The Impact of Microwave Irradiation Reaction in Medicinal Chemistry: A Review
Department of Chemistry, Raja Peary Mohan College, West Bengal, India.
Corresponding Author E-mail: ashupal33@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370101
Article Received on : 22-Jan-2021
Article Accepted on :
Article Published : 15 Feb 2021
The present review collects an update of the reactions in the area of medicinal chemistry using microwave irradiation. This review come up with an overview of most salient reactions performed under microwave irradiation in the field of drug discovery. Moreover, chemists are preferring to use this reaction rapidly in the academic as well as pharmaceutical laboratory during their drug discovery and making library of compounds. This reaction is much greener using less amount and readily recyclable solvents or sometimes reaction process without solvents and product become much cleaner, often yields are better than the conventional heating. Microwave irradiation is now very robust instrument used in company in the field of drug discovery, due to reduce the reaction time from hour to minute or even second and efficiently creation of compound libraries through combinatorial methodology associated with drug discovery so that new therapeutic agents bring to the market quicker. Hopefully, we will observe in the future the use of microwave irradiation drugs for the patients and this technology will utilize increase in number extensively in the field of medicinal chemistry. As this is an exceedingly rapid evolution area, this review offers significant expertise to the interested readers.
KEYWORDS:Conventional Method Robust Instrument; Drug Discovery; Microwave Irradiation
Download this article as:| Copy the following to cite this article: Pal A, Gayen K. S. The Impact of Microwave Irradiation Reaction in Medicinal Chemistry: A Review. Orient J Chem 2021;37(1). |
| Copy the following to cite this URL: Pal A, Gayen K. S. The Impact of Microwave Irradiation Reaction in Medicinal Chemistry: A Review. Orient J Chem 2021;37(1). Available from: https://bit.ly/2OD5AeB |
Introduction
Microwave is an electromagnetic radiation. In the electromagnetic spectrum the microwave radiation is located between IR and Radio wave. Microwave has wave lengths 1 mm – 100 km, frequencies 300 GHz – 3 KHz and energy 1.24 x 10-3 – 1.24 x 10 -6 eV.1,2
Table 1: Wave length, frequency and energy of electromagnetic radiation: 1 eV = 3.8 x 10 -23 KCal
|
S.No |
Radiation type |
Wave length |
Frequency |
Energy |
|
|
1 2 3 4 5 |
Gamma rays X-rays Far Ultraviolet rays Ultraviolet rays Visible rays |
<10 picometer 0.01-210 nm 10-200 nm 200-400 nm 400-800 nm |
1019 Hz 3×1019 – 3×1016 Hz 3×107 – 1.5×106 GHz 1.5×106 – 7.5×105 GHz 7.5×105 – 3.7×105 GHz |
>100 KeV 102KeV-124 eV 124 – 6.2 eV 6.2 – 3.1 eV 3.1 – 1.5 eV |
Ionizing radiation |
|
6 7 8 9 10
|
Near IR IR Far IR Microwave Microwave and Radiowave
|
0.8- 2.5 m 2.5- 15 m 15-1 mm 1mm -1 m 1 mm to 100 km |
3.7×105 – 1.2×105 GHz 1.2×105 – 2.0×104 GHz 2.0×104– 3.0×102 GHz 7.5×105 – 3.7×105 GHz 300 GHz – 3 KHz |
1.5 – 0.5 eV 0.5 – 0.08 eV 0.08 – 1.24×10-3 eV 1.24×10-3– 1.24×10-6 eV 1.24×10-3–1.24×10-11 eV |
Non-Ionizing radiation |
These are non-ionizing invisible short wave length radiation travel with the velocity of light. Direct chemical reaction like UV-Visible radiations in photochemical reaction microwave is not sufficiently energetic to cleave C-C bond to initiate chemical reaction. But surprisingly microwave radiated reaction is faster than that conventional chemical reaction. It was well known that microwaves heated water quickly. In organic synthesis microwave radiations have made its position as a non-conventional energy source as an alternative source of energy.
Microwaves are produced in magnetron. In 1920s it was discovered by an American engineer A. W. Hull and in the 1940s cooking food using microwaves irradiation was invented by accident by another American engineer Percy Spencer. When he was working on active radar set he observed that a peanut chocolate bar in his pocket started to melt. He understands that radar had helped to melt his chocolate bar. Then popcorn was the first cooked deliberately with Spencer’s microwave, and egg was the second experiment that blows up in the face when one experimenter worked on it. In 1947, for food processing microwave appliances “Radarange” come on to the market and in the 1950s both domestic and mercantilegad get started to appear for boiling, grilling, heating, roasting and making of foods. First microwave oven was launched in the 1955s by Tappan but in the 1970s and 1980s frequently used domestic microwave ovens come about. In 1970s, the building of the microwave generator was improved and simplified and the price of the domestic used microwave oven drop dramatically. After that the oven chamber grill changes significantly higher or bigger size to smaller according to demand. Use of microwave irradiations in organic synthesis (MIOS) has made its position as a non-conversional energy source. In 1986 the first article was published related to MIOS.
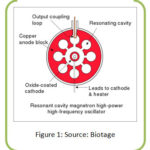 |
Figure 1: Source: Biotage. |
Magnetron consists of a heated cathode a solid metal rod acts at the center and a ring shaped anode having holes called resonant cavities and a powerful magnet whose magnetic field surrounding the cathode. From the heated cathode energized electrons from cathode try to move towards anode in a straight line, but due to presence of strong magnetic field, the moving electrons are encountered both by the electric and magnetic fields and the electron experience a force to follow a curved path and move fast around the space between the anode and cathode. As the electron nip past the cavities, the cavities resonate and emit microwave radiation. The electron pass the cavities, it transfers energy to the cavity and making cavity resonate like someone blowing flute on the open end produces sound to generate microwave wave instead of sound wave.
At present, in chemical reactions two types of heating was used:
Conventional Heating
Heating in this method is slow, non-selective, and less dependent and the outer surface is heated first then heated inside the reaction system. Heat is introduced into the reaction solvents, reagents, and substance come after the walls of the vessel.
Microwave Heating
In the beginning microwave-assisted synthesis was observed that rate of heating is faster and selective absorption by polar compounds and sometimes altered product distributions contrast to conventional heating experiments by the use of oil bath have led to speculation on the existence of so-called “specific” or “non-thermal” microwave effects. When a synthesis accomplished under MW irradiation was initiate completely different results from that of the convention-ally heated with the identical conditions. According to most of the chemists the rate of amplification was observed during the MW irradiation is absolutely kinetic or thermal effect.
 |
Figure 2: Source: Biotage Left image = oil bath, both taken after 1 min of heating, right image = microwave heating. |
Unlike the high energy radiations like-γ-rays, X-rays, UV-Visible radiations, MW radiations are non-ionizing radiations as they are not energetic enough to ionize an atom or a molecule. So MW cannot cleave a chemical bond to initiate a chemical reaction directly unlike photochemical reactions where UV-Visible radiations are used to initiate reaction.
The given temperature of a reaction system is able to reach quickly by the microwave irradiation with a very short induction period as a result the reaction reaches the desired temperature quickly and the reaction starts immediately, which is beneficial in cases where the catalyst/reagents is not stable. The microwave heats the reaction system direct and uniform way, with respect to conventional heating where the glass is heated first and in turn the glass heats the solution.
In microwave reactions the vials are used as sealed and reactions are carried out in closed systems which means in addition to temperature, reactions are carried out under increased pressure and the reaction is allowing to be heated high up the boiling point of the solvent being used therefore reaction time is reduced compared to classical procedure. Reactions under microwave conditions are much cleaner with easy work up improved yield with less side effects.
