Synthesis and Antimicrobial Study of Thiophene Clubbed Thiazolyl Carbohydrazides
Sadhana Dhondibhau Mhaske
Department of Chemistry, Dadapatil Rajale Arts, Science and Commerce College, Adinathnagar, 414505, Affiliated to SPPU, Pune, Maharashtra, India.
Corresponding Author E-mail: mhaskesadhana@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370412
Article Received on : 17-May-2021
Article Accepted on :
Article Published : 05 Aug 2021
Reviewed by: Dr. Fatma Çetin
Second Review by: Dr. Haresh Thakellapalli
Final Approval by: Dr. Bimal K. Banik
Thiophene containing thiazolyl carbohydrazide on reaction with various aryl isothiocynates yields thiosemicarbazides which were transformed into 1,2,4- substituted thiazoles by Hantzsch synthesis and characterized by spectral methods. Most of the synthesized new thiosemicarbazides are found to be promisingly effective against tested bacterial strains and exhibited moderate activitytested fungal strains. Most of the 1, 2,4- substituted thiazoles are weakly active against test organisms.
KEYWORDS:Acid hydrazide; Antimicrobial Activity; Thiosemicarbazides; Thiazole
Download this article as:| Copy the following to cite this article: Mhaske S. D. Synthesis and Antimicrobial Study of Thiophene Clubbed Thiazolyl Carbohydrazides. Orient J Chem 2021;37(4). |
| Copy the following to cite this URL: Mhaske S. D. Synthesis and Antimicrobial Study of Thiophene Clubbed Thiazolyl Carbohydrazides. Orient J Chem 2021;37(4). Available from: https://bit.ly/2VtTKY1 |
Introduction
Heterocyclic compounds containing sulphur1 are important entities present in various bioactive molecules in the field of medicinal and synthetic organic chemistry. Among the five membered ring containing sulphur heterocycles, thiazole2-5 and thiophene6-8 possess promising bioactivity profile. Compounds containing thiophene and thiazole9-14 moieties exhibited promising bioactivities like antimicrobial and antitumor activities.
Thiosemicarbazide and substituted thiosemicarbazides have been proved not only as efficient precursors for different heterocycles15 but also as potentially bioactive scaffolds16,-18. Synthesis of thiosemicarbazides from acid hydrazide derivatives18, 19 is one of the effective synthetic routes. Acid hydrazides can be synthesized from esters20, 21. Although thiazole and substituted thiazoles can be synthesized by various routes22-25, Hantzsch synthesis26-28 is an efficient and widely preferred route of thiazole synthesis. Phenacyl bromides are widely used in thiazole synthesis29. In our previous work we have reported antibacterial activities of bromine containing compounds30.
Activities associated with thiophene, thiazoles, thienyl-thiazoles, bromine containing compounds, synthetic and biological importance of thiosemicarbazides, phenacyl bromides and efficiency of synthetic routes prompted to club thiophene and thiazoles into carbohydrazides and evaluating them for antimicrobial potential.
Results and Discussion
Preparation of 4-Methyl-2-(thiophen-2-yl)-1,3-thiazole-5-carbohydrazide 4was carried outby known method20,21(scheme 1). The reaction of compound 4 and aryl isothiocynates 5a-j in ethanol resulted in substituted thiosemicarbazides 6a-j as shown in scheme 2. The band at 1660 cm-1in the IR spectrum of 6a was due to C=O stretching.In the1H NMR spectrum of 6a a singlet at δ 2.69 supports the presence of methyl group on thiazole ring, a triplet at δ 7.05 with J = 8.68 Hz for two protons is for two protons ortho to fluorine.
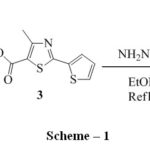 |
Scheme 1 |
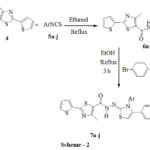 |
Scheme 2 Click here to View figure |
The presence of NH protons was confirmed by singlets at δ 9.71, 9.74 for two protons and at δ 10.18 for one proton. Thiosemicarbazides on condensation with 4-bromo-phenacyl bromide in ethanol were transformed into thiazoles 7a-j (Scheme 2).The bands at 3396 and 1666 cm-1 corresponding to N-H stretching and for highly conjugated C=O group respectively are seen in the IR spectrum of compound 7a. A singlet δ 2.71 was for a deshielded methyl group on thiazole ring, three multiplets at δ 7.14-7.33, 7.43-7.45 and 7.60-7.79 for aromatic protons and a downfield singlet at δ 10.55 for N-H proton are seen in the 1H NMR spectrum of the compound showed. Mass spectrometric analyses were in support of these compounds.
