Bioactivity Study of Thiophene and Pyrazole Containing Heterocycles
Nitin V. Kale1, Supriya P. Salve1, Bhausaheb K. Karale1, Sadhana D. Mhaske2, Sushama B. Dare 1, Anil E. Athare1 and Sushama J. Takate 1*
1Department of Chemistry, New Arts, Commerce and Science College, Ahmednagar, 414001, affiliated to SPPU, Pune, Maharashatra, India
2Department of Chemistry, Dadapatil Rajale Arts, Science and Commerce College, Adinathnagar, 414505, Affiliated to SPPU, Pune, Maharashtra, India.
Corresponding Author E-mail: sjtakate26@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370417
Article Received on : 17-Jul-2021
Article Accepted on : 19-Aug-2021
Article Published : 31 Aug 2021
Reviewed by: Dr. Mohammed Golam Rasul
Second Review by: Dr. Sutikno . M
Final Approval by: Dr. Nenad Ignjatovic
Chalcones3a-fwere prepared by reacting thiophene containing pyrazolyl aldehyde (2) with different 2-hydroxy acetophenones 1a-f. The compounds3a-f were transformed into different Pyrazolines 4a-f. The formation of chromene derivatives 5a-f occurred from the cyclization of 3a-f, which were then transformed into pyrazole derivatives 6a-f. Newly synthesized compounds have promising antibacterial activity against S.typhii and S.aureus, while weak activity against B.subtilis and E.coli. Compounds 5d and 6d had significant antifungal action towardsA. niger, while most of the compounds were moderately active towards T.viride. Some of the synthesized compounds showed promising α-amylase inhibitory activity at 1 mg/mL concentration.
KEYWORDS:Antimicrobial Activity; Chromone; 2-Hydroxyacetophenone; Pyrazole
Download this article as:| Copy the following to cite this article: Kale N. V, Salve S. P, Karale B. K, Mhaske S. D, Dare S. B, Athare A. E, Takate S. J. Bioactivity Study of Thiophene and Pyrazole Containing Heterocycles. Orient J Chem 2021;37(4). |
| Copy the following to cite this URL: Kale N. V, Salve S. P, Karale B. K, Mhaske S. D, Dare S. B, Athare A. E, Takate S. J. Bioactivity Study of Thiophene and Pyrazole Containing Heterocycles. Orient J Chem 2021;37(4). Available from: https://bit.ly/3zExGc8 |
Introduction
In the field of medicinal chemistry, most drugs have different heterocyclic scaffolds that show potential biological activities. Microbes are responsible for different human diseases. As these microorganisms develop resistance towards many of the present drug molecules, there is a need for continuous research on developing new potential medicinal agents. The presence of oxygen, sulphur and nitrogen containing heterocyclic nucleus imparts very effective pharmacological properties to therapeutic agents. In these scaffolds, the presence of Fluorine increases bioactivity of molecules several times1,2.
Thiophene derivatives have varied therapeutic applications. Thiophene containing heterocyclic compounds have created interest among the researchers owing to their vast spectrum of biological functions including antimicrobial3,4, antiparasitic5,6, antiviral7, anticancer8,9, enzyme inhibitors10, anti-inflammatory and analgesic11 properties. Some of the commercially available drugs that contain thiophene as an integral component are Suprophen and Tiaprofenic acid as an anti-inflammatory, Rolaxifene and OSI-930 as an anticancer, Methapyrilene as anti-histamine, Tienilic acid as anantihypertensive, Ticlopidine as antiplatelet, Olanzapine as antisychotic, Etizolam as anti-anxiety and Tigabine as anticonvulsant agents.
In recent years, pyrazole is the most studied heterocycle among the azole family due to its innumerable chemical, agrochemical and pharmacological12,13 properties. Pyrazole containing compounds are reported for anticancer14,15 antibacterial, antifungal16–18, antiviral19,20, antinflammatory18,21, anti FAAH(Fatty Acid Amide Hydrolase)22, anti- enzymatic(Anti-S IRT 1 and SIRT 2)23, analgesic24, 5α-Reductase inhibitor25, antioxidant26,27, and insecticidal28. The Pyrazole scaffold has fascinating medicinal potential and is found as a pharmacophoric group of the drug molecules available on the market such as anti-inflammatory agents Meprizole, Celecoxib and Lonazolac, Rimonabant acts as cannabinoid receptor and used to treat obesity, Difenamizole functions as an analgetic, Fomepizole inhibits alcohol dehydrogenase, Fezolamine acts as antidepressant, CDPPB functions as anti-psychotic and Sildenafil inhibits phosphodiesterase (Figure I).
Chalcones have attracted much attention from medicinal chemists, not only as synthon for biosynthetic perspectives but also as bioactive moiety29–32. Several heterocyclic rings can be obtained from chalcones through ring closure reactions. Chalcone shows diversified medicinal and biological activities such as antimalarial33,34, anticancer35,36, anti-inflammatory37,38, anti-tubercular39,40, Antioxidant41,42, anti-alzheimer43, antibacterial and antifungal44–46.
Chromones belong to the flavonoid family and are widely used in folk medicine because of their interesting biological activities47,48. The large number of bioactive molecules have chromone moiety as an essential pharmacophore49,50. Many of the biologically active chromones show anti-bacterial and antifungal51,52, anti-inflammatory53,54, anti-oxidant55,56 anticancer57–59, anti-HIV60, anti-obesity61, antiviral62, antidiabetic48, anticonvulsant63 and anti-tubercular activities64. In light of the significance of thiophene, pyrazole, chalcone and chromone in numerous areas, particularly in medicinal chemistry(biological activity), the present study focuses on synthesis of these scaffolds and their biological activities.