Table 2: Frequency of
|
Radiation type |
Frequency (MHz) |
Quantum energy (ev) |
Bond type |
Bond energy (ev) |
|
Gamma rays |
3.0×1014 |
1.24×106 |
C-C single bond |
3.61 |
|
X-rays |
3.0×1013 |
1.24×105 |
C=C double bond |
6.35 |
|
Ultraviolet |
1.0×109 |
4.1 |
C-O single bond |
3.74 |
|
Visible light |
6.0×108 |
2.5 |
C-O double bond |
7.71 |
|
Infrared light |
3.0×106 |
0.012 |
C-H bond |
4.28 |
|
Microwave |
2450 |
0.0016 |
OH bond |
4.80 |
|
Radiofrequencies |
1 |
4.0×10-9 |
Hydrogen bond |
0.04-0.44 |
Interaction of microwave with material
Microwave interacts with substances depending on the nature of the substance and the type of interactions may be reflection, transmission or absorption. On irradiation by microwave a metal strip generates spark on the surface of the metal. An electric conductive material reflects MW. Whereas, insulators, which are contemplated as substances with high dielectric properties like quartz, glass or polytetrafluoroethylene allows the transmission of most of the waves causing no heat. But the lossy dielectrics, which are substances that show dielectric losses and materials having permanent dipole moment, absorb most of the incident microwaves and convert them into heat. Only polar molecule can absorb MW radiation and get heated whereas the non-polar compounds do not absorb MW and are transparent to MW i.e., substance having permanent or induced dipole moment are microwave active.
How does heat generate by absorbing MW radiation
Microwaves interrelate straight with the compounds of the whole reaction mixture cause to a quick jump up in the temperature. The process is not restricted by the thermal conductivity of the reaction tube, the consequence is an instantaneous confine super heating of any material that will respond to either dipole orient or ionic conductivity. In the reaction tube both contents and vessel heated with better homogeneity and selective heating of polar molecules might be realized.
 |
Figure 3: Source: Biotage. Microwave irradiation tube. |
Use of microwave the acceleration of chemical reactions outcomes from the interactions between electromagnetic field and the material leading to the thermal and specific (non-thermal) effects. The compound must possess a dipole moment for microwave irradiation. A dipole is orients itself with an oscillating electric field and the electric field inverses in its every oscillation when a dipole is exposed to an alternating current and the dipole also rearrange itself to follow inverse electric field. The induced or permanent dipole respond to electric field and transfer power from electric field to the dielectrics and the dielectrics possess some residual energy as the polarization cannot follow an extremely rapid reversal pathway which is known as the hysteresis loss. The remaining energy induces heating in the dielectric material. This phenomenon is known as dielectric heating. The number of reversal of the dipole of the dielectric is related to the frequency of the electromagnetic radiation and in every reversal of the dipole there will be hysteresis loss. So a household microwave oven commonly used irradiation frequency (2450 MHz) oscillates 2 x 2450 × 106 times per second and each oscillation is associated with a residual hysteresis loss and this total energy is transformed into the heat energy.2-5
Kappe6 and Von Hippel.7reported some values for the dissipation factor and based on the values are showed in Table 3
Table 3: Dissipation factors for some solvents and materials (3 GHz and 25 oC)
|
Solvent |
tan δ |
Material |
tan δ |
|
Ethylene glycol |
1 |
Nylon 6,6 |
12.8·10−3 |
|
Ethanol |
0.25 |
Poly(vinyl chloride) |
5.5·10−3 |
|
Methanol |
0.64 |
Porcelain 4462 |
1.1·10−3 |
|
Acetic acida |
0.174 |
Borosilicate glass |
1.06·10−3 |
|
Water |
0.157 |
Ceramic F66 |
0.55·10−3 |
|
0.1 mol/l NaCl |
0.24 |
Polyethylene |
0.31·10−3 |
|
0.5 mol/l NaCl |
0.63 |
PTFE–PFA |
0.15·10−3 |
|
Toluenea |
0.040 |
Fused quartz |
0.06·10−3 |
So the basic concept of heating is the ability of the radiation to introduce polarization of charges within the dielectric material. The amount of energy absorbed by a domain depends on the microwave intensity and the absorption cross section of the domain. Molecules possessing a large absorption cross-section are selectively heated and such molecules molecular radiators. To summarize, it is principal note that MW irradiation dielectric constant and dielectric loss both the properties are a function of microwave frequency. However, both laboratory and domestic used microwave gadget generally operate at 2.5 GHz to admit a deeper penetration MW into the substances, result a more uniform heating.
Conduction Mechanism
Ionic conduction of dissolved ions in solutions is familiar that increases with the rise in the temperature. The increase in the dielectric properties with temperature is especially important in the microwave heating of solids, as it introduces the phenomenon of thermal runaway. Dipole rotation influences the offering for heating in the starting of the process when submitting sample ions to MW irradiation. However, the ionic conduction dominates the heating process due to increase in temperature. The main limitation of this method is that it is not applicable for materials that have high conductivity, since such materials reflect most of the energy that falls on them.5
Microwave heating in organic Synthesis.
It has been considered that MW is responsible for undergoing chemical reactions due to its thermal effect and non-thermal effect. When heat generated by MW is responsible for a chemical reaction, it is called thermal effect. When some other effect comes into play to accomplish a chemical reaction, it is considered as non-thermal effect. But the question arise that why MW-introduced reactions are often faster than conventional heating? This is a burning question and many theories and postulates are published in the literature. A short discussion is made in this review.
Thermal effects which includes (i) Overheating, (ii) Hot spot, and (iii) selective heating of solvents, catalyst, reagents, and susceptors.
Non-thermal effects of the highly polarizing field.
Overheating
When a liquid is heated by a normal oil bath, the liquid gets heated from the surface and gradually the whole liquid gets heated by convection and conduction process and finally reaches its boiling point when the vapour pressure of the liquid equals the atmospheric pressure and this observation is on the surface of the liquid. Interestingly, when a liquid is irradiated with microwave inverted heat transfer takes place from the interior of the irradiated medium to the exterior. When the boiling temperature reaches at the surface, the internal temperature is higher than the boiling point by 13-260C. In 1997 Mingos10 observed this effect in polar solvent on using microwaves, where overheating in the range 13–26 uC above the normal boiling point may occur (Fig. 2).
This overheating effect by MW in polar liquids by observing a temperature-dependent photochemical reaction was successfully justified by Klan et. al.[11] in 2001. Klan reported in a photochemical Norrish type II cleavage on valerophenone when irradiated in UV light (Scheme 1) with both ketones (1:1) were irradiated with UV light (at λ >280 nm) in solvents (MeOH and CH3CN) with similar photochemical environment for both substances. The fragmentation–cyclization ratio diversed from 5 to 8 and was attribute for given reaction conditions (Table 4). Commonly, by conventional heating, cyclization to cyclobutanol is a side reaction. But application of microwave irradiation increases the formation of cyclobutanol, which is more pronounced in acetonitrile.
 |
Scheme 1: Norrish type II reaction reported by Klan. |
Klan reported the photo-Fries rearrangement11 of phenylacetate under MW irradiation as well as electrode less discharge lamp (EDL) which gives two main products: 2- and 4-hydroxyacetophenone (Scheme 2). The formationof products are shown in Table 4.
 |
Scheme 2: Photo-Fries rearrangement reported by Klan. Click here to View scheme |
Table 4: Product ratios under different reaction conditions
|
Solvent |
Mode of heating |
Reaction Temp (0C) |
Overting (oC) |
A:B |
|
MeOH |
Conventional |
65 |
0 |
1.52 |
|
MeOH |
microwave |
75 |
10 |
1.32 |
|
CH3CN |
conventional |
81 |
0 |
1.12 |
|
CH3CN |
microwave |
90 |
.09 |
0.9 |
Hot spots
Temperature of certain zones within the sample, solid or solution becomes very high, these zones are called hot spots. The temperatures at those zones are 100-200 oC higher than the bulk temperature and sizes of the hot spots are around 100 μm. The difference in temperature was calculated by the help of some conversions observed, such as the conversion of c- to a-alumina and the melting of MoS2, that happen at temperatures much larger than the regular bulk temperature. The presence of hot spot was elaborated by Mingos studying the decomposition of H2S over γ-Al2O3 or MoS2-γ Al2O3.12 The efficiencies of conversion was obtained to be better by MW irradiation over conventional heating. During the transformation by microwave irradiation γ-Al2O3 to α- Al2O3 was observed. The decomposition temperature of H2S over γ-Al2O3 was 100-700 oC. Phase transfer of γ-Al2O3 to α- Al2O3 takes place above 1000 oC. The conversion of γ-Al2O3 to α- Al2O3 supports the formation of hot spots during microwave irradiation reaction.
 |
Scheme 3:The presence of hot spot was elaborated. |
It is well known that the transformation efficiency using MW was established to be better compared to oil bath heating which is assigned as the presence of hot spots under microwave irradiation. During this transformation by MW- irradation transition of γ-Al2O3 to α-Al2O3 was observed. The decomposition temperature of H2S over γ-Al2O3 was 100 – 700 oC. Phase transfer of γ-Al2O3 to α-Al2O3 takes place above 1000 oC. The conversion of γ-Al2O3 to α-Al2O3 supports the formation of hot spots during MW-irradiation process. Similarly some molten molybdenum disulfide was obtained by MW-irradiation (mp. of MoS2 is 1185 oC).