Antimicrobial Activity
In vitro antimicrobial studies of the compounds 6a-j, 7a-j was determined against three bacterial strains Escherichia coli, Salmonella typhii and Bacillus subtilis and two fungal strains Alternaria and Fusarium oxysorum (Table 1). For this agar well diffusion method was used. Ciprofloxacin and ketoconazole were used as reference antibacterial and antifungal agents while DMSO is used as negative control. The results were recorded as an average of three experimental sets and expressed as zone of inhibition in mm.
The results of antimicrobial study depicted that compounds 6a-jare promisingly active against three bacterial strains while 6a-f are active against Alternaria and 6b, 6i have activity against F. oxysporum. Among the remaining compounds 7i, 7h-j are active against Bacillus subtilis. Compounds 7c, 7g showed activity against Salmonella typhi. Compounds 7i, 7c exhibited fairly good activity against F. oxysporum. Rest of the compounds are weakly active or inactive at the 1 mg/mL concentration against experimental microbes. All the thiosemicarbazides showed promising antimicrobial activities while on cyclization activities have been diminished. The observed activities are may be due to presence of free C=S group in compounds 6a-f.
Table 1: Antimicrobial Activity (Zone of Inhibition at 1 mg/mL in mm)
|
Antibacterial activity |
Antifungal activity |
||||
|
Compounds |
Bacillus subtilis |
Escherichia coli |
Salmonella typhii |
Alternaria |
Fusarium oxysporum |
|
6a |
15 |
13 |
14 |
19 |
+ |
|
6b |
13 |
12 |
12 |
21 |
15 |
|
6c |
20 |
16 |
18 |
26 |
+ |
|
6d |
18 |
15 |
17 |
19 |
+ |
|
6e |
20 |
14 |
15 |
20 |
+ |
|
6f |
13 |
15 |
21 |
18 |
+ |
|
6g |
19 |
17 |
17 |
– |
+ |
|
6h |
15 |
14 |
13 |
+ |
+ |
|
6i |
13 |
13 |
15 |
20 |
16 |
|
6j |
11 |
12 |
15 |
+ |
+ |
|
7a |
12 |
+ |
– |
14 |
|
|
7b |
+ |
+ |
– |
– |
– |
|
7c |
– |
– |
11 |
– |
13 |
|
7d |
– |
– |
– |
– |
– |
|
7e |
– |
– |
– |
– |
+ |
|
7f |
– |
– |
– |
– |
+ |
|
7g |
+ |
– |
14 |
+ |
– |
|
7h |
17 |
– |
+ |
+ |
+ |
|
7i |
15 |
– |
– |
– |
|
|
7j |
15 |
– |
+ |
+ |
+ |
|
Ciprofloxacin |
35 |
40 |
39 |
– |
– |
|
Ketoconazole |
– |
– |
– |
34 |
38 |
Experimental Section
Physical constants were recorded using open glass capillary method. The IR and NMR spectra were recorded using IRAffinity-1S spectrophotometer (Shimadzu) and Bruker Avance II 400 MHz NMR spectrometer respectively. DMSO-d6 was used as a solvent and TMS as reference compound in NMR experiment on HP 1100 LC/MSD Mass Spectrometer and Perkin-Elmer analyzer were used for mass spectrometric analyses and elemental analyses respectively.
