A Mini-review on Ultra Performance Liquid Chromatography
Rabia Basharat1, Vijay Kotra1*, Lean Yen Loong1, Allan Mathews1, Mahibub Mahamadsa Kanakal1 , CH B Praveena Devi2, Shaik Nyamathulla3
, CH B Praveena Devi2, Shaik Nyamathulla3 , Ravi Varala4
, Ravi Varala4 , Long Chiau Ming5, KRS Sambasiva Rao6, B. Hari Babu7 and M. Mujahid Alam8
, Long Chiau Ming5, KRS Sambasiva Rao6, B. Hari Babu7 and M. Mujahid Alam8
1Faculty of Pharmacy, Quest International University, 30250 Ipoh, Malaysia.
2Department of Pharmacy, Joginpally B.R. Pharmacy College, Yenkapally, Moinabad Mandal, Hyderabad, Telangana-500075, India.
3Department of Pharmaceutical Technology, Faculty of Pharmacy, Universiti Malaya, 50603 Kuala Lumpur, Malaysia.
4Scrips Pharma, Hyderabad-500 076, Telangana, India.
5PAP Rashidah Sa’adatul Bolkiah Institute of Health Sciences, Universiti Brunei Darussalam, Gadong, Brunei Darussalam.
6Mizoram University (A Central University), Aizawal-796004, Mizoram, India.
7Department of Chemistry, Acharya Nagarjuna University, Guntur -522510, Andhra Pradesh, India.
8Department of Chemistry, College of Science, King Khalid University, PO Box 9004, Abha 61413, Saudi Arabia.
Corresponding Author E-mail: ravivarala@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370411
Article Received on : 08 May 2021
Article Accepted on : 16-Jul-2021
Article Published : 09 Jul 2021
Reviewed by: Dr. R.K. Lal
Second Review by: Dr. Guna Shekar
Final Approval by: Dr. Ioana stanciu
Chromatography is a widely used analytical tool for separating a mixture of compounds into individual component. High performance liquid chromatography (HPLC) is one of the most important methods used for the separation, identification and quantification of a compounds present in a mixture. It meets many criteria of analysis but its main drawbacks are it is relatively time consuming to run a chromatogram and consumes high amount of solvent compared to other analytical methods. There is a need to develop a method which can overcome these drawbacks of HPLC. Ultra performance liquid chromatography (UPLC) is the new approach which opens novel direction in the field of liquid chromatography. It works on similar principle but shows better performance than conventional HPLC. UPLC is a technique of liquid chromatography with improved runtime and sensitivity with less than 2 μm particle size. The UPLC separation process is carried out under very high pressure (up to100 MPa). Additionally, it reduces the cost of reagent with shorter run time as compared to conventional HPLC. This article updated until 2020, provides a general review on the principle, instrumentation and application of UPLC in different fields of science.
KEYWORDS:Instrumentation and Applications; Quantification; Resolution; Sensitivity; Separation; Ultra performance liquid chromatography
Download this article as:| Copy the following to cite this article: Basharat R, Kotra V, Loong L. Y, Mathews A, Kanakal M. M, Devi C. H. B. P, Nyamathulla S, Varala R, Ming L. C, Rao K. R. S. S, Babu B. H, Alam M. M. A Mini-review on Ultra Performance Liquid Chromatography. Orient J Chem 2021;37(4). |
| Copy the following to cite this URL: Basharat R, Kotra V, Loong L. Y, Mathews A, Kanakal M. M, Devi C. H. B. P, Nyamathulla S, Varala R, Ming L. C, Rao K. R. S. S, Babu B. H, Alam M. M. A Mini-review on Ultra Performance Liquid Chromatography. Orient J Chem 2021;37(4). Available from: https://bit.ly/3e3uFt0 |
Introduction
Modern analytical chemistry, playing a tremendous role related to chemical innovation, began in the 18th century, especially in many aspects of chemistry such as chemical synthesis, qualitative and quantitative analysis1. Nowadays, analytical chemists are working on different instruments such as mass spectroscopy (MS) Nuclear Magnetic Resonance (NMR) inductively coupled plasma, gas chromatography, HPLC and more recently UPLC. These analytical methods are not only useful for chemistry laboratories but also helpful for environmental and biological laboratories and have gained excellent benefits2. Amongst all the above analytical methods, HPLC has become most widely used analytical tools. In 1970s, there were various advancements in equipment and instrumentation. HPLC has started a revolution in biological, pharmaceutical chemistry and other fields of science3. The first commercially available UPLC system was demonstrated in 20044. Today ultra-performance liquid chromatography has overtaken HPLC as the standard platform5.
History of chromatography
Chromatography was discovered in early 20th century by M.S. Tswett who gave comprehensive details of the adsorption based separation of different compounds in complex mixtures of plant pigments. Almost 10 years later, L.S. Palmer and C. Dhere issued the similar separation processes. In 1931, Lederer purified xanthophylls on CaCO3 adsorption column by using M. S.Tswett’s method. Martin and Synge were awarded Nobel Prize for their discovery of partition chromatography in 19416. Until 1970s, separation process exploited thin layer, paper and column chromatography. However, the main disadvantages of these techniques are the lack of accuracy for quantitative work and poor resolution for similar compounds7,8.