Hihn et al. 13 reported the temperature distribution during the synthesis of coumaran-2-one in absence of solvent in which the volume was divided into three different layers of equal thickness. Generation of hot spot was also described by Hallberg in the synthesis of β,β-diarylated aldehydes by the reaction of enol ethers in a two phase (toluene/aq. HCl) system.14 Marken et al. 15 reported that the effect of 2.45 GHz MW radiation on electro-organic reaction. The effect of MW was studied during the oxidation of ferrocene in acetonitrile (0.1 M nBu4+Pf6–) with a Pt-electrode. Increase in power of MW led to increase in limiting current. This effect qualitatively attributed to formation of hot spot near the electrode surface. The hot spot temperature was found to be 118 oC which is considerably higher than both the boiling point of acetonitrile (81 oC) and the electrode temperature (47 oC). The solvent acetonitrile convection through the hot spot under these conditions becomes more effective for the process. So generation of hot spots can be considered in solid as well as in solution. They cannot be measured directly in that process.
Dąbrowska et al.16 was studied that laboratory reactors with microwave power capabilities of 600 W can heat 100 mL containers with water 100 times faster than convection heating and observed the overheating effects on reaction rates.
Selective heating
MW irradiation is a specofic mode of heating. Fundamentally, MW irradiation give rise to quick intense heating of polar molecules while polar materials do not absorb the radiation and are not heated.17 Such selective heating may occur in any one or more components of a reaction system. In solvents, catalysts and reagents selective heating has been utilized.
Solvents
Strauss studied18,19 a Hoffmann elimination of β-aroylethyltrimethylammonium iodide under different condition (Scheme 4). It has been found that the polymerized product was generated when the reaction was transformed in water at 105 oC. However, to avoid the polymerization reaction the synthesis was carried nicely by the help of MW irradiation in a two phase water/chloroform system when the temperatures of water and chloroform were 110 and 50 oC, respectively. After completion of the elimination reaction in aqueous phase at higher temperature, the formed phenyl vinyl ketone comes to the lower chloroform layer, where the alkene survives from polymerization due to lower temperature.
 |
Scheme 4: Hoffmann elimination of β-aroylethyltrimethylammonium iodide under different condition. |
To avoid polymerization this reaction was carried using two immiscible solvent systems like CHCl3/H2O under microwave irradiation where phenyl vinyl ketone produced with high yield. The difference dielectric constants of CHCl3 and H2O are responsible for different temperature under microwave irradiation. The temperature organic phase and aqueous phase were 50 oC and 110 oC respectively. After the reaction the product phenyl vinyl ketone comes to the organic layer where the elimination product survives from polymerization due to lower temperature.20
Catalysts
In heterogeneous reactions mixture presence of a polar catalyst sometimes become the site of selective heating. For example, Bogdal21,22 experimented the oxidation of alcohols using a solid chromium dioxide catalyst Magtrieve I (Scheme 5). The microwave heating of Magtrieve I rapidly reaches its temperature up to 360 oC within 2 minutes. When Magtrieve I and toluene were suspended into the reaction tube, the temperature of the catalyst becomes 140 oC at the same time period. This shows that the temperature of the Magtrieve I can be higher than the bulk temperature of the reaction mixture, which suggests that MW irradiation process might be superior to other conventional processes.
 |
Scheme 5: Heterogeneous reactions of alcohols using Magtrieve I. Click here to View scheme |
Auerbach23 was explained the overheating effect through both the equilibrium and none quilibrium molecular dynamics using zeolite–guest systems when Conner24 completed his experimental work with that system. All atoms in zeolite–guest at equilibrium condition are at the same temperature however when Na–Y zeolite is irradiated to microwave in the methanol–benzene binary mixture, the effective steady-state temperature of Na atoms is considerably larger than that of the rest of the groundwork, suggesting a thermal energy distribution and found temperatures are different for each component of Tmethanol & Tbenzene, Tzeolite. From this result it can be concluded that methanol dissipates energy to benzene, which is much slower approach to thermal equilibrium under steady-state conditions. Although, some disagreement also remains concerning the effects of MW heating in heterogeneous catalysis.25 Another group of authors have showed the changeof the catalyst’s electronic properties upon exposure to MW irradiation26-28 during explain the superior catalytic properties of catalysts under these conditions.
Reagents and products
Larhed29 studied the molybdenum and palladium-catalyzed asymmetric allylic alkylation of (E)-3-phenyl-2-propenyl acetate using allylic carbonate. The reaction has been accomplished with complete conversion, good reproducibility, high yields (87%) and excellent regioselectivity (ee = 98%) within few minutes (Scheme 6) using tetrahydro furan under microwave irradiation power of 250 W at 220 oC. This high yield is clearly suggested that when MW heating is used there is a significant contribution from sustained overheating which is not only for the increased boiling points at elevated pressure.
 |
Scheme 6: Molybdenum and palladium-catalyzed asymmetric allylic alkylation of (E)-3-phenyl-2-propenyl acetate. |
Susceptors
When the reagents and solvents in a reaction medium do not absorb microwave radiation, a chemically inert substance in that medium but capable of absorbing MW radiation is used to facilitate the reaction, such substances are called susceptors. It transfers the thermal energy to another compound which itself cannot absorb the radiation. This method has an interesting advantage because it differs from a catalyst where the energy is focused on the reaction surface of the susceptor.
The cyclization reaction of (+)-citronellal to (-)-isopulegol and (+)-neoisopulegol in absence of solvent or heterogeneous conditions graphite acts as a susceptor studied by Garrigues30 under MW heating (Scheme 7) in which the stereoselectivity of the transformation is changed. (-)-Isopulegol is all the timeis the main diastereoisomer, although under conventional heating a trace amount of (+)-neoisopulegol was produced by the MW heating, it is formed in 30% yield.
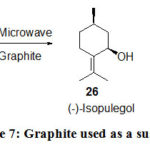 |
Scheme 7: Graphite used as a susceptor. |
Ionic liquids (IL) act as a susceptor either in solution or under homogeneous conditions. Ley 31 studied the reaction of amides to achieve thioamides where toluene is not a good solvent to perform under microwave conditions, but reactions can occur under conventional heating condition. N,N-Dimethylbenzamide forms corresponding thioamide by heating with O-ethyl phosphorodichloridothioate [PS(OEt)Cl2] for 30h in toluene whereas using IL it can be performed in 15 mins under microwave irradiation (Scheme 8).
 |
Scheme 8: Ionic liquids (IL) used as a susceptor for the Preparation of thioamides from amides. |
Lead beater 32 determined to explain the above experimental result using ionic liquids as aids for the MW heating under non polar solvent (Table 5). This reaction transform in a quick period and polar solvents can be heated to much higher temperatures than the boiling point of the solvent used in sealed vessels using small quantity of an ionic liquid.
 |
Table 5: Effect of ionic liquid. |
Non-thermal effects or specific MW effects.
Non-thermal effect of microwave is still yet uncertain. Several theories have been postulated the presence of non-thermal effect along with the thermal effect of MW, whereas other group of scientists stick purely to the thermal effect of microwave which are only responsible for making a reaction faster or selective compared to oil bath heating methods. Several theories and prediction have been made to explain the presence of non-thermal effect of microwave; at the same time other group of people has tried to refute all these considerations. A brief discussion with some examples has been made in this review.