Table: 2 Physical data of synthesized compounds.
|
Compounds |
Ar |
M. P. (°C) |
Yield (%) |
|
6a |
4-F-C6H4 |
188 |
73 |
|
6b |
4-CH3-C6H4 |
212 |
72 |
|
6c |
2-Cl-C6H4 |
272 |
75 |
|
6d |
3-Cl-C6H4 |
242 |
75 |
|
6e |
4-Cl-C6H4 |
194 |
78 |
|
6f |
2,4-di-Cl-C6H3 |
291 |
74 |
|
6g |
3,4-di-Cl-C6H3 |
280 |
77 |
|
6h |
2-F-C6H4 |
172 |
71 |
|
6i |
3-CH3-C6H4 |
222 |
73 |
|
6j |
2-OCH3-C6H4 |
182 |
72 |
|
7a |
4-F-C6H4 |
180 |
66 |
|
7b |
4-CH3-C6H4 |
174 |
67 |
|
7c |
2-Cl-C6H4 |
198 |
65 |
|
7d |
3-Cl-C6H4 |
204 |
60 |
|
7e |
4-Cl-C6H4 |
220 |
62 |
|
7f |
2,4-di-Cl-C6H3 |
178 |
64 |
|
7g |
3,4-di-Cl-C6H3 |
158 |
67 |
|
7h |
2-F-C6H4 |
162 |
65 |
|
7i |
3-CH3-C6H4 |
168 |
68 |
|
7j |
2-OCH3-C6H4 |
260 |
63 |
N-(Aryl)-2-{[4-methyl-2-(thiophen-2-yl)-1,3-thiazol-5- yl]carbonyl} hydrazinecarbothioamides, 6a-j
Compound 4 and aryl isothiocynates 5ain equimolar quantity were refluxed for 1hin 25 mL ethanol with TLC monitoring. The reaction mixture was left undisturbed for 15 min after completion of reaction. The solid product 6a was filtered and recrystallized from ethanol.Preparation of compounds 6b-j was achieved under same experimental condition. Physical data of compounds 6a-j is mentioned in Table 2 and analytical data isgiven below.
6a.IR: 3307, 3169 (N-H stretching frequency), 1660 (C=O stretching frequency), 1608 (C=N stretching frequency), 1543 (Ar C=C stretching frequency), 1213 (Ar-F stretching frequency), 827, 734 cm-1(=C-H bending);1H NMR: δ 2.69 (3H,s), 7.05 (2H,t,J = 8.68 Hz), 7.13-7.17 (1H,m), 7.36-7.58 (2H,m), 7.60-7.62 (2H,m), 9.71 (1H,s), 9.74 (1H,s), 10.18 (1H,s); MS: (M+1) 393; Analysis: C16H13FN4OS3: Cal.: C, 48.96; H, 3.34; N, 14.27; Observed: C, 48.97; H, 3.33; N, 14.24%.
6b.IR: 3257, 3151 (N-H stretching frequency), 1654 (C=O stretching frequency), 1598 (C=N stretching frequency), 867, 739 cm-1 (=C-H bending); 1H NMR: δ 2.31 (3H,s), 2.68 (3H,s), 7.12-7.18 (3H,m), 7.38-7.48 (4H,m), 7.55 (1H,s), 9.48 (1H,s), 9.59 (1H,s), 10.02 (1H,s); MS: (M+1) 389;Analysis: C17H16N4OS3: Cal.: C, 52.55; H, 4.15; N, 14.42; Observed: C, 52.54; H, 4.12; N, 14.39%.
6c.IR: 3317, 3163 (N-H stretching frequency), 1651 (C=O frequency), 1610 (C=C frequency), 835, 725 cm-1(=C-H bending); 1H NMR: δ 2.69 (3H,s), 2.77 (3H,s), 7.15-7.25 (4H, m), 7.35-7.50 (3H,m), 9.51 (1H,s) 9.62 (1H,s), 10.09 (1H, s); MS: (M+1) 409;Analysis: C16H13ClN4OS3: Cal.: C, 46.99; H, 3.20; N, 13.70; Observed: C, 46.96; H, 3.17; N, 13.68%.
6d.IR: 3319, 3225 (N-H stretching frequency), 1651 (C=O frequency), 1596 (C=C frequency), 834, 743 cm-1(=C-H bending);1H NMR: 2.71 (3H,s), 7.13-7.20 (4H,m), 7.32-7.51 (3H, m), 9.47 (1H,s), 9.58 (1H,s), 10.04 (1H,s); MS: (M+1) 409; Analysis: C16H13ClN4OS3: Cal.: C, 46.99; H, 3.20; N, 13.70; Observed: C, 46.98; H, 3.18; N, 13.69%.