Results and discussion
HPLC
It was first developed in the mid 1970’s and till now it is the most used method in analytical chemistry. Following the development of column packing material and detectors, the technique rapidly improved. In 1980’s HPLC was widely utilized specially for the separation of reaction mixture9. Some novel methods like use of computer, automation and reverse phase chromatography were developed along the time for enhanced separation methods, quantification and identification of mixtures10. In continuation of these advancements, by the year 2000, a tremendous development was taking place in different aspect of particle size of the stationary phase. HPLC is probably the most popular type of technique which is useful in quality control, pharmaceutical analysis, forensic analysis, clinical testing and environmental monitoring and other fields of science.
Principle
The separating principle of HPLC is derived on the difference in affinity of the compound to be separated toward the stationary and mobile phase. Detector can recognize analytes after leaving column and signals are recorded in the data system.
Instrumentation
HPLC consists of following components
Pump
To maintain constant flow of mobile phase through the column and manage the back pressure caused by the flow resistance of the packed column.
Injector
To introduce a liquid sample into the HPLC system by injection, usually in the range of 0.1 to 100 µl of volume.
Column
It is the heart of HPLC in which separation occurs. A variety of columns are used for different substances depending on the nature of the analytes.
Detector
HPLC detector is used to detect solute present in the eluent coming out from column. There are various types of detectors such as ultraviolet detector, fluorescence detector, mass spectrometer etc.11.
Applications of HPLC
HPLC is widely used in many fields of science for identification, quantification and purification of a compound. These include application in the fields of pharmaceutical science, environmental science, forensic science and clinical analysis. It is widely used in quality control and dosage form. It can be employed for the determination of pharmaceutical product shelf life and also for Identification of different active metabolites. HPLC is also helpful for the analysis of environmental material such as detection of phenolic compound in drinking water and for bio monitoring pollutant as well. Forensic applications for the quantitative analysis of the drug in blood sample and steroids identification method also require HPLC technique12.
UPLC
It opened an innovative direction for liquid chromatography covering three major areas including speed, sensitivity and resolution of evaluation by means of the use of packing material with particles size less than 2 µm. The device is created to handle very high pressure experienced by the column. Ultra-performance liquid chromatography also has the advantage of reducing solvent consumption compared to conventional high-performance liquid chromatography13.
Principle of UPLC
The ultra performance liquid chromatography is established on principle of Van Deemter equation14.
Equation of van Demeter is:
H=A+B/μ +Cμ
Where:
H= Plate height
A= Eddy diffusion
B= Longitudinal diffusion C= Equilibrium mass transfer
μ= Flow rate
Smaller plate height value corresponds to greater peak efficiency, as more plates can occur over a fixed length of column15,16 (Figure 1).
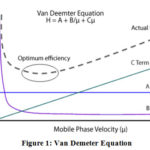 |
Figure 1: Van Demeter Equation. |
Shorter diffusion path length of smaller particles allows a faster movement of the solute in and out of the particles. Because of this the solute/analyte spends less time inside the particle where the peak diffusion occurs17-18(Figure 2).
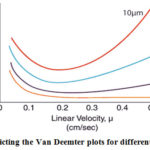 |
Figure 2: Depicting the Van Deemter plots for different particle sizes. |
It has been noticed that using a shorter column length allows much higher sample throughput without losing chromatographic quality of the analytical method19.
Instrument of UPLC
Ultra performance liquid chromatography instrumentation is basically similar to that of HPLC. It is designed to work under much higher pressure without disturbance and increased maintenance.For UPLC detection, new electronics and firmware are used to support the tunable UV/Visible detector at the high data rates. The tunable UV/VIS detector comprises a 10 mm flow cell path length with a volume of only half a litre.
The instrumentation of UPLC includes:
Sample injection
UPLC columns
Detectors
Sample injection
The injector is used to add a small amount of solution containing the sample in the mobile phase that is precisely measured. The injection must be done consistently and precisely. Conventional injection valves can be manual or programmed, and the injection procedure must be somewhat pulse-free to protect the column from excessive pressure instabilities. To decrease the risk of band spreading, the device’s swept volume should be kept to a minimum. To effectively benefit from the speed of UPLC, a short injection cycle time is required. Low volume injections with minimum carry over are required to increase sensitivity. In UPLC, the sample volume is usually 2-5 μl. For biological samples, direct injection techniques are now commonly used. Flow chart of UPLC shown below (Figure 3).
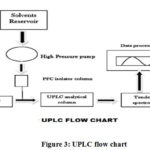 |
Figure 3: UPLC flow chart. |
Ultra performance liquid chromatography columns are made of small particles size 2 µm. Waters Associates develops and supplies most of the UPLC columns, some of which are described as follow:
Bridged Ethylene Hybrid (BEH) C18 column
This column provides high level of column ability, symmetrical peak and stability. It is compatible with mobile phase pH 1-12 and temperature 80 oC.
Example: Rapid assay for Cytochrome p450 isoenzymes degradation studies of Glimepirade (retention time: 8.2 minutes)20.
Bridged Ethylene Hybrid (BEH) C8 column
This column exhibits low hydrophobicity than C18 column due to its shorter alkyl chain length, thus resulting in rapid elution of analyte peaks.
