Optimization of Some Parameters on the Low Efficiency of Solvent Extraction of Uranium with Alamine 336
Abdoul-Rachid Chaïbou Yacouba1 , Salmana Laouali Ibrahim2
, Salmana Laouali Ibrahim2 , Abdoul Razak Moumouni Wage2
, Abdoul Razak Moumouni Wage2 and Ibrahim Natatou1,2
and Ibrahim Natatou1,2
1Department of Chemistry, Faculty of Sciences and Techniques, Agadez University, P.O.B. 199, Agadez, Niger.
2Department of Materials, Water, and Environment Laboratory, Faculty of Sciences and Techniques, Abdou Moumouni University, P.O.B. 10662, Niamey, Niger.
Corresponding Author E-mail: arachidchaibou@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370129
Article Received on : 26-12-2020
Article Accepted on :
Article Published : 21 Jan 2021
Solvent extraction of uranium from sulfate liquor acid ore with Alamine 336 from two suppliers has been studied. The influence of various parameters, such as sulfuric acid concentration on uranium bearing solutions, concentration of Alamine 336, and concentration of uranium was investigated. The decrease of uranium efficiency extraction at the plant was caused by the degradation of the organic phase. Degradation caused by the presence of vanadium in the uranium ore. Two theoretical stages could efficiency extract more than 90% of uranium from a solution containing 3881 mg/L at O/A ratio of 1.5:1. At the range of sulfuric acid concentration of 0.1 M to 0.2 M, Uranium efficiency was enhanced from 89% to 92 at the 1st contact and from 18% to 20% at the second contact. At this range of concentration, the UO2(SO4)2/2 species predominate. For sulfuric acid concentrations over 0.2 M the uranium efficiency decreased due to the presence of UO2(SO4)2/2 and UO2(SO4)3/4 species. Improving volume percentage of Alamine 336 in organic phase enhanced the uranium efficiency to 99%.
KEYWORDS:Alamine 336; Niger Plant; Optimization; Solvent Extraction; Uranium
Download this article as:| Copy the following to cite this article: Yacouba A. R. C, Ibrahim S. L, Wage A. R. M, Natatou I. Optimization of Some Parameters on the Low Efficiency of Solvent Extraction of Uranium with Alamine 336 Orient J Chem 2021;37(1). |
| Copy the following to cite this URL: Yacouba A. R. C, Ibrahim S. L, Wage A. R. M, Natatou I. Optimization of Some Parameters on the Low Efficiency of Solvent Extraction of Uranium with Alamine 336. Orient J Chem 2021;37(1). Available from: https://bit.ly/3iuvs7w |
Introduction
The production of nuclear fuel at a competitive price compared with other sources of energy is a permanent concern of the nuclear industry.
The solution of this situation must necessarily go through the research and exploitation of new uranium deposits, but also through the mastery of nuclear technology to design very safe, reliable and environment-friendly nuclear power plants.
The Republic of Niger is located in West Africa with an area of 1.267.000 km2 annually produces more than 3.000 tons of uranium. In 2016, national production was 3.479 tones. So, it has risen to the 4th position among uranium producing countries in the world 1. However, it remains one of the poorest countries with a GDP, which ranks it 143rd in the world on 195. Uranium constitutes the country’s main source of income in the industrial field.
The uranium sites discovered in Niger are located in the northwestern part of the country, on the western border of the Aïr massif. The main mining sites in exploitation are the Arlit site, the Akouta site and the Azelik site respectively exploited by SOMAÏR, COMINAK and CNNC (chinese).
ORANO (formerly known as AREVA) is the majority shareholder of SOMAÏR and COMINAK 2. At the Akouta Mining Company, uranium is found in the Guezouman sandstone formations, whose mineralized can vary from 1 to 15 m with an ore concentration between 0.2% and 0.6% in uranium. The sterile cover is 250 m. These companies purify and concentrate uranium, which the final products are respectively sodium diuranate (Na2U2O7) and magnesium diuranate (MgU2O7).
Generally confined in uranium ore, vanadium, zirconium, and molybdenum are troublesome elements for the Republic of Niger, which prefers to valorize uranium.
These uranium companies use liquid-liquid extraction as the process to recover uranium. Tertiary amines such as Alamine 336 are widely used in the extraction of uranium.