6e.IR: 3301, 3192 (N-H stretching frequency), 1652 (C=O stretching frequency), 1547 (Ar C=C stretching frequency), 837 cm-1; 1H NMR: δ 2.72 (3H,s), 7.02 (2H,d, J = 8.2 Hz), 7.14-7.21 (3H,m), 7.40-7.47 (2H,m), 9.49 (1H,s), 9.54 (2H,bs), 10.12 (1H,s); MS: (M+1) 409; Elemental analysis: C16H13ClN4OS3: Cal.: C, 46.99; H, 3.20; N, 13.70; Observed: C, 46.96; H, 3.18; N, 13.69%.
6f.IR: 3316, 3192 (N-H stretching frequency), 1652 (C=O stretching frequency), 1546 (Ar C=C stretching frequency), 837 cm-1(=C-H bending); 1H NMR: δ 2.68 (3H,s), 7.09-7.20 (4H,m), 7.36-7.47 (2H, m), 9.48 (1H,s), 9.57 (1H,s), 10.02 (1H, s); MS: (M+1) 443.Analysis: C16H12Cl2N4OS3: Cal. C, 43.34; H, 2.73; N, 12.64; Observed: C, 43.32; H, 2.71; N, 12.63.
6g.IR: 3312, 3192 (N-H stretching frequency), 1654 (C=O stretching frequency), 1546 (Ar C=C stretching frequency), 835 cm-1(=C-H bending); 1H NMR: δ 2.71 (3H,s), 6.84-6.9 (2H,m), 7.06-7.21 (4H,m), 7.38-7.51 (2H,m), 9.50 (1H,s), 9.59 (1H,s), 10.11 (1H,s); MS: (M+1) 443;Analysis: C16H12Cl2N4OS3: Cal.: C, 43.34; H, 2.73; N, 12.64; Observed: C, 43.33; H, 2.72; N, 12.63%.
6h. IR: 3304, 3193 (N-H stretching frequency), 1651 (C=O stretching frequency), 1555 (Ar C=C stretching frequency), 831 cm-1;1H NMR: δ 2.76 (3H,s), 7.06-7.20 (4H,m), 7.36-7.45 (3H,m), 9.51 (1H,s), 9.59 (1H,s), 10.15 (1H,s); MS: (M+1) 393;Analysis: C16H13FN4OS3: Cal.: C, 48.96; H, 3.34; N, 14.27. Observed: C, 48.98; H, 3.32; N, 14.25%.
6i.IR: 3307, 3169 (N-H stretching frequency), 1660 (C=O stretching frequency), 1608 (C=N stretching frequency), 1504 (Ar C=C stretching frequency), 1213, 827, 707 cm-1 (=C-H bending); 1H NMR: δ 2.34 (3H,s), 2.72 (3H, s), 6.96-9.98 (1H,m), 7.15-7.17 (1H, m), 7.19- 7.36 (3H,m), 7.39-7.42 (2H,m), 9.65 (1H,s), 9.68 (1H,s), 10.12 (1H,s); MS: (M+1) 389;Analysis: C17H16N4OS3: Cal.: C, 52.55; H, 4.15; N, 14.42; Observed: C, 52.53; H, 4.11; N, 14.38%.
6j. IR: 3321, 3192 (N-H stretching frequency), 1648 (C=O stretching frequency), 1549 (Ar C=C stretching frequency), 827 cm-1; 1H NMR: δ 2.76 (3H,s), 3.8 (3H,s), 6.84-6.9 (2H,m), 7.16-7.21 (2H,m), 7.37 (2H, d, J = 7.8Hz), 7.95-8.05(2H, m), 9.53 (2H, bs), 10.01 (1H,s); MS: (M+1) 404; Analysis: C17H16N4O2S3: Cal.: C, 50.47; H, 3.99; N, 13; Observed: C, 50.45; H, 3.97; N, 13.84%.
N‘-[(2Z)-4-(4-Bromophenyl)-3-phenyl-1,3-thiazol-2(3H)-ylidene]-4-methyl-2- (thiophen-2-yl)-1,3-thiazole-5-carbohydrazides, 7a-j.
