Effect of Leaves and Acids types on The Electrical Absorbance of DSSC using Nanostructured Zinc Oxide
Saeed Ahmed Al-Ghamdi
Department of Electrical Engineering, Faculty of Engineering Albaha University, Albaha, Kingdom of Saudi Arabia.
Corresponding Author E-mail:sasg2000@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370125
Article Received on : 07 nov 2020
Article Accepted on :
Article Published : 20 Jan 2021
Solar energy is the most promising sources of energy in KSA due to the constant high solar radiation throughout the year. This not used effectively due to the solar cells rising costs. Nano solar cells are the promising alternative in comparison to silicon solar cells due to its lower cost. leaves of green cabbage and orange plant used as sensitizers. The effect of extracted temperatures, and immersed periods on the energy conversion efficiency is obtained. Among the previous dyes, orange showed the best efficiency. The Nano dye sensitized solar cells (NDSSC’s) performance improved through controlling the extraction solution temperature and both the pre-treatments of the Nano zinc oxide glass substrate and its post-treatments films using hydrochloric, phosphoric, and nitric acids. The max. conversion efficiency is 0.141 at 60OC and 0.11 at 45OC for both orange and green cabbage respectively. The pre-treatment of the FTO with HCL, HNO3 and H3PO4 showed an efficiency improvement of 214%, 180% and 140% at 8 hrs using orange leaves dye, and 207%, 184% and 152% respectively at 6 hrs using green cabbage .
KEYWORDS:Energy Conversion Efficiency and Spectrum Absorption; Green Cabbage and Orange; Hydrochloric, Phosphoric, and Nitric Acids; Nano Dye Sensitized Solar Cells; Nano Zinc Oxide;
Download this article as:| Copy the following to cite this article: Al-Ghamdi S. A. Effect of Leaves and Acids types on The Electrical Absorbance of DSSC using Nanostructured Zinc Oxide. Orient J Chem 2021;37(1). |
| Copy the following to cite this URL: Al-Ghamdi S. A. Effect of Leaves and Acids types on The Electrical Absorbance of DSSC using Nanostructured Zinc Oxide. Orient J Chem 2021;37(1). Available from: https://bit.ly/3sOlWB1 |
Introduction
Petroleum derivatives considered a non-sustainable power source, sun considered power source, limited to silicon sunlight cells, high cost, complex generation process, and application limitations to astronautic and aerodynamic innovation,[1-2]. Abo-Dief et al. [3] produced biodiesel from used oil produced from natural vegetables and fruits. While Iwuji et al. [4] concluded that the titanium dioxide solar cell efficiency was 0.04%.
Derdakh et al. [5] stated that the Dye-sensitized solar cell (DSSC) efficiency enhancing controlling the economic conversion of solar energy into electrical energy. Syafinar et al. [6] found that the photocatalytic efficiency of inflorescence dyed TiO2 nanoparticle was 0.78%. They considered their investigation paves new way to develop the DSSC. The TiO2 semiconductor layer using a nitric acid post-treatment showed an increase in dye sensitized solar cell efficiency by 128%, [7]. Pamain et al. [8] successfully converted visible light into electrical output. They found that the cells efficiency and characteristics correlated to the dye absorption spectra. Dumbrava et al. [9] used natural dyes from chlorophyll and spinach as a sensitizer. Amad et al. [10] showed that using natural dyes as sensitizers are promising because of their environmental friendliness, low-cost production and designable polychrome modules.