Inhibition of the digestive enzymes is one of the approaches to manage diabetis. These enzymes catalyse the hydrolysis of starch into smaller carbohydrates like glucose65. This is one of the important steps in maintaining blood sugar level. In diabetics, hypoglycaemia can be achieved by inhibiting the enzyme alpha-amylase and the inhibitor can prove a potential anti-diabetic agent. Heterocycles like flavones, pyrazoles and thiophenes are reported for their α-amylase inhibitory activity 66-69
 |
Scheme I |
Result and Discussion
A well-known literature approach was used to synthesize various acetophenones(1a-f) and arylaldehyde (2) derivatives, as illustrated in scheme-I and scheme-II. The synthesis of chalcones,3a-f was carried out by Claisen-Schmidt condensation by using 1a-f and 2. Different spectral methods have verified the formation of 3a-f. I.R. spectra of compound 3d shows band at 3439, 1639 cm-1. HRMS shows a molecular ion peak at 459.0135support 3d formation. 1HNMR signal at 5.88 indicate olefinic proton and also the signal at δ 8.85 shows proton of pyrazole ring. In ethanol, the molecule 3a-f interacts with hydrazine hydrate to producebipyrazolyl phenols,4a-f. This formation of pyrazolines4a-f was confirmed by spectral technique. The I.R. Spectrum 3334cm-1 and Molecular ion peak at 473.0410 in HRMS validated formation of 4d. The most important confirmation of 4d formation is in 1HNMR spectra which shows two doublets at δ 3.17 and δ 3.71 confirmthe presence of diastereotopic protons of methylene group of pyrazoline ringwhile theδ 5.09 triplet of methine in pyrazoline ring. These signals strongly support the formation of 4a-f. Refluxing chalcone 3a-f in DMSO with a catalytic quantity of iodine yielded 2-substituted chromone 5a-f. The I.R. spectrum at 1653cm-1and the molecular ion peak at 456.9967 verify 5d. 1HNMR spectra validate chromone formation as there is absence of downfield signal above δ 10.0 implies absence of O-H and also singlet at δ 6.80 is due to 3-position proton chromone. Chromones5a-f when refluxed in ethanol and hydrazine hydrate were transformed into pyrazoles6a-f. The I.R. Spectrum at 3404cm-1 and In HRMS molecular ion peaks at 471.0243 supports 6d formation. The most significant confirmation is the presence of a singlet at δ 12.70 in1HNMR spectra of O-H proton
Amylase Inhibitory Activity
In a test tube 500 μL of test sample, 500 μL solution ofα-amylase whose concentration is 0.5 mg/mL and phosphate buffer of 0.2 mM concentration were mixed and kept undisturbed 10 min at room temperature. Then the contents of the test tube were allowed to react 1% starch solution in phosphate buffer having pH 6.9. Then dinitrosalicylic acid was used to extinguish reaction. After incubation of 5 min test tubes were cooled and the contents were diluted with distilled water. The absorbance of resulting solutions was recorded at the wavelength 540 nm. Then the results were compared with well-known inhibitor of the acarbose.
The results are recorded as % inhibition of enzyme activity.
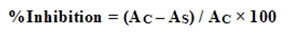
Where AC – Absorbance for Control and AS– Absorbance for Sample
Table 1: Amylase inhibitory Activity: (Concentration 1 mg/mL)
|
Compd |
% Inhibition |
|
6a |
34 |
|
6b |
31 |
|
6e |
09 |
|
6f |
10 |
|
Acarbose |
45 |
Microbial Analysis
In vitro tests were performed on the compounds 2, 3a-f, 4a-f, 5a-f, and 6a-jagainst four bacterial and two fungal strains(Table 2). The agar well diffusion technique was utilized in this experiment. Ciprofloxacin and fluconazole were utilized as antibacterial and antifungal reference drugs respectively, while DMSO was used as a negative control.All compounds are inactive towards gram positive bacteria Bacillus subtilis and have modest action against Escherichia coliand Staphylococcus aureus. Compound 3c, 5a and 5b showed good activityagainst Salmonella typhii while all other compounds have a moderate level of action.Compounds 5d and 6d were shown to have promising action against Aspergillus niger, while others had modest activity against Trichoderma viride.The results were averaged over three experimental sets and reported as zone of inhibition in millimeters
Table 2: Antimicrobial Activity (Zone of Inhibition at 1 mg/mL in mm).
|
Compd |
Antibacterial Activity |
Antifungal Activity |
||||
|
Bacillus subtilis |
Staphylococcus aureus |
Salmonella typhii |
Escherichia coli |
Aspergillus niger |
Trichoderma Viride |
|
|
2 |
– |
11 |
13 |
+ |
– |
– |
|
3a |
– |
13 |
12 |
+ |
– |
13 |
|
3b |
– |
15 |
13 |
+ |
– |
14 |
|
3c |
– |
14 |
18 |
10 |
– |
– |
|
3d |
– |
10 |
11 |
10 |
– |
– |
|
3e |
– |
11 |
12 |
+ |
– |
12 |
|
3f |
– |
12 |
12 |
10 |
– |
– |
|
4a |
– |
13 |
13 |
+ |
– |
11 |
|
4b |
– |
12 |
15 |
+ |
– |
14 |
|
4c |
– |
11 |
12 |
10 |
– |
13 |
|
4d |
– |
12 |
13 |
+ |
– |
– |
|
4e |
– |
13 |
12 |
+ |
– |
12 |
|
4f |
– |
12 |
13 |
+ |
– |
– |
|
5a |
– |
15 |
17 |
10 |
– |
15 |
|
5b |
– |
13 |
18 |
+ |
– |
21 |
|
5c |
– |
14 |
16 |
+ |
– |
– |
|
5d |
– |
13 |
16 |
+ |
21 |
20 |
|
5e |
– |
13 |
16 |
10 |
– |
– |
|
5f |
– |
14 |
15 |
10 |
– |
– |
|
6a |
– |
15 |
13 |
11 |
– |
– |
|
6b |
– |
12 |
12 |
11 |
– |
– |
|
6c |
– |
12 |
13 |
11 |
– |
– |
|
6d |
– |
13 |
13 |
11 |
22 |
21 |
|
6e |
– |
13 |
13 |
11 |
– |
17 |
|
6f |
– |
11 |
– |
– |
– |
– |
|
Ciprofloxacin |
28 |
23 |
40 |
26 |
– |
– |
|
Fluconazole |
– |
– |
– |
– |
28 |
29 |
 |
Scheme II |
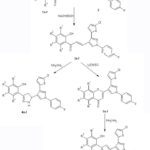 |
Scheme III |
Experimental
Open capillary technique was used for melting points which are uncorrected. IR Affinity-I Fourier transform infrared spectrophotometer (Shimadzu), Bruker Avance II 500 MHz spectrophotometer and Waters SYNAPT G2 HDMS were used to record the IR, 1H NMR and Mass spectra.Samples for NMR were prepared in DMSO-d6 and TMS was internal reference. Absorption frequencies in terms of chemical shift were expressed in δ ppm. Mass spectra were recorded on.