Example: Analysis of different aromatic amines.
Bridged Ethylene Hybrid (BEH) guard RP 18 column
It improves the peak shape for basic compounds and also fine tunable with 100% aqueous mobile phases.
Example: Study of profiles of Doxylamine10.
Hydrophilic interaction liquid chromatography (HILIC) Column
This column increases the time retention of polar compound and provide separation method for mixtures of ionizable/polar compounds.
Example: Separation of different classes of lipids.
Bridged Ethylene Hybrid 130 and 300 column
This column provides an improved characterization of peptide and protein, owing to the increase in resolving power.
Example: Analysis of amyloid β peptide in cerebral spinal fluid10.
Bridged Ethylene Hybrid phenyl column
Provides chemical stability, improved peak shape and reproducibility for wide range of analytes.
Example: Rapid analysis of 25 polymer additives is achieved by implementing UPLC method with Tandem Quadrupole along with BEH phenyl column
Amide column
The column is highly suited for analysis of carbohydrates due to compatibility with wide range of pH and elevated temperatures.
Example: UPLC-MS analysis of carbohydrates and separation of metformin
Bridged Ethylene Hybrid 300 C4 Column
This column is suitable for the high resolution separation of protein mixtures.
Bridged Ethylene Hybrid GLYCAN Columns
This column provides superior high resolution of glycoprotein.
Example: useful for analysis Transtuzumab, drug which is useful for breast cancer treatment.
Charged Surface Hybrid (CSH) C18 Column
It is a universal choice for C18 column under low pH, and mobile phase of weak ionic strength.
Charged Surface Hybrid Phenyl-Hexyl (CSH) Column
Suitable particularly for polyaromatic compounds. The CSH Phenyl-hexyl column provides excellent peak shape under low and high pH conditions.
Example: Analysis of Quinine, Labetolol, Diltiazem and Verapamil
Hollow Structural Sections (HSS) C18 Column
This is a general purpose silica-based C18 column which is applicable at low pH (Figure 4).
Example: Separation of xanthine alkaloids (Xanthine 0.31 min retention time, 7-methyl xanthine 0.49 min retention time, Theobromine 0.65 min retention time, Paraxanthine 0.78 min retention time, Caffeine 0.99 min retention time)
Hollow Structural Sections columns Cyano Column
This column provides low hydrophobicity.
Example: Analysis of analgesics/ steroids.
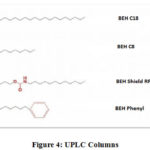 |
Figure 4: UPLC Columns. |
Detector
The UPLC detector used should be able to provide a high sampling rate with narrow attainable peaks (1s half-height peak width) and little dispersion of the peaks so that less separated solute is wasted on the column. The UPLC methodology delivers two to three times the separation sensitivity of the previous method HPLC because of the detector method. Acquity photodiode array (PDA) and Tunable Vis-UV (TUV) detectors are utilized in the UPLC, with Teflon AF providing an internally reflecting surface that improves light transmission efficiency by removing internal absorptions. Path lengths are 10 nanometers, acquisition speeds are 20 (PDA) and 40 (TUV), and total internal capacity is 500 nanoliters. Detection by mass spectrometry has also been used with UPLC21.
Advantages of UPLC
(Table 1) for comparison of characteristics of HPLC and UPLC): The main advantage of UPLC is high resolution performance and rapid resolving power as well as it is more selective and sensitive. With lower operating costs and shorter run times, it also reduces process cycle time and ensures end-product quality. The use of a unique column material with very small particle size boosts sensitivity and allows for rapid examination. It reduces solvent consumption and expends the scope of multiple residue methods.
It offers selectivity and sensitivity with minimum runtime.
Peak resolution is enhanced in many cases
Expands the scope of multi residue methods.
Less solvent consumption
Table 1: Differences between HPLC and UPLC in a nutshell
|
Characteristic |
HPLC |
UPLC |
|
Size of particle |
3-5 µm |
Less than 2µm |
|
Back pressure |
35-40 Mpa |
103.5Mpa |
|
Analytical column |
C18 |
BEHC18 |
|
Injection volume |
5 µm |
2 µm |
|
Temperature |
30 C |
65 C |
|
Run time |
10 min |
1.5 min |
|
Resolution |
3.2 |
3.4 |
|
Plate count |
2000 |
7500 |
|
Flow rate |
3.0 ml/min |
0.6 ml/min |
Disadvantages of UPLC
UPLC has many advantages but there is some drawback like high-pressure required more maintenance and shelf life of column is short22. When compared to standard HPLC, UPLC has greater back pressures, which reduces column life. In UPLC, increasing the column temperature lowers the problem of back pressure. Furthermore, particles smaller than 2 μm are typically non-generable and so have a limited application.
New technological advancement of UPLC
The most important advantage of ultra performance liquid chromatography is speed, able for high speed resolution23. UPLC with sub-2-μm porous stationary particles working with high linear velocity pressures > 9000 psi, was once combined with spectrometer properly working for the rapid separation of lipids and complex compound and their metabolites24.