Over the extraction cycles, the solvent loses its efficiency to extract uranium. This solvent is a mixture of Alamine 336 as extractant, isotridecanol as alcoholic modifier and kerosene as diluent. Some studies on the chemical mechanism of solvent degradation 3-5proved that the conversion of tri-n-octylamine were responsible of the low uranium rate recovering. Indeed, the presence of vanadium in the uranium bearing solutions on polyvanadates forms 6was majority responsible for that modification of Alamine 336. They also reported that the presence of other parameters like molecular dioxygen, chromium influenced the solvent degradation.
In this regard, we focused on the solvent extraction of uranium from uranium bearing solutions of Cominak plant. The effects of various parameters like contact number, ratio between organic and aqueous phase, uranium concentration, Alamine 336 concentration and sulfuric acid concentration were investigated in order to extract efficiency uranium.
Materials and Methods
The reactants
Alamine 336 (industrial grade, Cognis and BASF) was an anionic extractant with a flashpoint of 179 °C. Kerosene (industrial grade, Total) was used as diluent Table 1 shown some properties. Isotridecanol (analytical grade, VWR) was used to avoid the formation of the third phase with a flashpoint of 122.5 °C. The mixture of these reagents formed the organic phase.
Tri-octyl-phosphine oxide (TOPO) was a solvating or neutral extractant (purity > 99%, Merck), sulfuric acid (purity 95%, VWR), nitric acid (purity 65%, VWR), sodium fluoride (analytical grade, VWR) were used to prepare organic phase to re-extract uranium from aqueous samples. Pyridin (analytical grade, VWR), dibenzoylmethane (purity > 99%, Merck) were used to dose uranium.
Table 1: Kerosene propertie
|
Density (g/cm3) |
0.788 |
|
Viscosity (cP) |
1.64 |
|
Solubility in water at 20°C |
– |
|
Dielectric constant |
1.8 |
|
Boiling point (°C) |
150 |
|
Molecular weight (g/mol) |
170.34 |
|
Flash point (°C) |
54 |
The aqueous phases were prepared from uranium-bearing solutions, Fig.1 those last were obtained from the leaching of uranium ores process.
 |
Figure 1: Uranium solvent extraction process |
The solutions had a free acidity (H+) of 10 to 22 g/L and Table 2 shown the composition of some parameters. The experimental temperature was maintained at 30 °C.
Table 2: Composition of uranium-bearing solutions sample
|
Sample |
Concentration |
|
Uranium (mg/L) |
3698 |
|
Molybdenum (mg/L) |
52 |
|
Vanadium (mg/L) |
2423 |
|
Zirconium (mg/L) |
20 |
|
Iron (mg/L) |
465 |
|
Ph |
1 |
|
Redox potential (mV) |
496 |
Extraction procedure
The extraction process was done in mixer-settler. The shaking was set at 700 rpm with a Heidolph brand agitator. The pH was determined with Knick pH-meter 766-calimatic device and the redox with Hanna HI 98240 pH/ORP meters device. In the mixer-settler 100 mL of aqueous phase containing the metal and 100 mL of organic phase containing Alamine 336 were brought into 2 minutes, sufficient time for the transfer of metal from one phase to the other. At the end of the extraction, the two phases were separated by decantation.
The analysis of the aqueous samples was done after an adequate dilution. The test samples depended on the uranium concentration in the aqueous solutions (directly for weak concentrations and by dilution for high concentrations).
In pill jar containers of 60 mL according to the test samples, the following additions were made: solution of HNO3 at 10%; solution of NaF as oxidant; solution of TOPO as extractant. The whole were stirred for 10 minutes. After decantation, the organic phase (TOPO) was carefully removed. It was taken using a diluting device. The setting was of 1 mL of the organic phase for 3 mL of the DBM solution. The formation of a U-DBM complex of yellow coloring in pyridine medium was obtained.
The uranium was analyzed with a Varian Cary 50 uv-vis spectrophotometer at 405 nm.
The determination of molybdenum in the aqueous phase was carried out directly after an adequate dilution with solution of Al(NO3)3, solution of KCl, and solution of HNO3. Molybdenum was analyzed using a Varian AAFS240 atomic absorption spectrophotometer equipped with an acetylene-nitrous oxide burner at 313 nm.