Table 3: Physical data for synthesized compound.
|
Compd |
R1 |
R2 |
R3 |
R4 |
M.P.(oC) |
Yield(%) |
|
3a |
H |
H |
H |
H |
170 |
65 |
|
3b |
H |
CH3 |
H |
H |
172-174 |
61 |
|
3c |
H |
CH3 |
Cl |
CH3 |
220-222 |
63 |
|
3d |
H |
H |
Cl |
H |
262-264 |
66 |
|
3e |
Cl |
H |
Cl |
H |
280-282 |
68 |
|
3f |
H |
H |
CH3 |
H |
262-264 |
58 |
|
4a |
H |
H |
H |
H |
176-178 |
84 |
|
4b |
H |
CH3 |
H |
H |
188-190 |
78 |
|
4c |
H |
CH3 |
Cl |
CH3 |
242-244 |
86 |
|
4d |
H |
H |
Cl |
H |
252-254 |
82 |
|
4e |
Cl |
H |
Cl |
H |
294-296 |
84 |
|
4f |
H |
H |
CH3 |
H |
264-266 |
80 |
|
5a |
H |
H |
H |
H |
206-208 |
76 |
|
5b |
H |
CH3 |
H |
H |
218-220 |
78 |
|
5c |
H |
CH3 |
Cl |
CH3 |
222-224 |
80 |
|
5d |
H |
H |
Cl |
H |
232-234 |
82 |
|
5e |
Cl |
H |
Cl |
H |
256-258 |
78 |
|
5f |
H |
H |
CH3 |
H |
248-252 |
80 |
|
6a |
H |
H |
H |
H |
196-198 |
82 |
|
6b |
H |
CH3 |
H |
H |
204-206 |
78 |
|
6c |
H |
CH3 |
Cl |
CH3 |
208-210 |
72 |
|
6d |
H |
H |
Cl |
H |
202-204 |
76 |
|
6e |
Cl |
H |
Cl |
H |
218-220 |
82 |
|
6f |
H |
H |
CH3 |
H |
212-214 |
74 |
(2E)-3-[3-(5-Chlorothiophen-2-yl)-1-(4-fluorophenyl)-1H-pyrazol-4-yl]-1-(2-hydroxyphenyl)prop-2-en-1-one, 3a
In 25 mL of ethanol and 12 mL of 30 percent NaOH solution, 2-hydroxyacetophenone 1a (0.015mol) and 1,3-disubstituted-pyrazole-4-carbaldehyde2 (0.015mol) were dissolved and stirred at ambient temperature for 40-48 hours with TLC monitoring. The contents were transferred to a beaker containing crushed ice.Then it was acidified using dil. acetic acid and the product was filtered and purified using alcohol to get 3a. The compounds 3b-3f were prepared using the same procedure.
3a
Yellow Solid, IR (KBr):3124, 3066, 2926, 1685, 1639, 1514,831, 748cm-1;HRMS: m/z 424.8751 (M+) ;1HNMR: δ 11.8 (1H, s), 8.89 (1H, s), 7.95 (2H,dd, J=9.12,4.12Hz), 7.81 (1H,dd, J=8.88, 2.82Hz),7.71 (1H,ddd, J=8.92, 8.96, 2.84Hz),7.46 (2H,t, J=9.12Hz),7.37 (1H, d),7.28 (1H,ddd, J=8.96, 8.88, 2.90Hz), 7.21-7.24`(3H,`m),`5.92 (2H, q).
3b
Yellow Solid, IR(KBr): 3398, 3132, 3080, 1639, 1573, 1514, 833, 800cm-1; HRMS: m/z 438.9017(M+) ; 1HNMR: δ 10.2 (1H, s), 8.81 (1H, s), 7.87 (2H,dd, J=8.88, 3.88 Hz), 7.71 (1H,dd, J=8.64, 2.58Hz), 7.38 (2H, t, J=8.88Hz), 7.12-7.16 (2H, m), 7.09 (1H, dd, J=8.64, 2.56Hz), 5.84 (2H, q), 2.34 (3H, s).
3c
Yellow Solid, IR(KBr): 3352, 3124, 2924, 1672, 1639, 1585, 1514, 831, 752cm-1; HRMS: m/z 487.3733(M+) ; 1HNMR: δ 12.4 (1H, s), 8.93 (1H, s), 7.99 (2H,dd, J=9.14,4.14 Hz), 7.50 (2H,t, J=9.14Hz), 7.41 (1H, d), 7.24-7.28 (2H, m),i5.96i(2H,iq),i2.56i(3H,is),i2.48 (3H,is).
3d
Yellow Solid, IR(KBr):3446, 3132, 3070, 1639, 1573, 1514, 833, 800cm-1; HRMS: m/z 459.0135 (M+) ; 1HNMR: δ 11.3(1H, s), 8.85 (1H, s),7.91 (2H,dd, J= 9.4Hz), 7.79 (1H, d, J=2.7Hz), 7.69 (1H,dd, J=8.8, 2.72Hz), 7.42 (2H, t, J=9Hz), 7.33 (1H, d), 7.17-7.20 (2H, m), 5.88 (2H, AB-Quartet).