Comparison between HPLC and UPLC tabulated as below
Wu et al.25 developed novel analytical methods for the separation of 12 phthalates, and the findings were compared to UPLC and HPLC results. The mobile phase consisted of a methanol and water gradient. The PDA detector was used to detect UV at 225 nm.A Waters UPLC with an Acquity UPLC BEH phenyl column (50 x 2.1 mm, 1.7 m) was used to separate the samples. The flow rate was 0.4 mL/min and the total run time was 7 minutes. The HPLC analysis was carried out using Agilent 1100 equipment and an Agilent SB-phenyl column (250 x 4.6 mm, 5m). The flow rate was 1.0 mL/min and the total run time was 18 minutes.In comparison to HPLC, analysis time was decreased by a ratio of 2.5 and solvent usage was reduced by a factor of 6.4 (Figure 5).
 |
Figure 5: Comparison of HPLC and UPLC analysis of 12 phthalates Click here to View figure |
Many analytical laboratories place a premium on analysis time, solvent use, and analysis cost. The amount of time it took to optimize new procedures was also drastically decreased. UPLC and HPLC techniques for determining ascorbic acid (AA) in fruit beverages and medicinal preparations were devised and compared by Inga Kleczka et al. Both procedures were quick, with complete analytical times of 15 and 6 minutes for HPLC and UPLC, respectivelyThe new UPLC method Figure 5was found to give superior separation than HPLC methods while lowering the run time from 18 to 7 minutes26.
With the introduction of UPLC, new small particle chemistry and equipment technology for liquid chromatography has emerged, allowing for increased throughput and hence analysis speed without sacrificing chromatographic performance. The use of smaller particles in column packing allows for greater speed and peak capacity. It is possible to perform higher resolution procedures with the UPLC technology, using shorter columns, smaller packing particle sizes, and greater flow rates under high pressure. It goes without saying that boosting peak capacity increases data quality. Working with UPLC systems also results in a significant reduction in solvent usage and column equilibration time. In comparison to all previously employed chromatographic procedures, the injection volume in UPLC is lowered by almost five to ten times, resulting in a better peak band and lower carryover effects related to column diameter. The biggest downside of UPLC is the expensive cost of the device.
Applications of UPLC
Natural product and herbal medicine
UPLC has the ability to provide high quality of separation and detection capability of active compound which is present in mixture27.
Examples
UPLC is used for multiple components for quantitative analysis in example analysis of Hyangsapyeongwisan which is traditional medicine and used in gastric disease28.
Ginseng species29.
analysis of ascorbic acid and dehydroascorbic acid in liquid and solid vegetable samples30.
for detection of pyrrlozidine alkaloids in herbal medicines31.
Identification of Metabolites
UPLC/MS/MS32 offers unmatched sensitivity and accuracy in biomarker discovery33.
Examples
fungal secondary metabolites34.
UPLC-MSE was used for rapid detection and characterization of verapamil metabolites in rats35.
UPLC-DAD-MS/MS was used in the metabolic of the medicinal grass Eleusine indica36.
Drug Discovery
Useful in drug discovery process37. UPLC system by using acquity BEH C18 column that method is faster and sensitive as compare to HPLC method38.
Examples
mango leaf tea metabolites39.
analysis of fenofibrate in Human Plasma40.
Determination of Mesa amine related impurities from drug sproduct by reversed phase validated UPLC method41.
Method Development
Validation to reduce cost and improving opportunities for business success42-44.
Examples
butoconazole in active ingredient45.
cefditoren pivoxil in API (active pharma ingredient)46.
Glibenclamide in rat plasma47.
UPLC method determination of sofosbuvir and daclatasvir in human plasma for therapeutic drug monitoring48.
Combination study
UPLC coupled with photodiode and mass spectroscopy which can give rapid identification of compound along with sensitivity49. The coupling of UPLC with other devices different techniques is convenient and economical as compared to HPLC50-51.
Examples
evaluation of bisphenol52.
metabolites of Mequindox in holothurians53.
UPLC-DAD-MS/MS was used in the metabolic of the medicinal grass Eleusine indica54.
Impurity profile
Reversed phase UPLC methods are highly useful for quantitative determination of active pharmaceutical compound55.
Examples
impurities in Maraviroc56.
Determination of products and process impurities of asenapine maleate in asenapine sublingual tablets by UPLC57.
impurities of halobetasol propionate in cream58.
Quality control
Reversed phase ultra performance provide a sensitive, rapid, and accurate result with less reagents cost and utilized in internal quality control in different dosage type59.
Examples
UPLC-QTOF/MSE a recent approach for identifying quality control analysis of fluctuation of xueshuantong lyophilized powder in clinic60.
UPLC-Q-TOF/MS analysis and species differentiation for quality control of Nigella glandulifera61.
UPLC method was developed for the quality control of rhubarb-based medicines.
Amino acid determination
The UPLC also suitable for analysis of different amino acids by coupling with MS technologies62. The methods are reliable, fast with high sensitivity and reputability63.
Examples
Method validation for amino acids64.
Quantification of sulphur amino acids in aquatic invertebrates65.
for quantify amines and amino acids in human disease phenotyping65.
UHPLC-UV was applied for the analysis of total amino acid in infant formulas and adult nutritionals66.
Determination of Pesticides
Combination of UPLC-MS/MS is effective for determination of pesticides. The instrument technique provides highly accurate with less matrix result67.