The distribution coefficient relation of Eq. (1) and the extraction efficiency percentage relation of Eq. (2) were respectively determined by the following formulas:
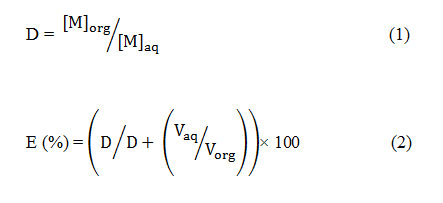
[M]org= Metal concentration in the organic phase (mg/L)
[M]aq= Metal concentration in the aqueous phase (mg/L)
Results and Discussions
Effect of Alamine 336 degradation
In order to study the behavior of the solvent during the extraction process, Alamine 336 from two suppliers (Cognis and BASF) and Alamine 336 (Cognis) regenerated from the plant after several extraction cycles were investigated. The aqueous phase had uranium concentration of 3698 mg/L and pH = 1. The redox potential was 498 mV and the organic phase had an Alamine 336 concentration of 0.1 M in kerosene. The extraction was carried out in 5 contacts with an O/A ratio of 0.5. The results are shown in Fig.2. The solvent formed from Alamine 336 (Cognis and BASF) exhibited the same extraction behavior. Whereas, the regenerate solvent the uranium extraction decreased. Fig.3 shown the total amount of uranium extracted with the 3 solvents after the 5 extraction cycles. The decrease of the extraction efficiency for the regenerated solvent may be due to the loss of Alamine 336 capacity to extract uranium. Indeed, according to Chagnes7works on the improvement resistance of solvent degradation of COMINAK plant, Alamine 336 had tended to transform into secondary amine, primary amine and other compounds. This degradation was due to the presence of powerful oxidants in ore such as vanadium 8and chromium 9.Abdoul-Rachid10reported also that beyond 700 mV, Alamine 336 effectively extracted vanadium, which automatically caused the decrease of uranium extraction efficiency.
For following works Alamine 336 supplied by Cognis was used.
 |
Figure 2: Effect of Alamine 336 degradation on uranium extraction (U = 3698 mg/L, Alamine 336 = 0.1 M, O/A = 1:2, pH = 1, T = 30 °C, E = 498 mV, agitation: 700 rpm, mixing time: 2 min) |
 |
Figure 3: Organic solvent charged after 5 cycles (U = 3698 mg/L, Alamine 336 = 0.1 M, O/A = 1:2, pH = 1, T = 30 °C, E = 498 mV, agitation: 700 rpm, mixing time: 2 min) |
Effect of contact number on uranium and molybdenum extraction from uranium bearing solutions
The contact number consists of maintaining a constant volume of organic and varying the volume of aqueous phase after each 2 min of agitation. The aqueous phase contained the following metals: U = 3698 mg/L, Mo = 65 mg/L, V = 2370 mg/L and pH = 1. The redox potential was 498 mV and the free acidity was H+ = 22 g/L. The organic phase had an Alamine 336 concentration of 0.1 M in kerosene. The extraction was carried out in 5 contacts. Fig.4 illustrates the effect of contact number on uranium extraction efficiency. Uranium extraction was effective at the first contact with 38%. Molybdenum efficiency extraction decreased from 7% to the first contact to 3% at second contact. At 498 mV Alamine 336 did not extract vanadium. The weak extraction efficiency may be due to these parameters low phase ratio O/A, sulfuric acid concentration of uranium bearing solutions.
 |
Figure 4: Effect of contact number on uranium and molybdenum from uranium bearing solutions (U = 3698 mg/L, Mo = 65 mg/L, Alamine 336 = 0.1 M, O/A = 1:2, pH = 1, T = 30 °C, E = 498 mV, agitation: 700 rpm, mixing time: 2 min) |
McCabe Thiele isotherm
In order to obtain the optimal contact number for the extraction, McCabe Thiele isotherm was plotted. The aqueous phase had a uranium concentration of 3881 mg/L. The free acidity was H+ = 12 g/L and redox potential was 498 mV. The organic phase had an Alamine 336 concentration of 0.1 M in kerosene. The ratios O/A were varied from 1:2, 1:1, 1.5:1 and 2:1. According, to results shown at Fig.5. Two theoretical stages were required to extract efficiency uranium from uranium bearing solutions 11. Collet 12studies on computer simulation and optimization of flow sheets reported also that with two feed inlets uranium could be recovery efficiency.
 |
Figure 5: Uranium extraction distribution isotherm Click here to View figure |
In order to increase the uranium extraction efficiency between the 1st and 2nd contact, parameters illustrated in section 3.4 to 3.7 were studied.