Similar to thermal effects of microwave, non-thermal effect may arise during the interaction between MW and material. In fact it is very difficult to make a separation the thermal and non-thermal effects of MW in mechanistic studies. The MW effects are increased in reactions with polar transition states. Some scientists suggested that changes in the thermodynamic properties under MW irradiation are the cause of microwave effects. Berlan et al.33 reported the rate of cycloaddition reactions with 2,3-dimethyl-1,3-butadiene and methyl vinyl ketone carried out under reflux either in dibutyl ether or in xylene (Scheme 9) were always faster when irradiated under microwave than the conventional heating process. The observed acceleration is more pronounced in relatively polar solvent xylene where dielectric losses are small than dibutyl ether. It has been predicted due to modification in ∆G* by the change of entropy of the system.
 |
Scheme 9: Cycloaddition reactions with 2,3-dimethyl-1, 3-buadiene and methyl vinyl ketone under MW irradiation. |
Gedye et. al.34 studied the Diels Alder reaction between cyclopentadiene and methyl acrylate (Scheme 10) where microwave radiation does not change the endo/exo selectivity. Higher the yield and faster reaction than those taking place under reflux can be explained due to the fact that the reactions processed by the MW heating at higher temperatures.
 |
Scheme 10: MW-irradation was observed that polar solvent works better. |
The rate of hydrolysis of adenosine triphosphate is 25 times faster under microwave irradiation than conventional heating at comparable temperature. The faster rate was attributed to direct absorption of radiation or by selective excitation of water of hydration over the bulk molecules. The rate enhancement may also be due to increase in kinetic energy of the solvent due to increase in kinetic energy of the solvent due to MW absorption. Bond35 and Strauss36, 37 reported individually the controlled systems esterification reactions either presence or absence of MW heating show identical and the yield of the final product depend only on the temperature profile—not on the mode of heating.
Halogenation of alkene in different solvents with tetrahalo methane under homogeneous conditions with MW-irradiation was studied by Hajek in 199738 and it was observed that polar solvent works better because of dielectric heating effect resulting from the effective coupling of MW with polar solvent. Whereas, under heterogeneous reaction conditions the presence of hot spot and selective heating as discuss earlier are responsible for observed acceleration in reaction rate.
Some authors39, 40 have described the direct activation either one or both reagents in the ring closing metathesis process is predicted that the rate increased in MW irradiation may be due to absorption of MW by the olefin as Kappe showed that absorption of MW heating by Grubb,s catalyst was little and the olefin acts as molecular radiator (Scheme 11). However, by conducting experiments under conventional heating conditions it was reported that it is less important whether a particular reactant gains the energy or the energy is transferred to the bulk solvent by the MW heating.41
 |
Scheme 11: MW radiation by Grubb,s catalyst. |
On the basis of some model experiment Miklavc42 calculated the rotational dependence of O + HCl (DCl) A OH (OD) + Cl reactions which is predicted marked accelerations in transformation may take placevia the rotational excitation on collision geometry. Earlier Berlinb43 and Strauss[18] proposed that the internal energy is distributed among translational, vibrational and rotational energies regardless of the mode of heating, since the dielectric heating increases the temperature of the system. Molecular agitation and mobility are also factors to consider MW effects.
During the decomposition of NaHCO3 by classical oil bath heating and by MW-irradiation, the activation energy of decomposition was found to be smaller in case of MW-irradiation. The application of MW field to dielectric materials induces quick rotation of polarized dipoles in the molecule. This evolve heat due to the friction while simultaneously increasing the probability of contact of molecules and atoms, thus decreases the activation energy and enhances reaction rate. In the Arrhenius pre-exponential factor ‘A’ which depends on the frequency of vibration of atoms at the reaction interface and this factor can be affected by MW without changing the activation energy. An increasing in ‘A’ alone may explain the acceleration of MW-induced reaction.
One instance which cannot be described by thermal effect44 of MW is the mutarotaion of α-D-glucose. Pagnota45 found that in ethanol-water (1:1), application of MW not only brings rapid equilibrium than conventional heating, but also changes the equilibrium proportion of the anomers and larger amount of α-D-glucose was found in the equilibrium than the thermal equilibrium.
Zhang46 was reported another interesting study on esterification of benzoic acid in methanol. The reaction under MW radiation at a frequency of 1 GHz has no MW irradiation action but only athermal microwave effect. But the rate of reaction under these conditions is faster using MW irradiation (Scheme 12).
 |
Scheme 12: Comparative study on esterification of benzoic acid in methanol. |
The study of [2+2] cycloaddition reaction between acid chloride and a Schiff base in presence of Et3N was found to differ in conventional heating and by irradiation with MW (Scheme 13). Under thermal condition the mode of addition of reagents played a great role in determining the population of stereoisomers of the products, whereas under MW condition mode of addition has no effect on the population of the stereomers.47,48
 |
Scheme 13: [2+2] Cycloaddition reaction between acid chloride and a Schiff base. |
This reaction under MW irradiation no isomerization of the cis-product (the kinetically controlled product) to the thermodynamically controlled trans-product occur.
The drug discovery process using microwave irradiation
Microwave assisted organic synthesis (MAOS) have an impact in different field of drug discovery and this heating is also being used to produce library of compounds in target discovery, screening, pharmacokinetics and even in the clinic. The wide scope of MW irradiation is firmly illustrated by its use in several fields in drug discovery,49 materials science, polymer chemistry,50 nanotechnology,51 organic synthesis, and bioconjugation fields.52 This review outlines upto date some selected applications with an attention on multistep microwave procedure in the drug discovery process.
Antimicrobial drugs
Besson et al. reported53 some novel antimicrobial agents, indolo[1,2-c]quinazolines and benzimidazo[1,2-c]quinazolines. Condensation reaction of the appropriate diamines (e.g., 2-(2-aminophenyl) indoleor 2-(2-aminophenyl) benzimidazole) with 2-yanobenzothiazoles under microwave irradiation (150 W) of the two starting compounds at 220 ◦C in the presence of graphite produced very good yield other than normal condition (Scheme 14).
 |
Scheme-14: Synthesis ofnovel antimicrobial agents under microwave irradiation. |
A.M. Naghla54 reported library of antimicrobial compounds using microwave irradiation with good yields (Scheme-15).
 |
Scheme-15: Preparation of antimicrobial compounds using microwave irradiation. |
Anti-Thrombotic agents
Muller et al. have reported55 some anthraquinone derivatives as non-nucleotide-derived competitive antagonists of platelet P2Y12 receptors. In the synthesis of the target compounds the key step was a copper(0)-catalyzed Ulmann coupling reaction of 1-amino-4-bromoanthraquinone derivatives with anilines using microwave irradiation heating between 80 – 120 oC for 5-20 min (Scheme 16). Following this approach, they prepared some non-nucleotide antagonists of human P2Y12 receptor.
 |
Scheme 16: Copper(0)-catalyzed Ulmann coupling reaction using microwave irradiation. |
Anti-obesity agents
Johnson and Johnson Pharmaceutical Research and Development have reported56 Melanin-concentrating hormone receptor 1 and prepared pyrazinone core which was performed in a fast and efficient way using microwave irradiation and the whole reaction time was less than 3h with five steps and out of five, three steps were carried out by microwave irradiation (Scheme 17).