Examples
for pesticide analysis in different fruit and vegetable63.
Analysis of residual pesticides and mycotoxins in cannabis68.
Pesticides analysis of vegetables by UPLC in combination with mass spectrometry69.
Conclusions
Ultra-Performance Liquid Chromatography (UPLC) provides much improvement over conventional HPLC. In fact, it has become the standard platform of HPLC. The main advantage is reduction of analysis time and solvent consumption. This is achieved by the use of small particle size and short column. An only drawback of UPLC could be high back pressure which can be decreased through increasing column temperature. Throughout UPLC technique is widely acceptable and offers significant improvement of speed, sensitivity and resolution compared with conventional HPLC.
Acknowledgement
Dr.RV thanks Dr.Ch.V.Rajasekhar, Scrips Pharma, Hyderabad, for his support.
Conflict of interest
Authors state no conflict of interest.
References
- Karayannis, M. I.; Efstathiou, C. E. Significant steps in the evolution of analytical chemistry – Is the today analytical chemistry only chemistry Talanta. 2012,102, 7-15. doi:10.1016/j.talanta.2012.06.003
CrossRef - Perkel, J. Advances in Analytical Chemistry: Processes, Techniques, and Instrumentation. 2017, pp.4-30. https://www.acs.org/content/dam/acsorg/membership/acs/benefits/extra-insights/analytical-chemistry-final.pdf.
- Thammana, M. A. Review on High Performance Liquid Chromatography (HPLC),Research & Reviews: Journal of Pharmaceutical Analysis, 2016, 5(2), 22-28.
- Narwate, B. M.;Ghule, P. J.; Ghule, A. V.; Darandale, A. S.; Wagh, J. G. Ultra Performance Liquid Chromatography: a New Revolution in Liquid Chromatography. Int. J. Pharm. Drug. Anal.,2014, 2(1), 25-34. http://www.ijpda.com/admin/uploads/vYTQr3.pdf.
- Cielecka-Piontek, J.; Zalewski, P.; Jelińska, A.; Garbacki, P. UHPLC: The greening face of liquid chromatography. Chromatographia. 2013, 76, 1429-1437. doi:10.1007/s10337-013-2434-6
CrossRef - William J. W. Partition Chromatography Revisited,IUBMB Life, 2001, 51, 329-330
CrossRef - Mimansha P.International Journal of Pharmacy and Pharmaceutical Research, 2018, 13 (4), 288-293.
- Coskun, O. Separation Tecniques: Chromatography. North Clin Istanbul. 2016, 3(2), 156-160. doi:10.14744/nci.2016.32757
CrossRef - Malviya, R.; Bansal, V.; Pal, O.P.; Sharma, P.K. High performance liquid chromatography: A short review, Journal of Global Pharma Technology. 2010, 2(5), 22-26.
- Seelam, S. C.; Priyanka, G.; Dhanalakshmi, K; Reddy, N. Switch from HPLC to UPLC: A novel achievement in liquid chromatography technique – A Review. Int. J. Pharm. Sci. Rev. Res. 2013, 21(1), 237-246.
- Sunil, A.; Anju, G.; Rajat, V. HPLC Detectors, Their Types and Use : A Review. Org.&Med. Chem. 2018, 6(5): 5556700. doi:10.19080/OMCIJ.2018.06.555700
CrossRef - Pramod, S. K.; Navnath, K. A.A brief review on ultra performance liquid chromatography.World J. of Pharm. Res., 2017,6(15), 407-422. DOI : 10.20959/wjpr201715-10136
- Patil, A. A review on ultra performance liquid chromatography. Asian J. Pharm. Technol. Innov.,2015, 3(10), 86-96.
- Patil, V. P.; Tathe, R. D.; Devdhe, S. J.; Angadi, S. S.; Kale, S. H. Ultra Performance Liquid Chromatography : A Review. Int. Res. J. Pharm.,2011, 2, 39-44.
- Modi, V.; Dubey, A.; Prajapati, P.; Basuri, T. A Review on Recent Advancement of LC-MS: Ultra High Pressure Liquid Chromatography -Mass Spectrometry (UHPLC-MS) and Its Applications. Int. J. Innov. Pharm. Sci. Res.,2016,4(5), 544-558.
- Devdhe, P. T.; Angadi, K. Ultra performance liquid chromatography: A review. Int. Res. J. Pharm.,2011, 2(6), 39-44.
- Palve, S. A.; Talele, S. G.; Chaudhri, G. A New Boon in Chromatography UPLC-A Review, Indo American J. of Pharm. Sci., 2015,2(3),676-683.
- Kumar, A.; Saini, G.; Nair, A.; Sharma, R. Review UPLC : A Preeminent Technique in Pharmaceutical Analysis. 2012, 69(3), 371-380.
- Pratima, N.; Shraddha, B.; Zibran, S. Review of Ultra Performance Liquid Chromatography and its applications. Int. J. Res. Pharm. Sci.,2013, 3, 19-40.
- Waters Corporation. Column Solutions Designed for UPLC Scientists. 2009.