Khashan and Abbas [11] considered Indium nitride (InN) nanoparticles (NPs) are a potentially important material for optoelectronic and high speed electronic devices. Saleh [12] declared that anatase-TiO2 nano-crystalline powder successfully synthesized using the chemical hydrolysis technique with some modifications. The highest conversion efficiency obtained for the DSSC fabricated using a mixture from purple cabbage and purple carrots. Khashan and Mohsin [13] synthesized carbon nitride (C3N4) nanoparticles (NPs) by pulse laser ablation of graphite in ammonium solution, and deposited on silicon substrates by spray. Khashan et al. [14] synthesized carbon nanostructures by laser ablation of graphite in de-ionized water. Ikeogu et al. [15] used TiO2 as photo anode and Green Cabbage as photosensitizer for fabricated dye sensitized solar cell. Ismail et al. [16] fabricated low cost ZnO/Si and ZnO/MgO/Si heterojunction (HJ) photo detectors using laser ablation and spray Pyrolysis techniques. Orabi et al. [17] treated used automotive engine oil using a microwave-induced pyrolysis process. Ismail et al. [18] synthesized embedding cadmium sulfide nanoparticles by a chemical method. While, Li et al. [19] used red cabbage that improves the DSSC performance. Amad et al. [10] improved the methodology of producing DSSC’s by the process of amalgamation with natural dyes. The experimental results found that the dye extract from Green Cabbage produced conversion efficiency (η) up to 0.1%. Sinha et al. [20] enhanced the efficiency of DSSC using ZnO and mixed natural dye.
The present work aimed at improving the DSSC’s performance through adjustment of the solution extraction temperature, the pre-treatments of the Nano zinc oxide glass substrate and post-treatments of the Nano zinc oxide film using hydrochloric (HCl), phosphoric (H3PO4), and nitric (HNO3) acids. The effect of both test sensitization temperatures and period has a greatest effect on the spectrum absorption carried out and investigated.
Materials and Methods
An electric blender pulverized the smaller, washed and dried pieces of green cabbage and orange leaves into powder to reduce the surface area. An electronic weighing balance used to weight 10g of the powder that mixed with 100 mL of ethanol and agitated using a magnetic stirrer for 1 hour to obtain a homogenous mixture. The homogenous mixture stand for 24 hours, filtered to obtain dye pigment, stored in a labeled sample bottle and mixed with 0.5 g of the Nano zinc oxide powder containing 3.5 mL of acetic acid. Mortar and pestle techniques used for forming the mixture into pasty, fine and homogeneous substance.
The NZnOfilm immersed in the natural dye solutions for three days to be absorbed then dried and mixed with two drops of iodide electrolyte that composed of a mixer of 2.0 g of iodine (I2), 3g of potassium iodide (KI) and 50 mL of deionized water. The solution diluted in 100 mL and stirred to dissolve. The counter electrodes (cathode) on the other side of the cell typically coated with graphite and attached with photo anodes glass plate. The two sides of photo electrodes clipped by binder clips to complete the assembled NDSSC. The complete NDSSC with each dye characterized using current-voltage characteristic to compare their efficiencies and both the voltage reading obtained and the current value was calculated using the Ohm’s Law.
Under sun light, the interaction between the dye molecules and the photons cause their photo-excitation, thereby generating electron hole pairs that injected into the conduction band of the semiconductor NZnO. The oxidized state of the dye containing the holes is rapidly reduced by the electrolyte, thus regenerating the dye, this prevents back transfer of electrons. The electrons travel through the semiconductor to the transparent electrode, the external load and eventually reach the counter electrode and regenerate the electrolyte the energy conversion (Fig. 1).
 |
Figure 1: Complete Dye-sensitized Solar Cell assembly. |
The experiments carried out at temperature of; 20ºC, 40ºC, 60ºC, 80ºC and 100ºC, and test period ranging from 2 hrs to 12 hrs using ethanol as an extracting solvent to get the optimum values of temperatures and test periods. The effect of post-treatments using hydrochloric, phosphoric, and nitric acids on NDSSC performance will be obtained. The short circuit current density (Jsc), open circuit voltage (Voc), fill factor (FF) and the under sunlight irradiation conversion efficiency are determined.
Results and Discussions
 |
Figure 2: Variation of sun light absorbance and wave length for cabbage and orange dyes. |
Figure 2. showed the absorption spectra analyses of green cabbage and orange extracts. Green cabbage has two peaks at 3600 and 900 nm and orange has a peak at 600nm which means that the two natural extracts absorbed light spectrum and hence fulfilled the primary criterion for their use as sensitizers in DSSCs in agreement with Martínez et al. [21].
