Vibrational Frequencies of Dichlorine Monoxide: A Mathematical Model
N. Srinivas and J. Vijayasekhar*
Department of Mathematics, GITAM Deemed to be University, Hyderabad, India.
Corresponding Author E-mail: vijayjaliparthi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370133
Article Received on : 10-Nov-2020
Article Accepted on : 15-Jan-2021
Article Published : 02 Feb 2021
Determinedstretching vibrational frequencies of a triatomic molecule, i.e..Dichlorine Monoxide (Cl2O) upto third overtone by Lie algebraic method.Fundamental vibrational wavenumbers calculated by Lie algebraic model are compared with available experimental values.
KEYWORDS:Dichlorine Monoxide(Cl2O); Lie algebraic method; Vibrational Spectra
Download this article as:| Copy the following to cite this article: Srinivas N, Vijayasekhar J. Vibrational Frequencies of Dichlorine Monoxide: A Mathematical Model. Orient J Chem 2021;37(1). |
| Copy the following to cite this URL: Srinivas N, Vijayasekhar J. Vibrational Frequencies of Dichlorine Monoxide: A Mathematical Model. Orient J Chem 2021;37(1). Available from: https://bit.ly/36yfN2d |
Introduction
Calculation of fundamental and higher overtones of molecule useful to understand molecular structure. We applied Lie algebraic method to calculate vibrational frequencies (wavenumbers) of molecule.This mathematical approach helps to calculate vibrational frequencies in higher overtones and moreover useful to compare in-vivo experimental results. The vibrational wavenumbers of the molecule are analyzedby means of traditional approaches, Dunham series expansion and Potential function approach. The noticabledemerit of these approaches are, requirement of huge experimental data to fit the parameters. We choose this method,as it overcomes this difficulty. In this method, vibrational frequencies are calculated using Hamiltonian operator[1-6].
Lie Algebraic Method for Cl2O
Dichlorine Monoxide (Cl2O) contains two chlorine (Cl) atoms and one oxygen atom with two Cl – O bonds. Symmetry point group is C2v with symmetry species A1 (Cl – O symmetric stretching), .B2 (Cl – O asymmetric stretching) and A1 (Cl-O-Cl bending). This framework explains the stretching bonds in Cl2O molecule using two Lie algebras i.e.( U1(2) and U2(2))
Below mentioned two chains, explains the molecular dynamical group.
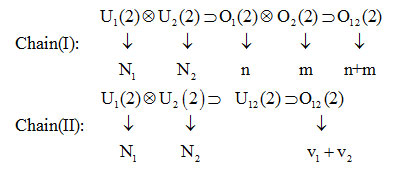
chain (I) represents the local and chain (II) represents the normal couplings. Quantum numbers in the chain (I) related to algebras, indicated by n, mand in the chain (II) are v1 , v2. Vibron numbers N1 and N2 are taken to be constants and are related to the maximum number of bound states of two Morse oscillators. Bending vibrations can be represented with the algebra U3(2) and combined with the algebra U1(2) U2(2) associates with the coupled stretching vibrations.Hamiltonian operator [9,10]
( stretching vibrations) of this molecule :
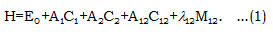
In equation (1), A1, A2, A12 and λ12 are the algebraic parameters, which are calculated from experimental spectra. The local stretching vibrations are denoted by (v1) and (v3), where as (v2) representing bending vibrations for Cl2O. The Hamiltonian in the equation (1) can be diagonalized to get vibrational frequencies. Where in the equation (1), C1, C2 are invariant operators of the uncoupled bonds with eigenvalues -4 (Ni vi – vi2 )(i =1,2) and Cij (i =1,2) operator for coupled bonds is diagonal with matrix elements