- Taleuzzaman, M.; Ali, S.; Gilani, S. J.; Imam, S. S.; Hafeez, A. Ultra Performance Liquid Chromatography (UPLC) – A Review. Austin J. Anal. Pharm. Chem., 2015, 2(6), 1056-1060
- Gaikwad, P. V.; Sawant, S. D.;Ghante, M. R.; Munot, N. M. Ultra Performance Liquid Chromatography : A Recent Novel Development in Hplc. Pharm. Glob. Int. J. Compr. Pharm.,2010, 1(2), 1-3.
- Ding, S.; Schoenmakers, I.; Jones, K.; Koulman, A.; Prentice, A.; Volmer, D. A. Quantitative determination of vitamin D metabolites in plasma using UHPLC-MS/MS. Anal Bioanal Chem.,2010, 398(2), 779-789. doi:10.1007/s00216-010-3993-0
CrossRef - Yang, D.; Yang, X.; Yan, H. UPLC-MS/MS Determination of Twelve Ginsenosides in Shenfu Tang and Dushen Tang. Int. J. Anal. Chem.,2019, 6217125. doi:10.1155/2019/6217125
CrossRef - Wu, T.; Wang, C.; Wang, X.; Xiao, H.; Ma, Q.; Zhang, Q. Comparison of UPLC and HPLC for Analysis of 12 Phthalates. Chromatographia 2008, 68(910), 803-806.
CrossRef - Kamal, S.; Sharad, W. Step-up in liquid chromatography from HPLC to UPLC: A comparative and comprehensive review. The Pharma Innovation Journal2018, 7(8), 342-347
- Cotruţ, R.; Bădulescu, L. UPLC Rapid Quantification of Ascorbic Acid in Several Fruits and Vegetables Extracted Using Different Solvents. Agric. Sci. Procedia.,2016, 10, 160-166. doi:10.1016/ j.aaspro.2016.09.047
CrossRef - Sharma, D. K.; Kim, S. G.; Lamichhane, R.; Lee, K. H.; Poudel, A.; Jung, H. J. Development of UPLC Fingerprint with Multi-Component Quantitative Analysis for Quality Consistency Evaluation of Herbal Medicine “hyangsapyeongwisan.” J. Chromatogr. Sci.,2016, 54(4), 536-546. doi:10.1093/chromsci/bmv182
CrossRef - Yuk, J.; Patel, D. N.; Isaac, G.;Smith, K.; Wrona, M.; Olivos, H. J.; Yu, K. Chemical profling of ginseng species and ginseng herbal products using UPLC/QTOF-MS. J. Braz. Chem. Soc.,2016, 27(8), 1476-1483. doi:10.5935/0103-5053.20160189
CrossRef - Yang, Z. R.; Wang, Z. H.; Tang, J. F.;Yan, Y.; Yue, S-J.; Feng, W. W.; Shi, Z-Y.; Meng, X-T.; Peng, C.; Wang, C-Y.; Meng, D-L.; Yan, D. UPLC-QTOF/MSE and bioassay are available approaches for identifying quality fluctuation of xueshuantong lyophilized powder in clinic. Front. Pharmacol.,2018, 9, 1-11. doi:10.3389/fphar.2018.00633
CrossRef - Luo, Z.; Li, X.; Wang, L.; Chang, C.; Fu, Q. Development of UPLC-Q-TOF-MS coupled with cation-exchange solid-phase extraction method for the determination of ten pyrrolizidine alkaloids in herbal medicines. Anal. Sci.,2019, 35(12), 1317-1325. doi:10.2116/ analsci. 19P230
CrossRef - Cuyckens, F.; Borgmans, C.; Bockx, M.; Kembuegler, R.; Malderen, H. V.; Petit, D. UPLC and Online Radioactivity Detection of Metabolites. Waters2010, 1-6.
- Xie, G. X.; Ni, Y.; Su, M. M.;Zhang, Y. Y.; Zhao, Ai. H.; Gao, X. F.; Liu, Z.; Xiao, P. G.; Liu, Z.; Xiao, P. G.; Jia, W. Application of ultra-performance LC-TOF MS metabolite profiling techniques to the analysis of medicinal Panax herbs. Metabolomics2008,4(3), 248-260. doi:10.1007/s11306-008-0115-5
CrossRef - Klitgaard, A.; Iversen, A.; Andersen, M. R.; Larsen, T. O.; Frisvad, J. C.; Nielsen, K. F. Aggressive dereplication using UHPLC-DAD-QTOF: Screening extracts for up to 3000 fungal secondary metabolites. Anal. Bioanal. Chem.,2014, 406(7), 1933-1943. doi:10.1007/s00216-013-7582-x
CrossRef - Srivastava, S.; Kumar, P. R.; Mishra, S. K. Identification ofMetabolites through GC/LC-MS Processed Data using Different Reference Libraries and Their Comparison. J. Pharm. Biomed. Sci. 2016, 6(6), 363-368. doi:10.20936/jpbms/160254
- Evelyn, M. C. P.; Livia, M. C.; Ivana, C. R. L.; P. F. de Aguiar.; Sônia, S. C. Metabolite Fingerprinting and Profiling of the Medicinal Grass.J. Braz. Chem. Soc., 2018, 29(12), 2522-2534.