3e
Yellow Solid, IR(KBr):3446, 3130, 3070, 1643, 1570, 1508, 835, 808cm-1; HRMS: m/z 473.7652(M+); 1HNMR: δ 10.8 (1H, s), 8.79 (1H, s), 7.85 (2H,dd, J=8.86, 3.86 Hz), 7.71 (1H,d, J=2.56Hz),7.61 (1H,d, J=2.58Hz),7.36 (2H,t, J=8.86Hz), 7.27 (1H, d), 7.11 (1H, d), 5.82 (2H, q).
3f
Yellow Solid, IR(KBr):3446, 3134, 1683, 1637, 1560, 1514, 831, 804cm-1; HRMS: m/z 438.9017(M+) ; 1HNMR: δ 11.3 (1H, s), 8.91 (1H, s), 7.97 (2H, dd, J=9.06,4.06 Hz), 7.83 (1H, d, J=2.76Hz),7.73 (1H,dd, J=8.86, 2.78Hz), 7.49 (2H, t, J=9.06Hz), 7.39 (1H, d), 7.22-7.26i(2H,im),i5.94i(2H,iq),i2.32i(3H,is).
2-[(3S)-3′-(5-Chlorothiophen-2-yl)-1′-(4-fluorophenyl)-3,4-dihydro-1′H,2H-3,4′-bipyrazol-5-yl]phenol,4a:
A mixture of 2 mL hydrazine hydrate, 15 mL ethanol and substituted pyrazolyl chalcone 3a (0.0015 mol) was taken in R.B. flask and refluxed for 4 hrs. Then by adding 2 mL of glacial acetic acid, heated forfurther 4 hrs. Once the reaction was finished the contents were taken into crushed ice. The resulting product had been filtered.On crystallization from ethanol, pure compound 4a was obtained. The compound 4b-4f were prepared using the same procedure.
4a
Yellow Solid, IR(KBr): 3309, 3084, 1593, 1514, 835, 802cm-1; HRMS: m/z 438.9050 (M+); 1HNMR: δi12.26 (s, 1H), 8.64i(s,i1H),i7.90-8.01 (m,i3H), 7.37-7.50 (m, 4H),7.32 (1H, ddd, J=8.84, 8.82, 2.68Hz), 7.22 (1H, d, J=8.92Hz),7.14 (1H,ddd, J=8.86, 8.80, 2.94Hz),6.97 (1H, dd, J=8.62, 2.94Hz),5.13 (1H,t, J=10.12Hz),3.75 (1H,dd, J=17.02, 10.12Hz), 3.21 (1H,dd, J=17.01Hz, 10.12Hz).
4b
Yellow Solid, IR(KBr): 3361, 3338, 3082, 1593, 1516, 829, 813cm-1; HRMS: m/z 452.9316 (M+) ; 1HNMR: δ 11.64i(1H,is), 8.56i(1H,is),T7.84-7.93i(3H, m), 7.29-7.44 (4H, m), 7.13 (1H, d, J=8.68Hz) 7.04 (1H,dd, J=8.62, 2.52Hz), 6.90 (1H, d, J=2.52Hz),5.05 (1H,t, 9.88Hz),3.67 (1H,dd, J=17.04, 9.88Hz),3.13 (1H,dd, J=17.05, 9.88Hz), 2.28 (3H, s).
4c
Yellow Solid, IR(KBr): 3292, 3078, 1514, 835, 802 cm-1;iHRMS:m/z 501.4032 (M+) ; 1HNMR: δ 10.74 (1H, s), 8.68 (1H, s), 7.94-8.05 (3H, m), 7.41-7.54 (3H, m),7.27 (1H,d, J=8.94Hz), 7.02 (1H, s),5.17 (1H,t, J=10.14Hz), 3.79i(1H,idd, J=16.94,i10.14Hz),3.25 (1H,dd,J=16.94,i10.14Hz), 2.40i(3H,is),i2.30i(3H,is).
4d
Yellow Solid, IR(KBr): 3334, 3084, 1514, 835, 817cm-1; HRMS: m/z 473.0410(M+) ; 1HNMR: δ 11.68 (1H, s), 8.6 (1H, s), 7.86-7.97 (3H, m), 7.33-7.46 (4H, m), 7.27 (1H,dd, J=8.72, 2.56Hz), 7.21 (1H,d, J=8.8Hz),6.94 (1H,d, J=8.5Hz),5.09 (1H,t, 10Hz),3.71 (1H,dd, J=17.01, 10Hz),3.17 (1H, dd, J=17.01, 10Hz).
4e:
Yellow Solid, IR(KBr): 3327, 3080, 1514, 835cm-1; HRMS: m/z 507.7951 (M+) ; 1HNMR: δ 12.08 (1H, s), 8.54 (1H, s), 7.80-7.91 (3H, m), 7.27-7.40 (4H, m), 7.21 (1H,d, J=2.42Hz), 7.13 (1H,d, J=8.66Hz),5.03 (1H,t, 9.86Hz),3.65 (1H,dd, J=15.97, 9.86Hz), 3.11 (1H,dd, J=15.96, 9.86Hz).
4f
Yellow Solid, IR(KBr): 3277, 3093, 1514, 821, 804cm-1; HRMS: m/z 452.9316 (M+) ; 1HNMR:δ 10.14 (1H, s), 8.66 (1H, s), 7.92-8.04 (3H, m), 7.39-7.52 (4H, m), 7.33 (1H,dd, J=8.72, 2.56Hz), 7.25 (1H,d, J=8.8Hz),7.00 (1H,d, J=8.5Hz),5.15 (1H,t, J=10Hz),3.77 (1H,dd, J=17.03, 10Hz),3.23 (1H,dd, J=17.03, 10Hz),2.30 (3H, s).