The photovoltaic performances of DSSCs using natural dyes as photosensitizer were determined by recording the current-temperature and (J-Temp.) and current-time (J-Time) curves as displayed in Fig. 3 and Fig. 4. It is clear that the optimum temperature for short circuit current is 60O for both cabbage and orange while for maximum circuit current, it is 42O for green cabbage and 60O for orange. But for test period, both the short circuit current and maximum current is optimum at 6 hrs. This is due to the dye extracted greater complete absorbance of light. The reason is that the dye extracted at 60 °C due the best stability and slowest degradation rate of pigments at 60ºC, [22].
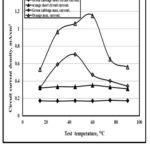 |
Figure 3: Variation of Jsc and Jm with temp. Click here to View figure |
 |
Figure 4: Variation of Jsc and Jm with time. |
Figures 5 and 6 illustrated the variation of both open circuit and max. voltage of both test temperature and period for both green cabbage and orange. The open circuit and max. circuit voltage for both green cabbage and orange are at 42OC and 60OC respectively. The highest Voc (0.33 V) and (0.46V) while Vmax. (0.31V) and (0.28V) with test temperatures for both green cabbage and orange respectively. But for test periods, these values were 0.46, 0.30, 0.42 and 0.21 for both the open circuit and max. voltage for green cabbage and orange.
 |
Figure 5: Variation of Voc andVm with temp. Click here to View figure |
 |
Figure 6: Variation of Voc and Vm with time. Click here to View figure |
Figures 7 and 8 showed the variation of Fill Factor % with both test temperatures and times for both green cabbage and orange. It is clear that the max. Fill Factor% is 55% and 32% at 60O and 28% at 8 hrs and 14% at 6 hrs for both orange and green cabbage respectively.
 |
Figure 7: Effect of FF% on test temperature. Click here to View figure |
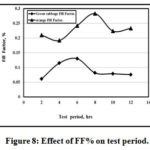 |
Figure 8: Effect of FF% on test period. Click here to View figure |
Figures 9 and 10 illustrated the effect of both test temperatures and periods on the power conversion efficiency of both green cabbage and orange. It is clear that the max. conversion efficiency is 0.141 at 60OC and 0.11 at 45OC for both orange and green cabbage respectively. While Fig. 9 showed that the max. conversion efficiency is 0.121 at 6 hrs and 0.075 at 8 hrs for both orange and green cabbage respectively.
 |
Figure 9: Variation of η% with test temperature. |
 |
Figure 10: Variation of η% with test period. |
The efficiencies of DSSCs sensitized with orange dyes are significantly higher than green cabbage due to :
The photo nodes of green cabbage has limited visibility as photosensitizer because it is a poor source of chlorophyll according to Ikeogu et al. [15].
Orange leaf is a rich source of chlorophyll compared to green cabbage, so it has higher visibility as photosensitizer and higher conversion efficiency compared to green cabbage.
Higher light absorption intensity and interaction of orange/Nano ZnO pigment compared to green cabbage/ NZnO produced better charge-transfer performance and higher efficiency, [22].
 |
Figure 11: Green cabbage conversion η at |
 |
Figure 12: Orange conversion η at various |
Figures 11 and 12 showed the relation between the conversion efficiency and test periods with hydrochloric (HCl), phosphoric (H3PO4) and nitric (HNO3) acids. The figures showed that the DSSCs conversion efficiency increased with acids pretreatment. The pre-treatment of the FTO with HCL showed an 214% and HNO3 and H3PO4 showed an improved efficiency of 180% and 140% at 8 hrs using orange leaves dye, while the pre-treatment of the FTO with HCL using orange leaves dye showed an improved efficiency of 207% and HNO3 and H3PO4 showed an improved efficiency of 184% and 152% respectively at 6 hrs. This is due to the bonding strength enhancement between the FTO substrate and the Nano ZnO layer and blocking the charge recombination between the electrons in the FTO and the holes in I-/Ι-3 redox couple that resulted in forming a dense layer of NZnO on FTO which impede carriers recombination between Ι-3 and FTO, thus, the short-circuit photocurrent density is improved, so the conversion efficiency is increased in agreement with Chang et al. [23].