- Wren, S. A. C.; Tchelitcheff, P. Use of ultra-performance liquid chromatography in pharmaceutical development. J. Chromatogr. A.,2006, 1119(1-2), 140-146. doi:10.1016/j.chroma.2006.02.052
CrossRef - Punugoti, R. A.; Jupally, V. R. Development and Validation of New Rp-Uplc Method for the Quantitative Determination of Olanzapine in Tablet Dosage Form.Asian J. of Pharm. Clinical Res., 2013, 6, 178-181.
- Alasvand, S.; Kim, H-J.; Haley-Zitlin, V. UPLC-QTOF-MS Method for Identification of Mango Leaf Tea Metabolites. Curr. Dev. Nutr. 2020, 4, 1736-1736. doi:10.1093/cdn/nzaa065001
CrossRef - Wabaidur, S. M.; Kazi, M.; Alothman, Z. A. Development of a Stability Indicating UPLC-MS/MS Method for Rapid and Reliable Determination of Fenofibrate in Marketed Product (Lypanthyl 200M) and Human Plasma. J. Pharm. Drug Dev. 2013, 1(1), 1-6. doi:10.15744/2348-9782.1.102
CrossRef - Liu, H.; Ren, C.; Han, D. UPLC-MS/MS method for simultaneous determination of three major metabolites of mequindox in holothurian. J. Anal. Methods Chem.,2018, 2768047 doi:10.1155/2018/2768047
CrossRef - Dong, M. W.; Zhang, K. Ultra-high-pressure liquid chromatography ( UHPLC ) in method development. Trends. Anal. Chem.,2014, 63, 21-30. doi:10.1016/j.trac.2014.06.019
CrossRef - Ramadanty, W. T.; Arozal, W.; Louisa, M.; Soetikno, V.; Purbadi, S.; Priyanto, P. Efficient validated method of UPLC-MS/MS to determine curcumin in rat plasma and ovarium. J. Appl. Pharm. Sci.,2019, 9(1), 58-65. doi:10.7324/JAPS.2019.90109
CrossRef - Chen, L.; Weng, Q.; Ma, J. A new UPLC-MS/MS method validated for quantification of jervine in rat plasma and the study of its pharmacokinetics in rats. J. Anal. Methods Chem.,2019, 5163625. doi:10.1155/2019/5163625
CrossRef - Pattanaik, P.; Subrahmanyam, K. V. UPLC method development and validation for terconazole in active ingredient. Int. J. Pharma. Res. Heal Sci.,2015, 3(2), 22-26. http://pharmahealthsciences.net/pdfs/ volume3-issue2/4_Vol3_Issue2_Suppl1.pdf.
- Garg, R.; Singh, N.; Srinivas, K. S.; Deb, B.; Ahmed A. Uplc method development and validation for cefditoren pivoxil in active pharmaceutical ingredient. J. Appl. Pharm. Sci.,2011, 1(7), 149-153.
- Alam, M. A.; Al-Jenoobi, F. I.; Al-Mohizea, A. M. Rapid, Validated UPLC-MS/MS Method for Determination of Glibenclamide in Rat Plasma. Int. J. Anal. Chem.,2018,2569027. doi:10.1155/2018/2569027
CrossRef - Al-Tannak, N. F.; Hemdan, A.; Eissa, M. S. Development of a Robust UPLC Method for Simultaneous Determination of a Novel Combination of Sofosbuvir and Daclatasvir inHuman Plasma: Clinical Application to Therapeutic Drug Monitoring. Int. J. Anal. Chem., 2018, 6535816. doi:10.1155/2018/6535816
CrossRef - Ramakrishna, U. V.; Sunder, S. R.; Kumar, R. K,; Sinha, S. N.Method development and validation for rapid identification of epigallocatechin gallate using ultra-high performance liquid chromatography. PLoS One.,2020, 15(1), 1-11. doi:10.1371/ journal. pone.0227569
CrossRef - Ma, F, Cui, Q, Bai, G. Combining UPLC/Q-TOF-MS/MS with biological evaluation for NF-κB inhibitors in uyghur medicine althaea rosea flowers. Front Plant Sci.,2019, 9,1-9. doi:10.3389/fpls.2018.01975
CrossRef - Malejko, J.; Nalewajko-Sieliwoniuk, E.; Szabuńko, J.; Nazaruk, J. Ultra-high Performance Liquid Chromatography with Photodiode Array and Chemiluminescence Detection for the Determination of Polyphenolic Antioxidants in Erigeron acris L. Extracts. Phytochem Anal.,2016, 27(5), 277-283. doi:10.1002/pca.2626
CrossRef - De Nys, S.; Putzeys, E.; Vervliet, P. A novel high sensitivity UPLC-MS/MS method for the evaluation of bisphenol A leaching from dental materials. Sci Rep.,2018, 8(1), 1-7. doi:10.1038/s41598-018-24815-z
CrossRef - Römpp, A.; Karst, U. Current trends in mass spectrometry imaging mass spectrometry imaging. Anal. Bioanal. Chem.,2015, 407(8), 2023-2025. doi:10.1007/s00216-015-8479-7
CrossRef - Kancherla, P.; Alegete, P.; Keesari, S. Stability-Indicating RP-UPLC Method Development and Validation for the Process Related Impurities of Nebivolol and Structural Characterization of Its Forced Degradation Products by LC-MS/MS. Br J Pharm Res.,2016, 14(6), 1-13. doi:10.9734/bjpr/2016/31354
CrossRef - Rashmitha, N.; Sharma, H. K.; Mukkanti, K. Avalidated stability-indicating HPLC method for the determination of impurities in florfenicol. Int. J. Res. Pharm. Biomed. Sci.,2012, 3(3), 1338-1345.