2-[3-(5-Chlorothiophen-2-yl)-1-(4-fluorophenyl)-1H-pyrazol-4-yl]-4H-chromen-4-one, 5a
Substituted pyrazolyl propenone3a (0.001mol) was refluxed in 12 mL of DMSO containing0.2 g Iodineat 135-145oC for 3-4 hrs and kept aside for 24 hrs. Then the mixture was transferred to smashed ice and filtered solid was treated with 15% sodium thiosulphate for removal of unreacted Iodine. The compound 5a was purified by recrystallizing it from ethanol. The compound 5b-5f were prepared using the same procedure.
5a
Faint Solid, IR(KBr): 3124, 3059, 1653, 1516, 833cm-1; HRMS: m/z 422.8592(M+) ; 1HNMR: δ 8.00-8.05 (4H, m), 7.65 (1H, dd, J=8.92, 2.88 Hz), 7.56 (1H,ddd, J=8.90, 8.84, 2.88Hz), 7.49-7.53i(4H,im),i7.29i(1H,d,iJ=3.92Hz), 6.84 (1H,is).
5b
Yellowish Brown Solid, IR(KBr): 3122, 3059, 1654, 1516, 833, 800cm-1; HRMS: m/z 436.8858(M+) ; 1HNMR: δ 7.92-7.97 (4H, m), 7.57 (1H,d, J=2.42Hz), 7.50 (1H, dd, J=8.56, 2.42Hz), 7.41-7.44 (3H, m),7.21 (1H,d, J=3.68Hz), 6.76 (1H, s), 2.38 (3H, s).
5c
Yellowish Brown Solid, IR(KBr): 3113, 3057, 1645, 1514, 833, 792cm-1; HRMS: m/z 485.3575(M+) ; 1HNMR: δ 8.04-8.09 (3H, m), 7.69 (1H, s), 7.53-7.57 (3H, m),7.33 (1H,d, J=3.94Hz), 6.88 (1H, s),i2.60i(3H,is),i2.54i(3H, s).
5d
Faint Yellow Solid, IR(KBr): 3140, 3066, 1653, 1541, 1517, 835, 796cm-1; HRMS: m/z 456.9967 (M+) ; 1HNMR: δ 7.96-8.01 (4H, m), 7.61 (1H,d, J=8.8 Hz), 7.45-7.49 (4H, m),7.25 (1H,d, J=3.8Hz), 6.80 (1H, s).
5e
Yellow Solid, IR(KBr): 3124, 3074, 1654, 829, 779cm-1; HRMS: m/z 491.7494 (M+) ; 1HNMR: δ 8.02-8.07 (4H, m), 7.51-7.55 (4H, m), 7.31 (1H,d, J=3.66Hz), 6.86 (1H, s).
5f
Yellow Solid, IR(KBr): 3140, 3066, 1653, 1541, 835, 796cm-1; HRMS: m/z 436.8858 (M+) ; 1HNMR (DMSO-d6): δ 7.90-7.96 (4H, m), 7.55 (1H,d, J=8.86Hz),7.39-7.43 (4H, m),7.19 (1H,d, J=3.86Hz), 6.74 (1H, s), 2.26 (3H, s).
2-[3′-(5-Chlorothiophen-2-yl)-1′-(4-fluorophenyl)-1′H,2H-3,4′-bipyrazol-5-yl]phenol, 6a
In ethanol 2-substituted-chromen-4-one, 5a (0.015mol) and hydrazine hydrate (0.005 mol) were dissolved and refluxed for 4hrs. TLC was used to monitor the reaction, and once it was finished, the reaction liquid stirred withcold water containing small amount of ice.After that, glacial acetic acid was used to neutralize the resulting mixture.Pure 6a was obtained by filtering the resultant product and recrystallizing it from ethanol. The compound 6b-6f were prepared using the same procedure.
6a
Yellow Solid, IR(KBr): 3336, 3128, 3068, 1514, 835, 800cm-1; HRMS: m/z 436.8891 (M+); 1HNMR: δ 13.12 (1H, s), 9.04 (1H, s), 8.14 (1H, d), 7.92-8.03 (3H, m), 7.37-7.49 (5H, m), 7.34 (1H,ddd, J= 8.66, 8.48, 2.24Hz), 7.26 (1H,d, J=9.20, 2.24Hz), 7.19 (1H, d).
6b
Yellow Solid, R(KBr): 3421, 3186, 1514, 835, 698cm-1; HRMS: m/z 450.9157 (M+) ; 1HNMR: δ 10.95 (1H, s), 8.96 (1H, s), 8.06 (1H, d), 7.84-7.95 (3H, m),I7.47i(1H,Idd, J=8.33,i2.25Hz),i7.29-7.39i(4H,im),I7.18i(1H,id,iJ=2.25 Hz),I7.13I(1H,Id),I2.19I(3H,Is).
6c
Yellow Solid, IR(KBr): 3313, 3113, 1514, 833, 802cm-1; HRMS: m/z 499.3873 (M+); 1HNMR: δ 11.37 (1H, s), 9.08 (1H, s), 8.18 (1H, d), 7.96-8.03 (2H, m),7.41-7.50 (4H, m), 7.30 (1H, s),7.24 (1H, d), 2.55 (3H, s), 2.41 (3H, s).
6d
Yellow Solid, IR(KBr): 3404, 3298, 3145, 1514, 833, 800cm-1; HRMS: m/z 471.0243 (M+) ; 1HNMR: δ 12.70 (1H,s), 9.0 (1H, s), 8.10 (1H, d), 7.88-7.99 (3H, m), 7.33-7.45 (5H, m), 7.22 (1H, d, J=9.08 Hz), 7.17 (1H, d).
6e
Yellow Solid, IR(KBr): 3419, 3086, 1514, 835, 802cm-1; HRMS: m/z 505.7792(M+) ; 1HNMR: δ 10.77 (1H, s), 9.06 (1H, s), 8.16 (1H, d), 7.94-8.06 (3H, m), 7.39-7.51 (5H, m), 7.22 (1H, d).