Effect of the ratio of organic phase and aqueous phase
The aqueous phase contained the following metals: U = 3881 mg/L, Mo = 60 mg/L, V = 2370 mg/L. The free acidity was H+ = 12 g/L and the redox potential was 496 mV. The organic phase had an Alamine 336 concentration of 0.1 M in kerosene. The extraction was carried out in 2 contacts. The effect of O/A was shown at Fig.6. The variation of ratio O/A increased the uranium extraction efficiency from 39.47% up to 99.67% at the 1st contact and from 5.16% up to 94% at the second contact.
 |
Figure 6: Effect of ratio O/A on uranium extraction (Alamine 336 = 0.1 M, pH = 1, T = 30 °C, E = 496 mV, agitation: 700 rpm, mixing time: 2 min) |
Effect of sulfuric acid concentration
The aqueous phase contained the following metals: U = 3619 mg/L, Mo = 50 mg/L, V = 2180 mg/L. The free acidity was H+ = 10 g/L and the redox potential 500 mV. The organic phase had an Alamine 336 concentration of 0.1 M in kerosene. The ratio O/A = 1:1. The sulfuric acid concentration of uranium bearing solutions was varied from 0.1 to 1 mol/L. Fig.7 illustrates the effect of sulfuric acid concentration on uranium extraction. For low sulfuric acid concentration (0.1-0.2) in the uranium bearing solutions, extraction efficiency increased from 88.91 up to 92% at the 1st contact and from 18.42% up to 20% at the second contact. This enhancement of extraction efficiency may be due to UO2 (SO4)2/2 species which was majority in the solution at these range of concentrations. For lower sulfuric acid concentration uranium was in its UO2 (SO4)2/2 and UO2(SO4) species in uranium bearing solutions 13. In similar conditions [(R3NH)4UO2(SO4)3]complex was obtained during the extraction of UO2 (SO4)2/2 by Alamine 336 14-15.
 |
Figure 7: Effect of sulfuric acid concentration on uranium extraction (Alamine 336 = 0.1 M, T = 30 °C, E = 500 mV, agitation: 700 rpm, mixing time: 2 min) |
The uranium extraction efficiency decreased when sulfuric acid concentrations were enhanced up to 0.2 M. Fig.8 shown the diagram of the different uranium species present in strong sulfuric acid solutions 16.Indeed, this drop of uranium extraction efficiency may be due to the different extraction mechanism because of the different speciation of uranium UO2 (SO4)2/2 and UO2 (SO4)4/3 present in uranium bearing solutions. E.C Avelar 17 studies on the modeling of the solvent extraction equilibrium of uranium reported that at higher concentration of sulfate ions UO2 (SO4)4/3 species were formed.
Therefore, the mechanisms of uranium extraction can described by the following equations:
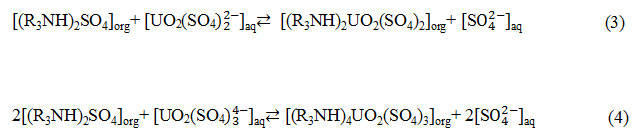
 |
Figure 8: Speciation diagram of uranium-sulfate-water system |
Effect of uranium concentration
Various industrial solutions loaded with uranium were used. Barren liquor from the second filtration with U = 450 mg/L, production liquor from the first filtration with U = 1064 mg/L, bearing solution with U = 3619 mg/L and uranium bearing solution from storage tank with U = 7112 mg/L. The redox potential was 500 mV. The organic phase had an Alamine 336 concentration of 0.1 M in kerosene. The ratio O/A = 1 and the extraction was carried out in 2 contacts. Fig. 9 illustrates the results. The extraction was effective for uranium concentration of 1000 mg/l with efficiency percentage exceeding 90%. The drop of the uranium percentage reflected the saturation of the complexing sites of Alamine 336. This saturation may be due to also to the UO2 (SO4)4/3 speciation and 4 molecules of Alamine 336 complex one molecules of uranium sulfate according to the 1:4 stoichiometry 18-19.
 |
Figure 9: Effect of uranium concentration variation (Alamine 336 = 0.1 M, pH = 1, T = 30 °C, E = 500 mV, agitation: 700 rpm, mixing time: 2 min) |
Effect of volume proportion of Alamine 336 in solvent formation
The aqueous phase contained the following metals: U = 3619 mg/L, Mo = 50 mg/L, V = 2180 mg/L. The redox potential was 500 mV and the free acidity was H+ = 10 g/L.