- Chilukuri, M.; Hussainreddy, K.; Narayanareddy, P.; Venkataramana, M. A.Validated stability-indicating UPLC method for the determination of impurities in Maraviroc. J. Chromatogr. Sci.,2014, 52(7), 609-616. doi:10.1093/chromsci/bmt085
CrossRef - Kumar, N.; Sangeetha, D.; Kalyanraman, L. Determination of degradation products and process related impurities of asenapine maleate in asenapine sublingual tablets by UPLC. IOP Conf Ser Mater. Sci. Eng.,2017, 263 (2), 022029. doi:10.1088/1757-899X/263/2/022029
CrossRef - Prakash, L.; Malipeddi, H.; Subbaiah, B. V.; Lakka, N. S. Impurity profiling and a stability-indicating UPLC method development and validation for the estimation of related impurities of halobetasol propionate in halobetasol propionate 0.05% (w/w) cream. J. Chromatogr. Sci.,2015, 53(1), 112-121. doi:10.1093/chromsci/bmu027
CrossRef - Ning, Z. W.; Zhai, L. X.; Peng, J. Simultaneous UPLC-TQ-MS/MS determination of six active components in rat plasma: Application in the pharmacokinetic study of Cyclocarya paliurus leaves. Chinese Med. (United Kingdom).,2019, 14(1), 1-11. doi:10.1186/s13020-019-0248-7
CrossRef - Lee, K. M.; Jeon, J. Y.; Lee, B. J.; Lee, H.; Choi, H. K. Application of metabolomics to quality control of natural product derived medicines. Biomol Ther.,2017, 25(6), 559-568. doi:10.4062/biomolther.2016.249
CrossRef - Yun, Q.; Liu, Q.; He, C. UPLC-Q-TOF/MS characterization, HPLC fingerprint analysis and species differentiation for quality control of Nigella glandulifera Freyn et Sint seeds and Nigella sativa L. seeds. Anal. Methods.,2014, 6(13), 4845-4852. doi:10.1039/c4ay00775a
CrossRef - Shen, X.; Ma, J.; Wang, X.; Wen, C.; Zhang, M. Toxicokinetics of 11 Gelsemium Alkaloids in Rats by UPLC-MS/MS. Biomed. Res. Int.,2020, 8247270, 1-13. doi:10.1155/2020/8247270
CrossRef - Pan YL, Li J, Li X, Chen JW, Bai GG. Determination of free amino acids in Isatidis Radix by HILIC-UPLC-MS/MS. Bull. Kor. Chem. Soc., 2014, 35(1), 197-203. doi: 10.5012/bkcs.2014.35.1.197.
CrossRef - Thera, J. C.; Kidd, K. A.; Dodge-Lynch, M. E.; Bertolo, R. F. Quantification of sulphur amino acids by ultra-high performance liquid chromatography in aquatic invertebrates. Anal. Biochem.,2017, 539, 158-161. doi:10.1016/j.ab.2017.10.022
CrossRef - Ahmetaj-Shala, B.; Olanipekun, M.; Tesfai, A.;MacCallum, N.; Kirkby, N. S.; Quinlan, G. J.; Shih, C-C.; Kawai, R.; Mumby, S.; Paul-Clark, M.; Want, E-J.; Mitchell, J. A. Development of a novel UHPLC-MS/MS-based platform to quantify amines, amino acids and methylarginines for applications in human disease phenotyping. Sci. Rep.,2018, 8(1), 13987. doi:10.1038/s41598-018-31055-8
CrossRef - Jaudzems, G.; Guthrie, J.; Lahrichi, S.; Fuerer, C. Total amino acids by UHPLC-UV in infant formulas and adult nutritionals, first action 2018.06. J AOAC Int.,2019, 102(5), 1574-1588. doi:10.5740/jaoacint.19-0036
CrossRef - Pizzutti, I. R.; Dias J V.; De Kok, A.; Cardoso, C.D.; Vela, G. M. E. Pesticide residues method validation by UPLC-MS/MS for accreditation purposes. J. Braz. Chem Soc.,2016, 27(7), 1165-1176. doi:10.5935/0103-5053.20160012
CrossRef - Shah, D.; Meruva, N.; Cleland, G. Multiresidue Analysis of Pesticides in Fruits and Vegetables UsingUPLC-MS/MS. Waters 2015. https://www.waters.com/webassets/cms/library/docs/720006039en.pdf
- Xu, F.; Yu, J. Y.; Wang, Q. S.; Fu, Y.; Zhang, H.; Wu, Y. L. Simultaneous determination of 25 pesticides in Zizania latifolia by dispersive solid-phase extraction and liquid chromatography-tandem mass spectrometry. Sci. Rep.,2019, 9(1), 1-8. doi:10.1038/s41598-019-46523-y
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