6f:
Yellow Solid, IR(KBr): 3292, 3124, 1514, 835, 815cm-1; HRMS: m/z 450.9157 (M+) ; 1HNMR: δ 12.15 (1H, s), 8.94 (1H, s), 8.04 (1H, d), 7.82-7.93 (3H, m), 7.27-7.39 (5H, m), 7.17 (1H,d, J=9.14 Hz),7.09 (1H, d), 2.12 (3H, s).
Conclusion
Flourine and thiophene containing different pyrazolyl compounds were prepared in this present work and spectroscopic evidence strongly supports the suggested compounds. Compound 6a and 6b are promising alpha-amylase inhibitory activity in comparison with reference compound Acarbose. These compounds can be considered as lead compounds as anti-diabetic agents. Results of the antimicrobial study show that all the synthesized compounds can be modified structurally to improve their antimicrobial profile.
Acknowledgement
The authors are thankful to Management of parent institute, Hon. Principal of the college, DST, Government of India for their support. The authors express their gratitude towards the Director, SAIF, Punjab University, Chandigarh, for spectroscopic investigations.
Conflict of Interest
There are no conflicts of interest declared by the authors.
References
- Gillis, E. P.; Eastman, K. J.; Hill M. D.; Donnelly, D. J.; Meanwell, N. A.; J. Med. Chem. 2015, 58(21), 8315–59 doi:10.1021/acs.jmedchem.7b01788.
CrossRef - Meanwell, N. A.; J. Med. Chem. 2018, 61(14), 5822–80, doi:10.1021/acs.jmedchem.5b00258.
CrossRef - Abdel-Rahman, S. A.; El-Gohary, N. S; El-Bendary, E. R. ; El-Ashry, S. M.; Shaaban, M. I.; Eur. J. Med. Chem. 2017, 140, 200–11 http://dx.doi.org/10.1016/j.ejmech.2017.08.066.
CrossRef - Ajdačić, V.; Senerovic, L.; Vranić, M.; Pekmezovic, M.; Arsic-Arsnijevic, V.; Veselinovic, A.; Bioorg. Med. Chem. 2016, 24(6), 1277–91 http://dx.doi.org/10.1016/j.bmc.2016.01.058.
CrossRef - Félix, M. B.; de Souza, E. R.; de Lima, M. do CA.; Frade, D.K.G; Serafim, V. de L.; Rodrigues, K. A. da F.; Bioorg. Med. Chem. 2016, 24(18), 3972–7 http://dx.doi.org/10.1016/j.bmc.2016.04.057.
CrossRef - Jacomini, A. P.; Silva, M. J. V.; Silva, R. G. M.; Gonçalves, D. S.; Volpato, H.; Basso, E. A.; Eur. J. Med. Chem, 2016, 124, 340–9.
CrossRef - Desantis, J.; Nannetti, G.; Massari, S.; Barreca, M.L.; Manfroni, G.; Cecchetti, V.; Eur. J. Med. Chem. 2017, 138, 128–39 http://dx.doi.org/10.1016/j.ejmech.2017.06.015.
CrossRef - Othman, D. I. A, Selim, K. B; El-Sayed, M. A. A.; Tantawy, A. S.; Amen, Y.; Shimizu, K.; Bioorg. Med. Chem. 2019, 27(19), 115026 https://doi.org/10.1016/j.bmc.2019.07.042.
CrossRef - Perin, N.; Rep. V.; Sović, I.; Juričić. Š.; Selgrad, D.; Klobučar, M.; Eur. J. Med. Chem. 2020, 185,111833. https://doi.org/10.1016/j.ejmech.2019.111833.
CrossRef - Mandawad, G. G.; Dawane, B. S.; Beedkar, S. D.; Khobragade, C.N.; Yemul, O. S.; Bioorg. Med. Chem. 2013, 21(1), 365–72 http://dx.doi.org/10.1016/j.bmc.2012.09.060.
CrossRef - Molvi, K. I.; Vasu, K. K.; Yerande, S. G.; Sudarsanam, V.; Haque, N.; Eur. J. Med. Chem. 2007, 42(8), 1049–58
CrossRef - Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman, New J. Chem. 2016, 41(1), 16–41 http://dx.doi.org/10.1039/C6NJ03181A.
CrossRef - Aziz, H.; Zahoor, A. F.; Ahmad, S. J.; Chil. Chem. Soc.2020, 65(1), 4746–53
CrossRef - Huang, Y. Y.; Wang, L. Y.; Chang, C. H.; Kuo, Y. H.; Kaneko, K.; Takayama, H.; Tetrahedron, 2012, 68(47), 9658–64 http://dx.doi.org/10.1016/j.tet.2012.09.054.
CrossRef - Bertuzzi, G.; Locatelli, E,; Colecchia, D.; Calandro, P.; Bonini, B. F.; Chandanshive, J. Z.; Eur. J. Med. Chem.2016, 117, 1–7 http://dx.doi.org/10.1016/j.ejmech.2016.04.006.
CrossRef - Nayak, N.; Ramprasad, J.; Dalimba, U.; J. Fluor. Chem.; 2016, 183, 59–68 http://dx.doi.org/10.1016/j.jfluchem.2016.01.011.
CrossRef - Viveka, S.; DineshaShama, P.; Nagaraja, G. K.; Ballav, S.; Kerkar, S.; Eur. J. Med. Chem.2015, 101, 442–51 http://dx.doi.org/10.1016/ j.ejmech.2015.07.002.
CrossRef - Li, Y. R.; Li, C.; Liu, J. C.; Guo, M.; Zhang, T. Y.; Sun, L. P.; Bioorg. Med. Chem. Lett.2015, 25(22), 5052–7
CrossRef - Jia, H.; Bai. F.; Liu. N.; Liang, X.; Zhan, P.; Ma, C.; Eur. J. Med. Chem.2016, 123, 202–10 http://dx.doi.org/10.1016/j.ejmech.2016.07.048.