The proportion of Alamine 336, alcohol (Isotridecanol) and kerosene were varied and were illustrated in table 3.
Table 3: Organic phase composition
|
Alamine 336 percentage |
Isotridecanol percentage |
Kerosene percentage |
|
2.5 |
3 |
94.5 |
|
5 |
10 |
85 |
|
7.5 |
3 |
89.5 |
|
10 |
10 |
80 |
The enhancement Fig.10 of Alamine 336 proportion in the organic phase formation increased the uranium extraction efficiency from 78.72% up to 99.58% at the first contact and from 4.61% up to 95.79% at the second contact. The addition of isotridecanol prevented the formation of a third phase. Zhu20reported that using equal concentration percentage of isodecanol and Aliquat 336 eliminated the third phase formation.
 |
Figure 10: Effect of Alamine 336 volume proportion on uranium extraction (Alamine 336 = 0.1 M, pH = 1, T = 30 °C, E = 500 mV, agitation: 700 rpm, mixing time: 2 min) |
Conclusion
The solvent extraction of uranium from sulfate liquor acid of Niger plant by Alamine 336 in kerosene modified with isotridecanol was investigated. The weak extractability of uranium was due to the degradation of Alamine 336, degradation caused by the presence of vanadium, which was an oxidant. According to McCabe Thiele isotherm, two theoretical stages were required to extract efficiency uranium. The variation of the O/A ratio from 0.5 to 2 had made it possible to recover more than 90% of uranium at the first contact. The variation in the H2SO4 concentration of the uranium liquor from 0.1 to 0.2 had also made it possible to recover more than 92% of uranium at first contact. For concentrations lower than 0.2 M the species UO2 (SO4)2/2 was predominant while for concentrations higher than 0.2 M it had coexistence of the UO2 (SO4)2/2 and UO2 (SO4)4/3 species. The variation of the volume proportions of Alamine 336 and kerosene had made it possible to raise the uranium efficiency more than 98% with a volume percentage of Alamine 336 of 10 in the organic phase.
Acknowledgements
The authors are thankful to director of Akouta Company COMINAK for his permission to do and publish this work.
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- IAEA/OECD. Uranium Resources , Production and Demand. NEA No. 7413. Red Book, 2018, 317 – 326.
- Billon, S. Clay minerals in uraniferous deposit of Imouraren (Tim Mersoï basin, Niger): implications on genesis of deposit and on ore treatment process. PhD Thesis, Poitiers University, France, 2014, 23-24. http://theses.univpoitiers.fr/notice/view/32762 [(04/11/20)]
- Zhu, Z.; Cheng, C.Y. A review of uranium solvent extraction: its present status and future trends. ALTA 2011 Uranium Conference: 7th Uranium Event, Australia, 2011, 215-220. https://www.altamet.com.au/wpcontent/uploads/2013/07/ALTA-Uranium-2011-Proceedings-Contents-Abstracts.pdf
- Munyungano, B.M. Solvent degradation-Rössing uranium mine. J. S. Afr. Inst. Min. Metall., SAIMM Conference, 2007, 107(7), 415-417.
- Sole, K.C.; Cole, P.M.; Feather, A.M.; Kotze, M.H. Solvent extraction and ion exchange applications in Africa’s resurging uranium industry: A review. Solvent Extr. Ion Exch., 2011, 29(5-6), 868–899. https://doi.org/ 10.1080/07366299.2011.581101
CrossRef - Chagnes, A.; Fosse, C.; Courtaud, B.; Thiry, J.; Cote, G. Chemical degradation of trioctylamine and 1-tridecanol phase modifier in acidic sulfate media in the presence of vanadium (V).Hydrometallurgy, 2011,105(3-4), 328–333. https://doi.org/10.1016/j.hydromet.2010.11.003
CrossRef - Chagnes, A.; Courtaud, C.B.; Thiry, J. Improved resistance to degradation of the extraction solvent used at the Cominak plant (Niger). SEPA Report No. 2, Contract No.: DIR ∕ 2008-196, 2009.