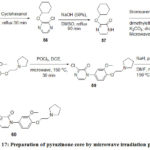 |
Scheme 17: Preparation of pyrazinone core by microwave irradiation process. |
Second way of the synthesis this group was developed pyrazinone core 3-alkyl-1-aryl- pyrazinone under microwave irradiations in all three steps with the reaction time of 4 hours (Scheme-18).
 |
Scheme 18: Second way preparation of pyrazinone core using microwave irradiation. |
This group was developed another way of the synthesis of 3-aminoalkyl-1-arylpyrazinones in three synthetic steps which was performed using microwave irradiation and a total reaction time of 70 min (Scheme 19). These synthetic routs are good example of use of microwave irradiation reaction can simplify chemistry and increase the efficiency of synthesis of potentially active compounds.
 |
Scheme 19: Synthesis of 3-aminoalkyl-1-arylpyrazinones using microwave irradiation. |
Infectious disease
Guo et.al reported57 the synthesis and biological studies of a set of pyridine dicarbonitriles as potential prion disease therapeutics and reduced the reaction time from 24 h to 1h at 90 oC using microwave irradiation (scheme 20) facilitating the purification and increasing the yield on an average by 15%.
 |
Scheme 20: Synthesis of pyridine dicarbonitriles using microwave irradiation. |
Erectile dysfunction
Flores-Togue et.al. have reported the efficiency of combining polymer-supported chemical transformation with MAOS of the well-known commercially important pharmaceutical drug, sildenafil (scheme 21).58,59
 |
Scheme 21: Polymer-supported synthesis with microwave-assisted organic synthesis. |
Antitubercular, antibacterial and antitumor drugs
Rizk et.al. described60 one-pot synthesis of pyrazolo[3,4-b] pyridine derivatives for antimicrobial, antibacterial61 and antitumor activities62 from the reaction of 5-amino-1-phenyl-3-(pyridin-3-yl)-1H-pyrazole with 4-anisaldehyde and p-substituted β-ketonitriles or with pyruvic acid and some aromatic aldehydes in acetic acid medium using microwave irradiation in multi-component system (Scheme 22).
 |
Scheme 22: Synthesis of pyrazolo[3,4-b] pyridine derivatives using microwave irradiation in multi-component system. |
Alzheimer’s disease
The multi component synthesis of a thiazolo [5,4-f]quinazoline was recently synthesized63 (Scheme 23) to improve yields with decrease reaction times and products that are more manageable to purification using MW heating.
 |
Scheme 23: Microwave assisted synthesis of a thiazol[5,4-f]quinazoline. |
Mohamed et al. reported number of fused five-membered aromatic ring analogues of tacrine containing halogens such as the pyrrolotacrines (Scheme 24), pyrazolotacrines (Scheme 25), and furanotacrines (Scheme 26) heterocyclic ring systems in many ways using MW heating for the preparation of Anti-Alzheimer drug.
 |
Scheme 24: synthetic route of pyarazolotacrine derivatives. |
 |
Scheme 25: Synthetic route for the preparation of pyrrolotacrines. |
 |
Scheme 26: Synthesis offuranotacrine using MW irradiation. |
PET imaging agents
MAOS efficiently used to synthesize short-lived compounds with extremely short reaction times that could not be otherwise be prepared using classical methods due to decrease their continuous decaying radioactivity.64 One most important application of activation by MAOS is in the preparation of radio labeled compounds for PET imaging agents for the treatment of cancer which contain short half-lives radioisotopes (Figure 4; e.g. 11C,122I and 18 F). In all cases MAOS have reduced reaction times by up to 50% successfully and in some cases, the radio chemical yields of the final product become doubled.
 |
Figure 4: Some PET with short half-lives compounds prepared using microwave irradiation. |
Dolle et.al65 used MW heating for the preparation of [18F]FPhEP, a novel α4β2-selective antagonist for imaging nicotinic acetylcholine receptors and the reaction time is reduced from 20 min to 90 s, to enhance the yield of the synthesis (Scheme 27).
 |
Scheme 27: synthesis of [18F] FPhEP using microwave irradiation. |
MAOS have immense impact on medicinal chemistry. Kappe et al. have reported 66 kinesin Eg5 inhibitor, monastrol (Figure 5; 109), those were synthesized successfully under microwave conditions. They got a higher yield of the inhibitor with an improved purity compared to classing heating method using a MW-assisted Biginelli reaction. Kidwai et al. have reported effectiveness in the synthesis of the novel antibacterialβ-lactams monastrol67 with microwaves heating during the preparation of quinolines (Fig. 5; 110)68 and cephalosporins (Fig. 5; 111).69 The synthesis of anticarcinogenic isoflavones (Fig. 6; 113),70 the antileukaemic alkaloid, convolutamydine-A (Fig. 6; 114)71 and the nitrogen mustard β-lactams and indoles72 have shown greater yield by microwave irradiation.
 |
Figure 5: Compound monastrol, quinolones, and cephalosporins. |
 |
Figure 6: Compound quinolones, anticarcinogenic isoflavones, convolutamydine-A. |
Medicinal and combinatorial chemistry
Hwang et. al reported isotopically labelled compounds (e.g. 11C, 122I and 18 F) prepared by MAOS for PET imaging agent for cancer treatment which is required extremely short reaction time.73
Born mannet al.74 have synthesized a series of c-Kit molecules using MW-assisted reaction as an environmental friendly heating tool in multi-component system by the reaction of 2,6-dichloro nicotinic acid chloride with the various zinc halides in presence of tetrakis (triphenyl phosphine) palladium in 2 mL of THF. Suzuki coupling reaction were carried out under MW heating at 150oC for 5 to 10 min produced 24 compounds at a time using CEM microwave reactor and the yield of the reactions are more than 25 percentages where in conventional heating the reaction time is three days and the of the reactions are less than 15 percentages and needs more solvent.
 |
Scheme 28: Synthesis of pyrozolo compounds under MW irradiation. |
Hallberg et al. have reported their quick preparation of modified HIV-1 protease inhibitors (Scheme 29; 120a-g) by the use of microwave-promoted coupling reaction. The commercially available aryl and heteroaryl boronic acids and palladium-catalyst were used during coupling reaction (Scheme 29) under MW heating in which the yield of desired compounds is very high.75
 |
Scheme 29: Synthesis of HIV protease inhibitor. |
Levita et al.76 reported the semi-synthesis of andrographolide analogues of anti HIV-1 drugs by modifying a synthesis method that has been successfully carried out by Hadi Poerwono et al. using microwave irradiation due to achieve several advantages.
 |
Scheme 30: The synthesis of 2-hydroxybenzylidene and rographolide (123), 3-hydroxybenzylidene and rographolide (124) and 4-hydroxybenzylidene and rographolide (125). |
Antiviral drug
Baran et al. described the total synthesis of ageliferin, an antiviral marine alkaloid by a tautomerization/ring expansion reaction from sceptrin.77 The product was successfully achieved by MW heating only in water within a minute and the yield of the reaction is 40% (Scheme 31). Whereas by the use of conventional heating takes longer time and led to decomposethe unstable compound.
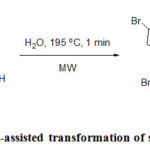 |
Scheme 31: Microwave-assisted transformation of sceptrin into ageliferin. |
Trost and Andersen recently have applied78 the concept in their approach to the orally bio available HIV inhibitor tipranavir (Scheme 32). Preparation of the key chiral intermediate 130 was obtained by asymmetric allylic alkylation beginning from carbonate 128. The yield of the product was obtained about 94 % by employing 10 mol % of the molybdenum precatalyst and 15 mol % of the chiral ligand 131 with 2.0 equivalents of sodium dimethyl malonate as the additive. The synthesis was transformed under sealed‐vessel MW heating at 180 °C for 20 min. Whereas classical heating (67 °C) required 24 h and produced almost same chemical yield of intermediate 130, albeit with slightly higher enantiomeric purity (96 % ee).
 |
Scheme 32: Rearrangement of a squaric acid derivative to a vinylketene. |
Trost et al. have synthesized successfully furaquinocin antibiotics by the microwave‐assisted squaric acid/vinylketene rearrangement for the preparation of dimethoxynaphthoquinone 135 which is a protected analogue of furaquinocin E (Scheme 33).79 The rearrangement reaction from the compound 133 to 134 using oil bath heating was unsuccessful. The same conversion was attempted under MW irradiation at 180 °C in toluene led to complete conversion with an admissible yield of 136 (58 %) after oxidation to the naphthoquinone.
 |
Scheme 33: Rearrangement reactionunder MW irradiation from squaric acid derivative to a vinylketene. |
Anticancer agents
Banik et.al described an efficient synthesis of 3-substituted-4-phenyl-1-(9,10-dihydrophenanthren-3-yl)azetidin-2-ones using Staudinger cycloaddition under microwave irradiation.80 The diastereoselectivity of the product formation depends on temperature, the nature of substituent on lactam nitrogen, the power level of the microwave irradiation, and the polarity of the solvent.
