Antibacterial Activity of Environmentally Sustainable Polyurethane Based Composites from Castor Oil
Department of Chemistry, Women Christian College, Nagercoil, Tamil Nadu, India, Affiliated to Manonmaniam Sundaranar University, Abishekapatti, Tirunelveli- 627012, Tamil Nadu, India.
Corresponding Author E-mail: subasjeba@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370334
Article Received on : 22-Apr-2021
Article Accepted on : 22-Jun-2021
Article Published : 28 Jun 2021
In this present work, soft and hard antibacterial polyurethane sheets of different composition have been synthesized from castor oil based polyurethane and which was reinforced with natural and synthetic fibres such as jute, sisal, hemp and glass. The activity of polyurethane sheets and selected antibiotics was evaluated against four bacterial pathogens including Staphylococcusaureus, Streptococcus mutans, E.coli, Pseudomonas aeruginosausing modified disc diffusion method. Among the ten samples, hard polyurethanes displayed potential activity against bacterial pathogens E.coli and Streptococcus mutans. Polyurethane sheets showed the highest activity against E.coli which is comparable with zone of inhibition exhibited by streptomycin. Further studies are needed to improve the polyurethane sheets for medical applications.
KEYWORDS:Antibacterial; Castor Oil; Disc Diffusion; Pathogens; Polyurethane Sheets; Streptomycin
Download this article as:| Copy the following to cite this article: Jeba D, Sheeja K. R. Antibacterial Activity of Environmentally Sustainable Polyurethane Based Composites from Castor Oil. Orient J Chem 2021;37(3). |
| Copy the following to cite this URL: Jeba D, Sheeja K. R. Antibacterial Activity of Environmentally Sustainable Polyurethane Based Composites from Castor Oil. Orient J Chem 2021;37(3). Available from: https://bit.ly/2T6vU3G |
Introduction
Increasing world population demands increasing amount of chemicals, materials, polymers for daily usage. Most of the polymers and chemicals are derived from fossil resources but the major problem of using this fossil based material is undesirable environmental problem, frequent oscillation in oil price and limited fossil resources (Wang et al., 2020). Bio-based materials are now gaining considerable attention in the promotion of sustainable chemistry in the manufacturing of materials by its substitution of petroleum-derived raw materials in the production of different products needed in various industries (Corcueraet al., 2010). These bio-based materials are a great substitute to fossil based materials due to renewable feedstock use, easy degradation, easy accessible source and use of low energy for production (van der Meer, 2017). Vegetable oils from both plants and animals (fish oils) are considered to be the most important form of renewable sources. (Meier et al., 2007; Xia, Larock, 2010). Compared to other bio-based materials, Vegetable oils are very cheap, most abundant, renewable natural resources available in large quantities from various oilseeds, such as castor, palm, linseed, soya bean, coconut, sunflower, canola oils (Javniet al.,2003).
Multidrug resistant microbial strains and the strains with reduced susceptibility to antibiotics are increasingcontinuously. This leads to the non selective use of broad-spectrum antibiotics, organ transplantation, immunosuppressive agents, and intravenous catheters. In the hospital environment, they are the cause of chronic, nosocomial and medical device-related infection (zohraet al., 2018). Both gram positive and gram negative bacteria can form Biofilm on medical devices, but the most common forms are Enterocccusfaecalis, Staphylococcus aureus, Streptococcus sps, E.coli, Klebsiellapneumoniae, Proteussps and Pseudomonas aeruginosa (Chen et al.,2013). They cause prosthetic heart valve infections, catheter biofilm infections, hospital-acquired, surgical site and blood stream infections (Pahariket al., 2016; Khan et al.,2008). The main purpose of this study was to develop a antibacterial polyurethane sheets for medical applications.
Materials and Methods
Materials
Pure castor oil was purchased from a local market in Tholayavattam. 4,4’-methylene diphenylisocyanate for polyurethane sheet preparation and the catalyst dibutylindilaurate and polyethylene glycol and methyl ethyl ketone were obtained from Madras Scientific Supplies. Synthetic fibres purchased from Siya Chemicals and natural fibres collected from the surroundings and prepared by myself.
Preparation of soft polyurethane sheets
Castor oil based polyurethane was obtained by reacting 4,4’-methylene diphenylisocyanate with castor oil has dissolved in methyl ethyl ketone at mole ratio 1:4. Here unsaturated fatty acid in castor oil reacted with additional hydroxyl group from methyl ethyl ketone, castor oil could be converted to polyol. Transesterification reaction of castor oil was allowed to carried out in a three-necked round bottomed flask equipped with a reflux condenser at 60oC for 45minutes under nitrogen atmosphere to avoid any oxidation reaction (Ibrahim et al., 2015). Then it was mixed well with few drops of catalyst dibutyltindilaurate which catalyst polymerization reaction. For synthesizing fibre incorporated sheet 0.001m length fibre (sisal, jute, hemp and jute) was mixed separately with polyurethane mixture and poured in a glass mould to cast a neat sheet. The polyurethane sheets were allowed to cure for 2h in flat surface.
 |
Scheme 1: Synthesis of castor oil based Polyurethane. |
Preparation of hard polyurethane sheets
Hard polyurethane sheet and fibre reinforced hard polyurethane sheets were prepared by the above method but at the time of adding catalyst additionally polyethylene glycol was added.