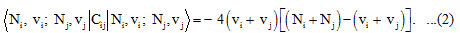
The Majorana operator Mij ( i =1,2)in the equation (1), describes local mode interactions in pairs and provide diagonal and non-diagonal matrix elements
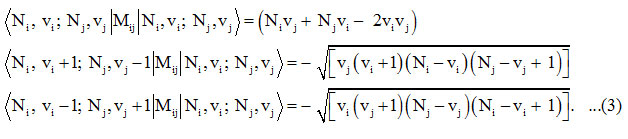
The Hamiltonian matrix for the local modes is given by
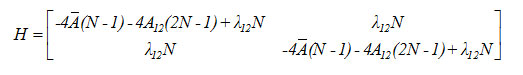
Results and Discussion
The parameters for stretching bonds of the water molecule in equations (1), (2) and (3) as Ni = N, Ai = A, Aij = A12, λij = λ12 ( i =1,2). The vibron number Ni ( i =1,2). calculated from the relation [9]
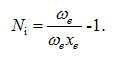
Here ωe and ωexe are the spectroscopic constants [11]. The value of the parameter is obtained by using the energy equation [9,10] for the single-oscillator fundamental mode, which can be calculated from
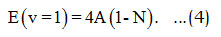
Using the equation (4), Ā can be evaluated as,
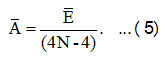
In equation (5), Ā and Ē are the average values of the algebraic parameters A’s and E’s. Theinitial value of λ12 , estimate by the relation [10],
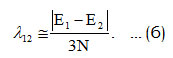
In order to calculate accurate results, a numerical regression fitting method is used to fit the algebraic parameters A, λ12 starting from the values as given by equations (5) and (6). The initial value of A12 assumming as zero.
Table 1: Stretching vibrational frequencies (in cm-1) of Cl2O
|
vib. quantum no. |
observed [11] |
calculated |
|
(1 0 0) |
639 |
638.274 |
|
(0 0 1) |
686 |
686.932 |
|
(2 0 0) |
– |
1245.407 |
|
(0 0 2) |
– |
1357.652 |
|
(3 0 0) |
– |
1911.094 |
|
(0 0 3) |
– |
2043.086 |
|
(4 0 0) |
– |
2537.335 |
|
(0 0 4) |
– |
2687.320 |
|
N= 154, A= -1.9091, A12 = 0.9328,=0.1863 |
||
Conclusion
Stretching vibrational frequencies of Cl2O upto third overtone are calculated and compared with experimental data in fundamental mode.Vibrational frequencies (stretching) of Cl2O are in close agreement with experimental values in fundamental mode.
References
- Iachello, F.Chem. Phys. Lett. 1981, 78(3),581-585.
CrossRef - Iachello, F. ; Levine, R. D. J. Chem. Phys. 1982, 77, 3046-3055.
CrossRef - Frank, A.; Iachello, F.; Lemus, R.Chem. Phys. Lett.1986, 131(4-5), 380-383.
CrossRef - Karumuri, S. R.; Vijayasekhar, J.;Sreeram, V.; Uma Maheswara Rao, V.; Basaveswara Rao, M. V. J. Mol. Spectrosc. 2011, 269, 119-123.
CrossRef - Karumuri, S. R.;Srinivas, G.;Vijayasekhar, J.; Rao, V. U. M.;Srinivas, Y.; Sunil Babu, K.; Kumar, V. S. S.;Hanumaiah, A.Chin. Phy. B. 2013, 22(9), 090304(1-8).
CrossRef - Karumuri, S.R.; Sravani, K.G.; Vijayasekhar, J.; Reddy, L.S.S. Acta Phys. Pol. A. 2012, 122 (1), 49 52.
CrossRef - Balla, M.R.; Jaliparthi, V.S. Mol. Phys. 2020. DOI: 10.1080/00268976.2020.1828634
CrossRef - Frank, A.; Lemus, R.; Bijker, R.Ann. Phys.1996, 252,211-238.
CrossRef - Iachello, F.; Oss, S. J. Mol. Spectrosc. 1990, 142(1), 85-107.
CrossRef - FENG, Zhaochi.; XIONG, Guang.; YANG, Qihua.; XIN, Qin.; LI, Can.Chin. Sci. Bull. 1999, 44(21), 1961-1964.
CrossRef - Irikura, K.K. J. Phys. Chem. Ref. Data.2007, 36 (2), 389-398.
CrossRef - Shimanouchi, T. Tables of Molecular Vibrational Frequencies Consolidated Volume I, National Bureau of Standards, 1972, 1-160.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