CrossRef - Fioravanti, R.; Desideri, N.; Biava, M.; Droghini, P.; Atzori, E. M.; Ibba, C.; Bioorg. Med. Chem. Lett.2015, 25(11), 2401–4 http://dx.doi.org/10.1016/j.bmcl.2015.04.006.
CrossRef - Khloya, P.; Kumar, S.; Kaushik, P.; Surain, P.; Kaushik, D.; Sharma, P. K.; Bioorg. Med. Chem. Lett.2015, 25(6), 1177–81 http://dx.doi.org/10.1016/j.bmcl.2015.02.004.
CrossRef - Aghazadeh, T. M.; Baraldi, P. G.; Ruggiero, E.; Saponaro, G.; Baraldi, S.; Romagnoli, R.; Eur. J. Med. Chem.2015, 97, 289–305 http://dx.doi.org/10.1016/j.ejmech.2015.04.064.
CrossRef - Therrien, E.; Larouche, G.; Nguyen, N.; Rahil, J.; Lemieux, A. M.; Li, Z.; Bioorg. Med. Chem. Lett.2015, 25(12), 2514–8 http://dx.doi.org/10.1016/j.bmcl.2015.04.068.
CrossRef - Abd-El, Gawad, N. M.; Hassan G. S.; Georgey, H. H.; Med. Chem. Re. 2012, 21(7) 983–94
CrossRef - Banday, A. H.; Shameem, S. A.; Jeelani, S. S.; Steroids.2014, 13–9, http://dx.doi.org/10.1016/j.steroids.2014.09.004.
CrossRef - Kenchappa, R.; Bodke, Y. D.; Chandrashekar, A.; ArunaSindhe, M.; Peethambar, S. K.; Arab. J. Chem.2017, 10, S3895–906 http://dx.doi.org/10.1016/j.arabjc.2014.05.029.
CrossRef - Ambethkar, S.; Padmini, V.; Bhuvanesh, N.; J. Adv. Res.2014, 6(6), 975–85, http://dx.doi.org/10.1016/j.jare.2014.11.011.
CrossRef - Liu, X. H.; Zhao, W.; Shen, Z. H.; Xing, J. H.; Yuan, J.; Yang, G.; Bioorg. Med. Chem. Lett.2016, 26(15), 3626–8 http://dx.doi.org/10.1016/j.bmcl.2016.06.004.
CrossRef - Rani, A.; Anand, A.; Kumar, K.; Kumar, V.; Expert. Opin. Drug Discov.2019, 14(3), 249–88 https://doi.org/10.1080/17460441.2019.1573812.
CrossRef - Zhuang, C.; Zhang, W.; Sheng, C,; Zhang, W.; Xing, C.; Miao, Z.; Chem. Rev. 2017, 117(12), 7762–810
CrossRef - Gaonkar, S. L.; Vignesh, U. N.; Res. Chem. Intermed.2017, 43(11), 6043–77
CrossRef - Nowakowska, Z.; Eur. J. Med. Chem.2007, 42(2), 125–37.
CrossRef - Kumari, A.; Karnatak, M.; Singh, D.; Shankar, R.; Jat, J. L.; Sharma, S.; Eur. J. Med. Chem.2019, 163, 804–29 https://doi.org/10.1016/j.ejmech.2018.12.007.
CrossRef - Kalaria, P. N.; Karad, S. C.; Raval, D. K.; Eur. J. Med. Chem.2018, 158, 917–36 https://doi.org/10.1016/j.ejmech.2018.08.040.
CrossRef - Pontes, O.; Costa, M.; Santos, F.; Sampaio-Marques, B.; Dias, T.; Ludovico, P.; Eur. J. Med. Chem.2018, 157, 101–14 https://doi.org/10.1016/j.ejmech.2018.07.058.
CrossRef - Karthikeyan, C.; NarayanaMoorthy, N. S. H.; Ramasamy, S.; Vanam, U.; Manivannan, E.; Karunagaran, D.; Recent. Pat. Anticancer Drug. Discov. 2014, 10(1), 97–115.
CrossRef - Bhale, P. S.; Chavan, H. V.; Dongare, S. B.; Shringare, S. N.; Mule, Y. B.; Nagane, S.; Bioorg. Med. Chem. Lett.2017, 27(7), 1502–7 http://dx.doi.org/10.1016/j.bmcl.2017.02.052.
CrossRef - Li, J.; Li, D.; Xu, Y.; Guo, Z.; Liu, X.; Yang, H.; Bioorg. Med. Chem. Lett.2017, 27(3), 602–606, 2018, 28(23–24), 3822, https://doi.org/10.1016/j.bmcl.2018.10.027.
CrossRef - Gomes, M. N.; Braga, R. C.; Grzelak, E. M.; Neves, B. J.; Muratov, E.; Ma, R.; Eur. J. Med. Chem. 2017, 137, 126–38 http://dx.doi.org/10.1016/j.ejmech.2017.05.026.
CrossRef - Chetty, S.; Ramesh, M.; Singh-Pillay, A.; Soliman, ME. S.; Bioorg. Med. Chem. Lett. 2017, 27(3), 370–86 http://dx.doi.org/10.1016/j.bmcl.2016.11.084.
CrossRef - Niu, H.; Wang, W.; Li, J.; Lei, Y.; Zhao, Y.; Yang, W.; Eur. J. Med. Chem.2017, 138, 212–20 http://dx.doi.org/10.1016/j.ejmech.2017.06.033.
CrossRef - Tajammal, A.; Batool, M.; Ramzan, A.; Samra, M. M.; Mahnoor, I.; Verpoort, F.; J. Mol. Struct.2017, 1148, 512–20 http://dx.doi.org/10.1016/j.molstruc.2017.07.042.
CrossRef - Wang, L.; Wang, Y.; Tian, Y.; Shang, J.; Sun, X.; Chen, H.; Bioorg. Med. Chem. 2017, 25(1), 360–71 http://dx.doi.org/10.1016/j.bmc.2016.11.002.