Antimicrobial assay
Preparation innoculum
Antibacterial activity of polyurethane sheet were tested against gram positive bacteria Staphylococcusaureus (MTCC 737), Streptococcus mutans (MTCC 890) and gram negative bacteria E.coli (MTCC 118), Pseudomonas aeruginosa (MTCC 1688),were purchased from Microbial Type Culture Collection and Gene Bank (MTCC)Chandigarh. The bacterial strains were pre-cultured in Muller Hinton broth over night in a rotary shaker at 37oC. Afterward, each strain was adjusted at a concentration of 108 cells/ml using 0.5 McFarland turbidity standards to yield a bacterial suspension of 1.5×108 cfu/ml. (Bhalodia and Shukla, 2011).
Antibacterial Test
Modified disc diffusion method was used to screen antibacterial activity of polyurethane sheets. Molten cooled Muller hinton agar plates were swabbed with Pathogenic Bacteria culture. Finally, synthesized polyurethane sheets were cut into pieces with the weight of 0.05g under sterile condition. Then the small pieces of sheets were placed on the surface of Mullar-Hinton medium and the plates were kept for incubation at 37°C for 24 hours. At the end incubation, inhibition zones were examined around the disc and measured with transparent ruler in millimetres (Kohneret al., 1994; Mathabeet al., 2006). here, standard streptomycin disc used as positive control and sterile distilled water used as negative control.
Result and Discussion
Polyurethane sheet preparation
Soft and hard polyurethane sheets and fibre reinforced sheets were successfully synthesized. During the formation of polyurethane isocyanate was completely converted to amide which is non toxic and improves the polyurethane sheet to become more biocompatible. Addition of fibres to polyurethane composites increase the physical and mechanical property of the polyurethane sheets which makes the sheets more strong and flexible (Sangeethaet al, 2015 and Merliniet al, 2011).
Antibacterial assay
Evaluation of antibacterial activity of polyurethane sheets were determined by modified disc diffusion method against different microorganisms. These organisms are regularly encountered in infectious diseases. All the polyurethane sheets have gd antibacterial activity against gram negative bacteria E.coli expect PUSR1G, PUHR1, PUHR1S, all the other samples showed antibacterial activity against Streptococcus mutans. Sample PUHR1 alone showed activity against Staphylococcusaureus. Comparison between soft and hard polyurethane sheets, hard polyurethane sheets showing good antibacterial potential against pathogens. Better antibacterial activity was seen in fibre reinforced polyurethane sheets than non-fibre included sheets. These results were shown in Table:1. Unexpectedly, no decrease in viability in P.aeruginosawas detected for all polyurethane composites (Figure:1 A – D).
Table 1: Antibacterial activity of polyurethane sheets
|
Samples |
Sample code |
Zone of inhibition (mm in diameter) |
|||
|
Staphylococcus aureus (G+) |
Streptococcus mutans (G+) |
E.coli (G-)
|
Pseudomonas aeruginosa (G-) |
||
|
Soft polyurethane sheets |
PUSR1 |
– |
12 |
10 |
– |
|
PUSR1J |
– |
13 |
11 |
– |
|
|
PUSR1S |
– |
11 |
12 |
– |
|
|
PUSR1H |
– |
9 |
8 |
– |
|
|
PUSR1G |
– |
– |
10 |
– |
|
|
Standard control |
Positive control |
14 |
23 |
21 |
19 |
|
Negative control |
– |
– |
– |
– |
|
|
Hard polyurethane sheets |
PUHR1 |
7 |
– |
9 |
– |
|
PUHR1J |
– |
10 |
14 |
– |
|
|
PUHR1S |
– |
– |
10 |
– |
|
|
PUHR1H |
– |
12 |
12 |
– |
|
|
PUHR1G |
– |
13 |
14 |
– |
|
|
Standard control |
Positive control |
16 |
23 |
13 |
17 |
|
Negative control |
– |
– |
– |
– |
|
Keywords:Positive control (Streptomycin), Negative control (sterile distilled water), “-“ No Zone, mm (Millimetre), G+ (Gram Positive Organism), G- (Gram Negative Organism).