In a 50 mL RBF, thiosemicarbazide 6a (0.001 mol) and 4-bromophenacyl bromide (0.001 mol) were dissolved in 25 mL ethanol. A TLC monitored reaction completed in 3 h. The reaction mixture was cooled to room temperature and crude compound 7a was filtered, dried and purified by recrystallization from ethanol. Compounds 7b-j were prepared under similar experimental conditions. Physical data of compounds 7a-j is mentioned in Table 2 and analytical data isgiven below.
(2H,m), 7.60-7.79 (2H,m), 10.55 (1H,s); MS: (M+1) 571;Analysis: C24H16N4S3OBrF: C, 50.44; H, 2.82; N, 9.80; Observed: C, 50.47; H, 2.84; N, 9.83%.
7b.IR: 3361, 2921 (N-H stretching frequency), 1614, 1570 (C=C stretching frequency), 841, 759, 701 cm-1 (=C-H bending); 1H NMR: δ 2.31 (3H,s), 2.75 (3H,s), 6.57 (1H,s), 7.08-7.18 (4H,m), 7.20-7.36 (5H,m), 7.48 (2H,d,J = 8.5 Hz), 10.56 (1H, s); MS: (M+1) 567; Analysis: C25H19N4S3OBr: Cal.: C, 52.91; H, 3.37; N, 9.87; Observed: C, 52.92; H, 3.38; N, 9.86%.
7c.IR: 3351, 2927 (N-H stretching frequency), 1626, 1581 (C=C stretching frequency), 835, 763, 704 cm-1(=C-H bending); 1H NMR: δ 2.70 (3H,s), 6.58 (1H,s), 7.10 (2H,d,J = 8.2 Hz), 7.13-7.35 (7H,m), 7.47 (2H,d,J = 8.2 Hz), 10.70 (1H,s); MS: (M+1) 587;Analysis: C24H16N4S3OBrCl: Cal.: C, 49.03; H, 2.74; N, 9.53; Observed: C, 49.06; H, 2.72; N, 9.51%.
7d.IR: 3361, 2919 (N-H stretching frequency), 1622, 1582 (C=C stretching frequency), 845, 813, 702 cm-1 (=C-H bending); 1H NMR: δ 2.69 (3H,s), 6.57 (1H,s), 7.09-7.20 (6H,m), 7.26-7.38 (3H,m), 7.46 (2H,d, J= 8.1 Hz), 10.61 (1H,s); MS: (M+1) 587; Analysis: C24H16N4S3OBrCl: Cal.: C, 49.03; H, 2.74; N, 9.53; Observed: C, 49.04; H, 2.72; N, 9.55%.
7e. IR: 3361, 2908 (N-H stretching frequency), 1619, 1581 (C=C stretching frequency), 843, 813, 702 cm-1(=C-H bending); 1H NMR (DMSO-d6): δ 2.71 (3H,s), 6.62 (1H,s), 7.06-7.16 (4H, m), 7.19-7.35 (5H,m), 7.42 (2H,d, J = 8.4 Hz), 10.58 (1H,s); MS: (M+1) 587;Analysis: C24H16N4S3OBrCl: Cal.: C, 49.03; H, 2.74; N, 9.53; Observed: C, 49.05; H, 2.76; N, 9.56%.
7f.IR: 3352, 2933 (N-H stretching frequency), 1631, 1583 (C=C stretching frequency), 855, 803, 702 cm-1(=C-H bending); 1H NMR: δ 2.70 (3H,s), 6.59 (1H, s), 7.05-7.20 (5H,m), 7.24-7.36 (3H,m), 7.42 (2H,d, J = 8.2 Hz), 10.52 (1H,s); MS: (M+1) 621;Analysis: C24H15N4S3OBrCl2: Cal.: C, 46.31; H, 2.43; N, 9.00; Observed: C, 46.33; H, 2.45; N, 9.03%.
7g.IR: 3361, 2934 (N-H stretching frequency), 1644, 1576 (C=C stretching frequency), 833, 707 cm-1; 1H NMR: δ 2.72 (3H,s), 6.54 (1H,s), 7.09 (2H,d, J = 8.4 Hz), 7.21-7.37 (6H,m), 7.48 (2H,d, J = 8.4 Hz), 10.58 (1H,s); MS: (M+1) 621;Analysis: C24H15N4S3OBrCl2: Cal.: C, 46.31; H, 2.43; N, 9.00; Observed: C, 46.30; H, 2.46; N, 9.01%.