CrossRef - Besharati S. T.; Keivanloo, A.; Kaboudin, B.; Yoshida, A.; Yokomatsu, T.; Tetrahedron, 2018, 74(19), 2350–8 https://doi.org/10.1016/j.tet.2018.03.055.
CrossRef - Yadav, P.; Lal, K.; Kumar, L.; Kumar, A.; Kumar, A.; Paul, A. K.; Eur. J. Med. Chem.2018, 155, 263–74 https://doi.org/10.1016/j.ejmech.2018.05.055.
CrossRef - Lal, K.; Yadav, P.; Kumar, A.; Kumar, A.; Paul, A. K.; Bioorg. Chem. 2018, 77, 236–44 https://doi.org/10.1016/j.bioorg.2018.01.016.
CrossRef - Ribnicky, D. M.; Poulev, A.; Watford, M.; Cefalu, W. T.; Raskin, I.; Phytomedicine, 2006, 13(8), 550–7.
CrossRef - Nurul, I. M.; Jung, H. A.; Sohn, H. S.; Kim, H. M.; Choi, J. S.; Arch. Pharm. Res. 2013, 36(5), 542–52.
CrossRef - Nazhand, A.; Durazzo, A.; Lucarini, M.; Romano, R.; Mobilia, M. A.; Izzo, A. A.; Nat. Prod. Res. 2020, 34(1), 137–52 https://doi.org/10.1080/14786419.2019.1678618.
CrossRef - Gaspar, A.; Matos, M. J.; Garrido, J.; Uriarte, E.; Borges, F.; Chem. Rev. 2014, 114(9), 4960–92 http://www.ncbi.nlm.nih.gov/pubmed/24555663 Kumar, S.; Koh, J.; Int. J. Mol. Sci.2012, 13(5), 6103–16.
CrossRef - He, J.; Li, Z. H.; Ai, H. L.; Feng, T.; Liu, J. K.; Nat. Prod. Res.2019, 33(24), 3515–20 https://doi.org/10.1080/14786419.2018.1486313.
CrossRef - Huo, H. X.; Gu, Y. F.; Zhu, Z. X.; Zhang, Y. F.; Chen, X. N.; Guan, P. W.; Phytochemistry, 2019, 158, 46–55 https://doi.org/10.1016/j.phytochem. 2018.11.003.
CrossRef - Venkateswararao, E.; Manickam, M.; Boggu, P.; Kim, Y.; Jung, S. H.; Bioorg. Med. Chem.2015, 23(10), 2498–504 http://dx.doi.org/10.1016/ j.bmc.2015.03.045.
CrossRef - Demetgül, C.; Beyazit, N.; Carbohydr. Polym.2018, 181, 812–7 https://doi.org/10.1016/j.carbpol.2017.11.074.
CrossRef - Reis, J.; Cagide, F.; Valencia, M. E.; Teixeira, J.; Bagetta, D.; Pérez, C.; Eur. J. Med. Chem.2018, 158, 781–800 https://doi.org/10.1016/j.ejmech.2018.07.056.
CrossRef - Demir, S.; Özen, C.; Ceylan-Ünlüsoy, M.; Öztürk, M.; Bozdağ-Dündar, O.; J. Heterocycl. Chem.2019, 56(4), 1341–51
CrossRef - Duan, Y. di.; Jiang, Y. yan.; Guo, F. xia.; Chen, L. xiao.; Xu, L. lu.; Zhang, W.; Fitoterapia, 2019, 135, 114–29
CrossRef - Zhao, L.; Yuan, X.; Wang, J.; Feng, Y.; Ji, F.; Li, Z.; Bioorg. Med. Chem.2019, 27(5), 677–85 https://doi.org/10.1016/j.bmc.2019.01.027.
CrossRef - Ungwitayatorn, J.; Wiwat, C.; Samee, W.; Nunthanavanit, P.; Phosrithong, N.; J. Mol. Struct.2011, 1001(1–3), 152–61 http://dx.doi.org/10.1016/j.molstruc.2011.06.035.
CrossRef - Iyengar, R. R.; Lynch, J. K.; Mulhern, M. M.; Judd, A. S.; Freeman, J. C.; Gao, J.; Bioorg. Med. Chem. Lett.2007, 17(4), 874–8.
CrossRef - Zhang, Y.; Zhong, H.; Lv, Z.; Zhang, M.; Zhang, T.; Eur. J. Med. Chem. 2013, 62, 158–67
CrossRef - Abbott, B. M.; Thompson, P. E.; Bioorg. Med. Chem. Lett.2006, 16(4), 969–73
CrossRef - Mujahid, M.; Gonnade, R. G.; Yogeeswari, P.; Sriram, D.; Muthukrishnan, M.; Bioorg. Med. Chem. Lett.2013, 23(5), 1416–9 http://dx.doi.org/10.1016/j.bmcl.2012.12.073.
CrossRef - Dhital, S.; Warren, F. J.; Butterworth, P. J.; P. R. Ellis and M. J. Gidley, 2014, doi.org/10.1080/10408398.2014.922043.
- Liu, X.; Luo, F.; Li, P.; She, Y.; Gao, W.; Food Res. Inter. 2017doi: 10.1016/j.foodres.2017.03.023
- Ibrahim, S. R. M.; Mohamed, G. A.; Abdel-latif, M. M. M.; EI-Messerrey, S. M.; Al Musayeib, N. M.; Shehata, I. A.; Starch, 2015, DOI 10.1002/star.201500068.
CrossRef - Bhosale, M. R.; Deshmukh, A. R.; Pal, S.; Srivastava, A. K.; Mane, R. A.; Bioorg. Med. Chem. Lett.25, 2015, 2442-2446
CrossRef - Kumar, P.; Duhan, M.; Kadyan, K.; Sindhu, J.; Kumar, S.; Sharma, H.; Med. Chem. Comm.2017, DOI: 10.1039/C7MD00080D.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









