunel, M, Molecules. 2007, 12(8), 1720-1730.
CrossRef - Anu kajal; Suman Bala; Sunil kamboj; Neha sharma; Vipin Saini. 2013, 2013, Article ID:893512.
CrossRef - Isac sobana Raj, C; Allen Ganaraj, G; Antillin Princela, M, Asian Journal of Chemical and PharmaceuticalResearch. 2015,3(1):208-214.
- Shashidhar Reddy, N; Shankara, BS; Murali Krishna, P; Basavaraj, C.B; Mahesh, Int Journal of Inorganic chemistry. 2013, ID 614628.
- Mohammad Shakir; ambreen Abbasi; Asad, U; Shahper, B;Spectro-chimica Acta part A molecular and Biomolecular spectroscopy.2010, 78, no. 1, 29-35.

This work is licensed under a Creative Commons Attribution 4.0 International License.