7h.IR: 3354, 2921 (N-H stretching frequency), 1623, 1573 (C=C stretching frequency), 842, 803, 700 cm-1(=C-H bending); 1H NMR: δ 2.71 (3H,s), 6.56 (1H,s), 7.08-7.21 (5H, m), 7.24-7.38 (4H,m), 7.46 (2H,d, J = 8.4 Hz), 10.57 (1H,s); MS: (M+1) 571;Analysis: C24H16N4S3OBrF: Cal.: C, 50.44; H, 2.82; N, 9.80; Observed: C, 50.46; H, 2.84; N, 9.82%.
7i.IR: 3351, 2919 (N-H stretching frequency), 1603, 1572 (C=C stretching frequency), 845, 713, 702 cm-1 (=C-H bending); 1H NMR: δ 2.32 (3H,s), 2.70 (3H,s), 6.56 (1Hs,), 7.09 (2H,d, J = 8.4 Hz), 7.16-7.35 (7H,m), 7.49 (2H, d,J = 8.4 Hz), 10.54 (1H,s); MS: (M+1) 567;Analysis: C25H19N4S3OBr: Cal.:C, 52.91; H, 3.37; N, 9.87; Observed: C, 52.94; H, 3.39; N, 9.85%.
7j.IR: 3381, 2936 (N-H stretching frequency), 1605, 1575 (C=C stretching frequency), 815, 723, 703 cm-1 (=C-H bending); δ 2.72 (3H,s), 3.81 (3H,s), 6.52 (2H,s), 6.98-6.16 (4H,m), 7.20-7.34 (5H,m), 7.44 (2H,d, J = 8.4 Hz), 10.57 (1H, s); MS: (M+1) 582; Analysis: C25H19N4S3O2Br: Cal.: C, 51.46; H, 3.28; N, 9.60; Observed: C, 51.48; H, 3.29; N, 9.63%.
Conclusions
In present study thiophene and thiazole containing carbohydrazides are synthesized quantitatively and spectroscopicdata well support the proposed compounds. Among the N-(Aryl)-2-{[4-methyl-2-(thiophen-2-yl)-1,3-thiazol-5- yl]carbonyl}hydrazinecarbothioamide compounds, 6a-j showed promising bactericidal activity against B. subtilis, E. coli, S. typhi. Most of the compounds from 6a-j series are effective against fungal species Alternaria except 6g. Most of the compounds from this series are weakly active against Fusarium oxysporum. Compounds from 7a-j series are either inactive or showed less activity against all the test organisms. Overall majority of the compounds reported in the present work can be developed into more active agents by performing structural modifications.
Acknowledgement
The author is thankful to Management and Principal of Dadapatil Rajale Arts, Science and Commerce College, Adinathnagar, and also thankful to Guide Hon. Dr. B. K. Karale for providing research facilities and valuable guidance. The author is grateful to the Director, SAIF, Punjab University, Chandigarh, for their support in the form of spectroscopic investigations.
Conflict of Interest
The author declares no conflict of interest.
References
- Pathania, S.; Narang, R. K.; Rawal, R. K.Eur. J. Med. Chem., 180, 2019, 486-508.
CrossRef - Mishra, I.; Mishra, R.; Mujwar, S.; Chandra, P.; Sachan, N. A. J. Heterocycl. Chem., 2020, DOI: 10.1002/jhet.3970.
CrossRef - Abdul, R.; Cihangir, T. Eur.J. Med. Chem., 97, 2015, 911-927.
CrossRef - Borcea, A-M.; Ionut, I.; Crisan, O.; Oniga, O. Molecules, 26 (624), 2021, http://doi.org/10.3390/molecules/26030624.
CrossRef - Gumus, M.; Yakan, M.; Koca, I. Future Med. Chem., 2019, 11(15), 1979–1998.
CrossRef - Singh, A.; Singh, G.; Bedi, P. M. J. Heterocycl. Chem.,2020, DOI: 10.1002/jhet.3990.
CrossRef - Pramodh, B.; Prathap, K. N. C.; Hema, N. K.; Warad, I.; Loknath, N. K. J. Mol. Struc.,2021, 1229, 129587.