 |
Scheme 34: Synthesis of β-lactam under MW irradiation. |
Gomha et al. reported81 an efficient and a novel approach for the synthesis of some novel 4-heteroaryl-pyrazoles (Figure 7), which have reported hitherto in a multicomponent synthesis under MW irradiation and to assess their anticarcinogenic effects against hepato cellular carcinoma (HepG-2) and human lung cancer (A-549) cell lines and showed that oxazoles are the most active compounds against the lung carcinoma cell line (A549), compared with cisplatin.
 |
Figure 7: Synthesis of some novel 4-heteroaryl-pyrazoles. |
Jovanovic-Santa et al. reported82 an efficient microwave-assisted synthesis of 2-methoxybenzoyl ester without solvents which shows important potency against MDA-MB-231 cells and maximum compounds are used to show their strong growth inhibition property against PC-3 cells. The most inhibition potency showed the salicyloyloxy stigmastane derivative(Scheme 35).
 |
Scheme 35: Microwave-assisted synthesis of 2-Methoxybenzoyl ester. |
G. Achaiah, RV. Malla et al. have been synthesized83 fourteen new 4-alkyl/ary//aryl-2,5-bis-(carbomethoxy/carbomethoxy)-1,4-dihydro-2,6-dimethyl pyridines carboethoxy/carbomethoxy derivatives under conventional and MW irradiation from a one-pot three-component reaction mixture consisting of alkyl acetoacetate, aldehyde and ammonium acetate. Al most all compounds revealedtheir cytotoxicity against MCF-7 and A549 cancer cell lines and exhibit moderate-to-good anticancer activity against these cell lines (Scheme-36).
 |
Scheme 36: Synthesis of 1,4-dihydro-2,6-dimethyl pyridines under classical and MWheating method. |
H.T. Buu Bui and his coworkers reported84 twelve novel 2-quinolizinyl benzimid azole and 2- aphthalyl benzimid azole type compounds with molecular variation generates by the substituents at positions 5- and 6 (aza, H, CH3, Cl, NO2, NH2, OCH3), via the condensation of 4-oxo-4H-quinolizinecarbaldehyde or naphthalenecarbaldehyde with substituted o-phenylenediamines, o– nitroaniline, and 2,3-pyridinediamine and use of sodium metabisulfite or sodium hydrosulfiteas catalyst by the MW condition (Scheme-37).
 |
Scheme 37: Synthesis of quinolizinyl benzimidazole and 2-naphthalyl benzimid azole type compounds by the microwave condition. |
L. Gaina et al. reported85 some novel 1,3-selen azole compound sunder microwave assisted Hantzsch condensation reactions. Anti-proliferative effects for these compounds were tested against leukemia cell lines (HL60 and CCRF-CEM) and carcinoma cell lines (HCT116, MDA-MB231 and U87MG). The result showed moderate cytotoxicity for all the experimented cell lines (Scheme 38).
 |
Scheme 38: Synthesis of 1,3-selenazole compounds under microwave assisted Hantzsch condensation reactions. |
N. Kumar et al. designed and synthesized86 three different series of amide derivatives of ferulic acid by MW heating in absence of solvent. All the compounds were found stable upto 100 oC during their thermal stability test but at higher temperature these compounds decomposed through single step. These compounds were tested their anticancer activity against breast (MDA-MB-231 and MCF-7), cervical (HeLa), lung (A549) and liver (HepG2) human cancer cell lines (Scheme-39) and these compounds exhibit noticeable activity.
 |
Scheme 39: Synthesis of a series of amide derivatives under microwave-assisted reaction Click here to View scheme |
Aromatase inhibitors
Potter et al. synthesized87 a series of novel and potent aromatase inhibitors,active heterocyclic compounds using Suzuki cross coupling methodology under microwave irradiation.
 |
Scheme 40: Synthesis of a series of heterocyclic compounds by microwave-assisted reaction. |
Antiarrhythmic, anticonvulsant, antineuralgic, trigeminal neuralgia and skeletal muscle relaxant.
 |
Figure 8: Some medicinally interesting hydantoin analogs. |
Hydantoin compounds have showed a wide range of medicinal properties (Figure 8), such as, phenytoin acts many purposes, like anticonvulsant, antiarrhythmic, trigeminal neuralgia,antineuralgic and skeletal muscle relaxant.88 Sulfahydantoin has been identified on the respect of inhibition of serineproteases.89 The glucopyranosylidene-spiro-thiohydantoin is described90 that it inhibits efficiently of muscle and liver glycogen phosphorilases.91 Mei-Jung Lin and Chung-Ming Sun92 have developed a hybrid strategy using both microwave irradiation and combinatorial approach. Thiohydantoins were synthesized by the following route is shown in Scheme 41.
 |
Scheme 41: The general synthetic route of thiohydantoins synthesis of by microwave-assisted reaction. |
Quinolines are well known for their salient biological activities and also for the development of polymers and conjugated molecules that integrate increased electronic or nonlinear optical properties with good instinctive properties. K won reported the synthesis of some quinoline derivatives using Fried lander coupling condensation between an acetophenone and a 2-aminoacetophenone in the presence of diphenylphosphate (0.1–0.5 equiv.) within 4 min under MW irradiation in solvent free condition.93 This yields of the products are up to 85% when MW irradiation technology is used, and the yield achieved not more than 24%with classical heating by the same conditions. (scheme 42).
 |
Scheme 42: Solvent free synthesis of quinoline derivatives under MW irradiation. |
Menéndez described solvent free microwave-assisted synthesis of 2-styrylquinolines by condensation of 2-methylquinolines with cinnamaldehydes or benzaldehydesin the presence of acetic anhydride94 (Scheme 43) which are valuable derivatives for Alzheimer patients as imaging agents for β-amyloid plaques on human brain sections.
 |
Scheme 43: Preparation of 2- styrylquinolines using MW. |
Conclusion
In this review we tried to give an outline of microwave assisted synthesis and it has already exercised a huge impact on medicinal chemistry. Microwave dielectric heating has many advantages compared to conventional heating in the medicinal synthesis. MW heating decreased reaction times from days to minutes, achieved higher yields, heating rates become higher than those achieved from classical heating. There is no direct touch between the energy source and the reacting substances. Sometime achieve magical transformation which is impossible to realize by oil bath heating.Microwaves interacts selectively between the reactants and solvents and in many cases in the absence of solvents i.e. solvent free reactions, provides clean reactions without the formation side products which are impossible using conventional heating. Such an enormous superior new technology is engage in the field of medicinal chemistry. This new technology can facility the bottlenecks within the drug discovery arena. Before chemists were used simple or‘adapted’ domestic instruments for their reaction system. Last twenty years, the appearance of new several commercial instruments with advance technology available to the bench of chemist. These facilities of new technology helps to overcome the problems of re productibility, control ability and safety experienced with the domestic microwaves during the transformation of reactions. This technology is utilizing in the laboratory and helps on the fields of medicinal chemistry with screening and making library of compounds for the drug design and drug development. Though this modern technology has huge facility in the laboratory but the major drawbacks of this relatively new technology is cost management of equipment. The recent price of microwave reactors for the reaction purpose is still many times higher than that of classically heating instruments though prices for microwave reactors have come down considerably since their first introduction in the late 1990s. Now be of the opinion that the use of microwave heating in medicinal chemistry will be normalize in the coming years since this technology assure the requirements of both the pharmaceutical laboratory and academic purpose.
Acknowledgement
The authors are thankful to the Principal, Raja Peary Mohan College, Uttarpara for his encouragement.
Conflicts of Interest
The authors announce that there is no conflict of interest.
References
- Bufflur microwave cooking and processing; engineering fundamentals for the food scientist. Van Nostrand Reinhold: New York, 1993.
- Galema, S. A.; Halstead, B. S. J.; Mingos, D. M. P. Chem. Soc. Rev., 1998, 2, 213–232.
- Horikoshi, S.; Hamamura, T.; Kajitani, M.; Yoshizawa-Fujita, M.; Serpone, N. Org. Process Res. Dev., 2008, 12, 1089–1093.