 |
Figure 1: (A) Antibacterial activity of polyurethane sheets against Staphylococcus aureus |
 |
Figure 1:(B) Antibacterial activity of polyurethane sheets against Streptococcus mutans |
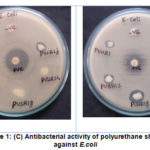 |
Figure 1: (C) Antibacterial activity of polyurethane sheets against E.coli |
 |
Figure 1:(D) Antibacterial activity of polyurethane sheets against Pseudomonas aeruginosa. |
Conclusion
Fibre reinforced polyurethane sheets were prepared by adding castor oil and 4,4’-methylene diphenylisocyanate and natural and synthetic fibres. These polyurethane sheets possess good antimicrobial activity, suggesting that such polyurethane sheets could be a good candidate material applied in tissue engineering, wound dressings, biomedical devices and food packing bags, while simultaneously reducing the bacterial colonization. Further studies must be needed to confirm their biocompatibility and cytotoxicity for their uses in biomedical and industrial applications.
Acknowledgement
We are grateful and thankful to the following organizations to carry out the analysis work. Inbioticslab for antibacterial studies and SEM Instrumentation Centre, Alagappa University, Karaikudi
Conflict to Interest
The authors declare that they have no conflict of interest.
References
- Kohner,P.C; Rosenblatt,J.E;Cockerill, F.R; Comparison of agar dilution, broth dilution and disk diffusion testing of Ampicillin against Haemophilus spp. by using in house and commercially prepared media J. Clin. Microbiol.1994,32, 1594 -96.
CrossRef - Ibrahim, S; Ahmad, A; Mohamed, N.S; synthesis and characterization of castor oil-based polyurethane for potential application as host in polymer electrolytes, Bull.Mater.Sci.,2015, 38(5),1155-1161.
CrossRef - Mathabe, M.C;Nikolova,R.V.; Lall,N; Nyazema,N.Z; Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province, South Africa, Journal of Ethnopharmacology, 2006, 105,286-293.
CrossRef - Sangeetha, N.J; Malar Retna,A; Preparation of Chain Extended Polyurehane and its Composites Based on Castor Oil and Coir Fiber, Green Chemistry and technology letters,2015,1(1) 67-70.
CrossRef - MerliniClaudia,ValdirSoldi,GuilhermeM.O.Barra, Influence of fiber surface treatment and length on physico-chemical properties of short random banana fiber-reinforced castor oil polyurethane composites, Polymer Testing,2011,30 (8) 833-840.
CrossRef - Bhalodia,N. R;Shukla,V. J; Antibacterial and antifungal activities from leaf extracts of Cassia fistula: an ethnomedicinal plant. J. Adv. Pharm. Technol. Res.2011 2, 104–109. doi: 10.4103/2231-4040.82956.
CrossRef - WangZhongkai , Mitra S.GanewattaChuanbingTang, Sustainable polymers from biomass: Bridging chemistry with materials and processing,progress in polymer science, 2020, 101, 101197, https://doi.org/10.1016/j.progpolymsci.2019.101197
CrossRef - Van der Meer, Yvonne , Sustainable bio-based materials: opportunities and challenges, proceedings of the biotech france 2017 international conference, paris-france, June 2017,28-30,.
CrossRef - Corcuera,M. A; Rueda, L; B. Fernandez d’Arlas, A. Arbelaiz, C. Marieta, I. Mondragon, and A. Eceiza, “Microstructure and properties of polyurethanes derived from castor oil,” Polym. Degrad. Stab.,2010, 95(1),2175–2184,.
CrossRef - Javni,W; Zhang,Z.S; J. Petrovic, Appl. Polym. Sci. 2003,88 (2003),2912–2916.
CrossRef - Meier, M.A; Metzger, J.O;Schubert ,U.S; Plant oil renewable resources as green alternatives in polymer science. ChemSoc Rev; 2007,3611,1788-1802.
CrossRef - ZhraKhatoon,Christoper D. Mctiernan, Eric.J.Suuronen, Thien-FahMah and Emilio I Alacron, bacterial Biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon, 2018,74(12).
CrossRef - M.Chen, Q.Yu, H. Sun novel strategy for the prevention and treatment of biofilm related infections. Int.J.Ml.Sci.2013,14(9) ,18488-18501.
CrossRef - A.E. Paharik, A.R. Horswill, the staphylococcal biofilm: adhesion, regulation and host response. Microbil.Spectr. 2016, 4(2).
CrossRef - S.U. Khan Rehman, A. Alim, B.Sultan, S. Sultan, Infection in orthopedic implant surgery, its risk factors and outcome. J.Ayub.Med.Coll.2008,20(1),23-25.

This work is licensed under a Creative Commons Attribution 4.0 International License.