CrossRef - Shah, R.; Verma, P. K. Chem. Cent. J., 2018, 12, 137, https://doi.org/ 10.1186/s13065-018-0511-5.
CrossRef - Mabkhot, Y. N.; Barkat, A..; AI-Majid, A. M.; Alshahrani, S.; Yousuf, S.; Choudhary, M. I. Chem. Cent. J., 2013, 7 (112), https://doi.org/ 10.1186/1752-153X-7-112.
CrossRef - Fadda, A. A.; Tawfik, E. H.; Selim, Y. A. Polycycl. Aromatic Compds., 2018, DOI: 10.1080/10406638.2018.1555174.
CrossRef - Bandock, S.; Fadaly, W.; Metwally, M. A.;Eur. J. Med. Chem., 2010, 45(9), 3692-3701.
CrossRef - Radwan, A. S.; Khalid, M. A. A. J. Heterocycl. Chem., 2019, https://doi.org/10.1002/jhet.3493.
CrossRef - Moharab, R. M.; Khalil, E. M.; Mayhoub, A. E.; Amira, E. M. A. J. Heterocycl. Chem., 2019, https://doi.org/10.1002/jhet.3870.
CrossRef - Rizk, O. H.; Shaaban, O. G.; Wahab, A. E. A. The Open Med. Chem. Journal, 2017, 11, 38-53.
CrossRef - Acharya, P. T.; Bhavsar, Z. A.; Jethava, D. J.; Patel, D. B.; Patel, H. D.;J. Mol. Struc., 2021, 1226, Part A, 129268.
CrossRef - Aly, A. A.; Hassan, A. A.; El-Shaimaa, S. M. J. Heterocycl. Chem., 2018, 55, 2196-2223.
CrossRef - Patel, D. B.; Darji, D. G.; Patel, K. R.; Rajani, D. P.; Rajani, S. D.; Patel, H. D. J.Heterocycl. Chem., 2020, 57(3), 1183-1200.
CrossRef - Karale, B. K.; Takate, S. J.; Salve, S. P.; Zaware, B. H.; Jadhav, S. S. IndianJ. Chem., 2014, 53B, 339-344.
- Majumdar, P.; Pati, A.; Patra, M.; Behera, R. K.; Behera, A. K.;Chem. Rev., 2014, 114, 2942-2977.
CrossRef - El Rayes, S. M. Molecules, 2010, 15, 5, 6759-6772.
CrossRef - Rollas, S.; Gulerman, N.; Erdeniz, H. II Farmaco, 57, 2002, 171-174.
CrossRef - Hussein, W.; Turan-Zitouni, G. MOJ Bioorg. Org. Chem.,2018, 2(2), 52-55.
CrossRef - Pathania, S., Rawal, R. K. Chem. Heterocycl. Compds., 2020, 56, 445-454.
CrossRef - Nayak, S.; Gaonkar, S. L. MiniRev. Med. Chem., 2019, 19 (3), https://doi.org/10.2174/1389557518666180816112151.
CrossRef - Ali, S. H.; Sayed, A. R. Synth. Commun., 2021, 51(5), 670-700.
CrossRef - Yogi, P.; Ashid, M.; Hussain, N.; Khan, S.; Joshi, A. Asian J. Chem., 2016, 28(4), 927-932.
CrossRef - Takate, S. J.; Shinde, A.D.; Karale, B. K.; Akolkar, H.; Nawale, L.; Sarkar, D.; Mhaske, P. C. Bioorg. Med. Chem. Lett., 2019, 29, 1199-1202.
CrossRef - Karale. B. K.; Takate, S. J.; Salve, S. P.; Zaware, B. H.; Jadhav, S. S. Indian J. Chem., 2015, 54B, 798-804.
- Aly, A. A.; EI-Sheref, E. M.;Brown, A. B.; Brase, S.; Nieger, M. J. Sulfur Chem., 2019, 40, 641-647.
CrossRef - Takate S. J.; Salve, S. P.; Dare, S. B.; Karale, B. K.; Akolkar, H. N.; Falke, D. B., Ghungurde R. B.; Mhaske, S. D. Indian J. Heterocycl. Chem., 2020, 30, 525-530.

This work is licensed under a Creative Commons Attribution 4.0 International License.