CrossRef - Gedye, R. N.; Smith, F. E.; Westaway, K.C.; Can. J. Chem., 1988, 66, 17–26.
CrossRef - Kingston, H. M.; Jassie, L. B.; ACS, Washington D. C., 1988, 2, 110–122.
- Kappe, C.O. Angew Chem Int Edn., 2004, 43, 6256.
CrossRef - Von Hippel, A.R. Dielectric materials and applications, John Wiley, New York, 1954
- Kappe, C. O. Chem. Soc.Rev., 2008, 37, 1127-1139.
CrossRef - Kingston, H.M.; Haswell, S. J. American Chemical Society, Washington, 1997.
- Baghurst, D. R.; Mingos, D. M. P. J. Chem Soc., Chem. Commun., 1992, 674.
CrossRef - Kla´n, P.; Litera´k, J.; Relich, S. J. Photochem. Photobiol. A, 2001, 143, 49.
- Zhang, X.; Hayward, D. O.; Mingos, D. M. P. Chem. Commun., 1999, 975.
CrossRef - Goncalo, P.; Hihn, J.-Y.; Vienet, R.; Nika, P.; Vebrel, J. J. Nature,2001, 13, 19.
- Nilsson, P.; Larhed, M.; Hallberg, A. J. Am. Chem. Soc., 2001, 123, 8217.
CrossRef - Tsai, Y. C.; Coles, B. A.; Compton, R. G.; Marken, F. J. Am.Chem. Soc., 2002, 124, 9784.
CrossRef - Dąbrowska, S.; Chudoba, T.; Wojnarowicz, J.; Łojkowski, W. Crystal,2018, 8, 379.
CrossRef - Mingos, D. M. P.; Whittaker, A. G. Microwave Dielectric Heating Effects in Chemical Synthesis, in Chemistry under Extreme or non-classical Conditions, ed. R. van Eldik and C. D. Hubbard, John Wiley & Sons, New York, 1997, 479–545.
- Strauss, C. R.; Trainor, R. W. Aust. J. Chem., 1995, 48, 1665.
CrossRef - Raner, K. D.; Strauss, C. R.; Trainor, R. W. J. Org. Chem.,1995, 60, 2456.
- Nilsson, P.; Larhed, M.; Hallberg, A. J. Am. Chem. Soc., 2001,123, 8217.
CrossRef - Bogdal, D.; Lukasiewicz, M.; Pielichowski, J.; Miciak , A.; Bednarz, Sz. Tetrahedron, 2003, 59, 649.
CrossRef - Lukasiewicz, M.; Bogdal, D.; Pielichowskia, J. Adv. Synth. Catal., 2003, 345, 1269.
CrossRef - Blanco, C.; Auerbach, S. M. J. Am. Chem. Soc., 2002, 124, 6250.
CrossRef - Turner, M. D.; Laurence, R. L.; Conner, W. C.; Yngvesson, K. S. AIChE J., 2000, 46, 758.
CrossRef - Will, H.; Scholz, P.; Ondruschka, B.; Chem.-Ing.-Tech., 2002, 74, 1057.
CrossRef - Klimov, A. Y.; Bal’zhinimaev, B. S.; Makarshin, L. L.; Zaikovskii, V. I.; Parmon, V. N. Kinet. Katal., 1998, 21, 511.
- Perry, W. L.; Katz, J. D.; Rees, D.; Paffet, M. T.; Datye, A. K. J. Catal., 1997, 171, 431.
CrossRef - Kaiser, N. F. K.; Bremberg, U.; Larhed, M.; Moberg, C.; Hallberg, A. Angew. Chem. Int. Ed., 2000, 39, 3595.
CrossRef - Ley, S. V.; Leach, A. G.; Storer, R. I. J. Chem. Soc., Perkin Trans. 1, 2001, 358.
CrossRef - Leadbeater, N. E.; Torrenius, H. M. J. Org. Chem., 2002, 67, 3145.
CrossRef - Berlan, J.; Giboreau, P.; Lefeuvre, S.; Marchand, C. Tetrahedron Lett., 1991, 32, 2363.
CrossRef - Gedye, R. N.; Rank, W.; Westaway, K. C.; Can. J. Chem., 1991, 69, 706.
CrossRef - S. D. Pollington, G. Bond, R. B. Moyes, D. A. Whan, J. P. Candlin and J. R. Jennings, J. Org. Chem., 1991, 56, 1313.
CrossRef - Raner, K. D.; Strauss, C. R. J. Org. Chem., 1992, 57, 6231.
CrossRef - Constable, D.; Raner, K.; Somlo, P.; Strauss, C.; J. Microwave Power Electromagn. Energy, 1992, 27,195.
CrossRef - Ha´ jek, M. Czech. Chem. Commun., 1997, 62, 347.
CrossRef - Mayo, K. G.; Nearhoof, E. H.; Kiddle, J. J. Org. Lett., 2002, 4, 1567.
CrossRef - Grigg, R.; Martin, W.; Morris, J.; Sridharan, V. Tetrahedron Lett., 2003, 44, 4899.
CrossRef - Garbacia, S.; Desai, B.; Lavaster, O.; Kappe, C. O. J. Org.Chem., 2003, 68, 9136.
CrossRef - Miklavc, A. Chem. Phys. Chem., 2001, 552.
CrossRef - Wroe, R.; Rowley, A. T. J. Mater. Sci., 1996, 31, 2019.
CrossRef - Pagnota, M.; Pooley, C. L. F.; Gurland, B.; Choi, M. J. Phys. Org. Chem., 1993, 6, 407.
CrossRef - Zhang, Z. B.; Zhou, L. X.; Zhang, M.; Wu, H.; Chen, Z. J. Synth. Commun., 2001, 31, 2435.
CrossRef - Bose, A. K.; Banik, B. K.; Manhas, M. S. Tetrahedron Lett.,1995, 36, 213.
CrossRef - Bose, A. K.; Jayaramen, M.; Okawa, A.; Bari, S. S.; Robb, E. W.; Manhas, M. S. Tetrahedron Lett., 1996, 37, 6989.
CrossRef - Garella, D.; Borretto, E.; Di Stilo, A.; Martina, K.; Cravotto, G.; Cintas, P. Med. Chem. Comm., 2013, 4(10), 1323–1343.
CrossRef - Kempe, K.; Becer, C. R.; Schubert, U. S. Macromolecules,2011, 44(15), 5825–5842.
CrossRef - Varma, R. S. Pure and Applied Chemistry.,2013, 85(8), 1703–1710.
CrossRef - Tabasso, S.; Montoneri, E.; Carnaroglio, D.; Caporaso, M.; Cravotto, G. Green Chemistry.,2014, 16(1) 73–76.
CrossRef - (a) Alexandre, F. R.; Berecibar, A.; Wrigglesworth, R.; Besson, T. Tetrahedron Lett., 2003, 44, 4455–4458. (b) Besson, T.; Guillard, J.; Rees, C.W. Tetrahedron Lett., 2000, 41, 1027–1030.
CrossRef - Naglah, A. M.; Awad, H. M.; Bhat, M.A.; Al-Omar, M. A.; Amr, A.E. Hindawi Journal of chemistry, 2015, 1-8.
CrossRef - Baqi, Y.; Atler, K.; Kose, M.; Glanzel, M.; Muller, C.E. J.Med.Chem., 2009, 52(12) 3784-3792.
CrossRef - Gehlert, D.R.; Rasmussen, K.; Shaw, J. J. Pharmacol. Exp. Ther, 2009, 319(2), 429-438.
CrossRef - Guo, K.; Mutter, R.; Heal, W. J.Med.Chem., 2008, 43(1) 93-106.
CrossRef - Flores-Togue, H.A.; Priviero, F.B.M.; Teixeira, C.E. J. Med. Chem., 2008, 51(9) 2807-2815.
CrossRef - Supuran, C.T.; Mastrolorenzo, A.; Barbaro. G. Cur. Pharm. Des., 2006, 12(27), 3459-3465
CrossRef - El-borai, M.A.; Rizk, H.F.; Abd-Aal, M.F.; El-Deeb, I.Y. Eur. J. Med. Chem., 2012, 48, 92-96.