- Chagnes, A.; Courtaud, B.; Thiry, J.; Bayardon, J.; Jugé, S.; Cote, G. Influence of phase modifiers on the degradation of Tri-n-octylamine/dodecane extracting mixture by an acidic solution of Vanadium (V).Solvent Extr. Ion Exch., 2012, 30(1), 67–76. https://doi.org/10.1080/07366299.2011.566524
CrossRef - Chagnes, A.; Cote, G. Chemical degradation of amixture of tri-n-octylamine and 1-tridecanol in the presence of chromium(VI) in acidic sulfate media. Metals, 2018, 8(1), 1–10. https://doi.org/ 10.3390 /met8010057
CrossRef - Abdoul-Rachid, C.Y.; Salmana, L.I.; Adamou, Z.; Ibrahim, N.; Ibrahim, M. Comparative study of solvent extraction of uranium with Alamine 336 and Aliquat 336: Application to the uranium-bearing solutions of Niger Republic. Eur. Sci. Journal, ESJ, 2018, 14(9), 76-92. https://doi.org/ 10.19044/esj.2018.v14n9p76
CrossRef - Chagnes, A.; Courtaud, B.; Thiry, J.; Cotea, G. Computer simulation of flow-sheets for the solvent extraction of uranium: A new route to delay the effect of chemical degradation of the organic phase during uranium recovery from acidic sulfate media.J. Chem. Technol. Biotechnol., 2009, 84(12),1899–1907. https://doi.org/10.1002/jctb.2263
CrossRef - Collet, S.; Chagnes, A.; Courtaud, B.; Thiry, J.; Cote, G. Solvent extraction of uranium from acidic sulfate media by Alamine® 336: Computer simulation and optimization of the flow-sheets.J. Chem. Technol. Biotechnol.,2009, 84(9), 1331–1337. https://doi.org/10.1002/jctb.2184
CrossRef - Chagnes, A.; Courtaud, B.; Thiry, J. Formulation of a new extraction solvent for the Cominak plant (Niger).SEPA report, Contract: DIR ∕ 2011, 2011.
- Ramadevi, G.; Sreenivas, T.; Navale, A.S.; Padmanabhan, N.P.H. Solvent extraction of uranium from lean grade acidic sulfate leach liquor with alamine 336 reagent.J. Radioanal. Nucl. Chem.,2012, 294(1), 13–18. https://doi.org/10.1007/s10967-011-1507-y
CrossRef - Jin-Young, L.; Jyothi, R. K.; Ho-Seok, J.; Joon-Soo K. Extraction and separation of hexavalent molybdenum from acidic sulfate solutions using Alamine 336 as an extractant. Period. Polytech. Chem. Eng.,2010, 54(1), 27–31. https://doi.org/10.3311/pp.ch.2010-1.04
CrossRef - Vercouter, T.; Vitorge, P.; Amekraz, B.; Moulin, C. Stoichiometries and thermodynamic stabilities for aqueous sulfate complexes of U(VI).Inorg. Chem.,2008. 47(6), 2180–2189. https://doi.org /10.1021 / ic701379q
CrossRef - Avelar, C.; Alvarenga, C.L.G.; Resende, G.P.S.; Morais, C.A.; Mansur, M.B. Modeling of the solvent extraction equilibrium of uranium (VI) sulfate with Alamine 336.Brazilian J. Chem. Eng.,2017, 34(1), 355–362. https://doi.org/10.1590/01046632.20170341s20150301
CrossRef - Khanramaki, F.; Shirani, A.S.; Safdari, J.; Torkaman, R. Investigation of liquid extraction and thermodynamic studies on uranium from sulfate solution by Alamine 336 as an extractant.Int. J. Environ. Sci. Technol.,2018, 15(7), 1467–1476. https://doi.org/10.1007/s13762-017-1473-1
CrossRef - Zaheri, P.; Davarkhah, R. Selective separation of uranium from sulfuric acid media using a polymer inclusion membrane containing alamine 336. Chem. Pap., 2020, 1–9. https://doi.org/ 10.1007/s11696-019-01029-9
CrossRef - Zhu, Z.; Pranolo, Y.; Cheng, C.Y. Uranium solvent extraction and separation from Vanadium in alkaline solutions.Sep. Sci. Technol.,2013, 48(9), 1402–1408.
https://doi.org/10.1080/01496395.2012.738277
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