CrossRef - Pridham, T.G.; Lindenfelser, L.A.; Shotwell, O.L.; Stodola, F.; Benedict, R.G.; Foley, C.; Jacks, P.W.; Zaumeyer, W.J.; Perston, W.H.; Mitchell, J.W. Phytopathology.,1956, 46, 568-577.
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R.; Ntl. J. Cancer Inst., 1990, 82, 1107-1112.
CrossRef - Foucourt , A.; Hédou, D.; Dubouilh-Benard , C.; Désiré, L.; Casagrande, A. S.; Leblond, B.; Loäec, N.; Meijer, L.; Besson, T. Molecules.,2014, 19, 15546-15571.
CrossRef - Wathey, B.; Tierney, J.; Lidström P.; Westman, J. DDT.,2002, 7(6), 373-380
CrossRef - Capello, C.; Fischer, U.; Hungerbuhler, K.; Green Chem., 2007, 9, 917.
CrossRef - Kappe, C.O.; Shishkin, O.V.; Uray, G.; Verdino, P. Tetrahedron.,2000, 56, 1859.
CrossRef - Kidwai, M.; Kumar, K. J. Indian Chem. Soc., 1998, 75,102.
- Kidwai, M.; Bhushan, K.; Saxena, R.K.; Gupta, R. Bioorg. Med.Chem., 2000, 8, 69
CrossRef - Kidwai, M.; Mishra, P.; Bhushan, K.; Saxena, R.K.; Gupta, R.; Singh, M. Indian Journal of Chemistry., 1999, 38B, 993.
- Chang, Y. C.; Nair, M.G.; Santell,; R.C.; Helferich, W.G. J. Agric. Food Chem., 1994, 42, 1869.
CrossRef - Jnaneshara, G. K.; Bedekar, A.V.; Despande, V.H.; Synth. Commun., 1999, 29, 3627.
CrossRef - Kidwai, M.; Mishra, P.; Bhushan, K.; Saxena, R. K.; Gupta, R.; Singh, M. Indian Journal of Chemistry., 1999, 38B, 1132–1135.
- Kuang, R.; Epp, J. B.; Ruan, S.; Yu, H.; Huang, P.; He, S.; Tu, J.; Schechter, N. M.; Turboy, J.; Froelich, C.J.; Groutas, W.C. J. Am. Chem. Soc., 1999, 121, 8128-9.
CrossRef - Bornmann, W.; Maxwell, D.; Pal, A.; Peng, Z.; Estrov, Z. PCT Int. Appl. 108pp, 2008. CODEN: PIXXD2 WO 2008005877 A2 20080110.
- Alterman, M.; Anderson, H. O.; Garg, N.; Ahlsen, G.; Lovgren, S.; Samuelsson, B.; Hallberg,A. J. Med. Chem,. 1999, 42, 3835–3844.
CrossRef - (a) Megantara, S.; Halimah, H.; Putrianty, A.; Hadi Tjahjono, D.; Kartasasmita, R. E.; Immaculata Iwo, M.; Levita, J.; Ibrahim, S.; J. App. Pharm. Sci., 2018, 8(03), 009-013. (b) Poerwono, H.; Hattori, Y.; Kubo, H.; Higashiyama, K. Indo. J. Chem., 2007, 7(2), 202-207.
CrossRef - Baran, P. S.; O’Malley, D. P.; Zografos, A. L. Angew. Chem., Int. Ed., 2004, 43, 2674-2677.
- Trost, B. M.; Andersen, N. G. J. Am. Chem. Soc., 2002, 124, 14320–14321.
CrossRef - Trost, B. M.; Thiel, O. R.; Tsui, H.-C. J. Am. Chem. Soc., 2003, 125, 13155 –13164.
CrossRef - Bandyopadhyay, D.; Banik, B. K. in Green Synthetic Approaches for Biologically Relevant Heterocycles., 2015.
- Gomha, S. M.; Edrees, M. M.; Faty, R.A. M.; Muhammad, Z. A.; Mabkhot , Y. N. Chemistry Central Journal., 2017, 11, (37), 1-12.
CrossRef - Katarina, M. P.G.; Evgenija, A. D. Mihaly, S.; Janos, G.; Janos. J. C. Steroids.,2015, 94, 31-40.
- Srinivas, N. A., Mahendar, P.; Sadanandam, A.; Achaiah, G.; Malla, R. V.; Medicinal Chemistry Research.,2013, 22, 147-155.
- Hue, T. B. B.; Quy, T. K. H.; Won, K. O.; Duy, D. V.; Yen, N. T. C.; Cuc, T. K. T.; Em, C. P.; Phuong, T. T.; Loan, T. T.; Hieu. V. M. Tetrahedron Letters.,2016, 57, 887-891.
- Adriana, I. G.; Luiza, G.; Victor, K.; Luminita, S.; Thomas, E.; Valentin, Z. Molecules., 2013, 18, 4679-4688.
CrossRef - Kumar, N.; Kumar, S.; Abbat, S.; Nikhil, K.; Sondhi, S. M.; Bharatam, P. V.; Roy, P.; Pruthi, V. Medicinal Chemistry Research.,2016, 25, 1175-1192.
- Jackson, T.; Lawrence Woo, L.W.; Trusselle, M.N.; Purohit, A.; Reed, M. J.; Potter, B.V.L.; ChemMedChem., 2008, 3, 603-18.
CrossRef - (a) Brouillette, W.J.; Jestkov, V.P.; Brown, M.L.; Akhtar, M.S.; DeLorey, T.M.; Brown, G. B. J. Med. Chem., 1994, 37, 3289. (b) Scicinski, J. J.; Barker, M. D.; Murray, P. J.; Jarvie, E.M. Bioorg. Med. Chem. Lett., 1998, 8, 3609-14.
CrossRef - (a) Osz, E.; Somsak, L.; Szilagyi, L.; Kovacs, L.; Docsa, T.; Toth, B.; Gergely, P. Bioorg. Med. Chem. Lett., 1999, 9, 1385-90. (b) Kuang, R.; Epp, J. B.; Ruan, S.; Yu, H.; Huang, P.; He, S.; Tu, J.; Schechter, N.M.; Turboy, J.; Froelich, C.J.; Groutas, W.C.A. J. Am. Chem. Soc., 1999, 121, 8128-9.
CrossRef - (a) Kuang, R.; Epp, J. B.; Ruan, S.; Chong, L. S.; Venkataraman, R.; Tu, J.; He, S.; Truong, T.M.; Groutas, J. C. Bioorg. Med. Chem. Lett., 2000, 8, 1005-16. (b) He, S.; Kuang, R.; Venkataraman, R.; Tu, J.; Truong, T.M.; Chan, H.; Groutas, C.; Bioorg. Med. Chem. Lett., 2000, 8, 1713-7.
CrossRef - (a) DeWitt, S.W.; Kiely, J.S.; Stankovic, C.J.; Schroeder, M.C.; Reynolds, D. M.; Pavia, M.R.; Proc. Natl. Acad. Sci. USA., 1993, 90, 6909-13. (b) Wu, S.; Janusz, J. M. Tetrahedron Lett.,2000, 41, 1165-9. (c) Albericio, F.; Bryman, L. M.; Garcia, J.; Michelotti, E. L.; Nicolas, E.; Tice, C. M. J. Comb. Chem., 2001, 3, 290-300. (d) Lamothe, M.; Lannuzel, M.; Perez, M. J. Comb. Chem.,2002, 4, 73-8. (e) Nefzi, A.; Giulianotti, M.; Truong, L.; Rattan, S.; Ostresh, J.M.; Houghten, R.A.; J. Comb. Chem., 2002, 4, 175-8.
- Lin, M.J.; Sun, C.M. Tetrahedron Lett.,2003, 44, 8739-42.
CrossRef - Song , S. J.; Cho, S. J.; Park, D. K.; Kwon, T. W.; Jenekhe , S. A. Tetrahedron Lett.,2003, 44, 255-257.
CrossRef - Staderini, M.; Cabezas, N.; Bolognesi, M. L.; Menéndez, J. C. Synlett., 2011, 17, 2577-2579.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