Conclusions
The following conclusions are obtained;
The constructed NDSSCs showed highest Voc (0.33 V) and (0.46V) while Vmax. (0.31V) and (0.22V) with test temperatures for both green cabbage and orange. But for test periods, these values were 0.46V, 0.30V, 0.42V and 0.21V for both the open circuit and max. voltage for orange and green cabbage respectively.
The optimum temperature for short circuit current is 60O for both cabbage and orange while for max. circuit current, it is 42O for green cabbage and 60O for orange. But for test period, both the short circuit current and max. current is optimum at 6 hrs. The Fill Factor% is 55% and 32% at 60O and 28% at 8 hrs and 14% at 6 hrs for both orange and green cabbage respectively.
The max. conversion efficiency is 0.141 at 60OC and 0.11 at 45OC for both orange and green cabbage respectively.
The pre-treatment of the FTO with HCL, HNO3 and H3PO4 showed an efficiency improvement of 214%, 180% and 140% at 8 hrs using orange leaves dye, and 207%, 184% and 152% respectively at 6 hrs using green cabbage.
Acknowledgement
Author is thankful to Albaha University for sponsoring and supporting this research under the project No. (10-1441) and enabling him to develop this work.
References
- Abualnaja, K. M.; Abo-Dief, H. M.; Abu-Ali, O. A.; Amin, M. T.; and Mohamed, A. T. Proceedings of Academics World Int. Conference, Jeddah, KSA, 14-15 December 2019, 14-19.
- Mohd, R.; Khan, B.; Gupta; P., Parvaz, M.; Ahmad, U.; Singh, P.; Singh, R.; Bhattacharya, B.; Khan, Z. Journal of Materials Science & Surface Engineering, 2017, 5, pp. 722-728.
- Abo-Dief, H. M.; Emam, A. S.; Abualnaja, K. M.; Mohamed; A. T. Oriental Journal of Chemistry, 2018, 34, 1011-1015.
CrossRef - Iwuji, C.; Ghann, W.; Iwuji, O.; Uddin, J. Nanoscience Journal, 2018, 1, 1-4.
- Derdakh, C.; Zerrouki, S.; Henni, A. Mater. Biomater. Sci., 2019, 02, 024–027.
- Syafinar, R.; Gomesh, N.; Irwanto, M.; Fareq, M.; Irwan, Y. M. Energy Procedia, 2015, 79, 896 – 902.
CrossRef - Dawoud, A. M.Sc. Thesis, Islamic University of Gaza, 2016.
- Pamain, A.; Pogrebnaya, T.; King’ondu, C. Research Journal in Engineering and Applied Sciences, 2014, 3, 332-336.
- Dumbrava, A.; Lungu, J.; Ion, A. Scientific Study & Research Chemistry & Chemical Engineering, Biotechnology, Food Industry, 2016, 17, 013 – 025.
- Amad, L.; Jenny, S.; Ahmed, A.; Brown, N.; Yadav, S.; Brown, D.; Ghann, W.; Gayrama, A.; Jiru, M.; Uddin, J. Nano science and Nano engineering, 2016, 3, 25-32.
CrossRef - Khashan, K.; and Abbas, S. International Journal of Modern Physics, 2016, 30, 20-30.
CrossRef - Saleh, H. Australian Journal of Basic and Applied Sciences, 2018, 12, 17-23.
- Khashan, K.; and Mohsin, M. Surface Review and Letters, 2015, 22, 4-8.
CrossRef - Khashan, K.; Jabir, M; and Abdulameer, F. Surface Review and Letters, 2019, 26, 10-19.
CrossRef - Ikeogu, I.; Adeniyi, O.; Aboje, A. International Journal of Engineering Research and Advanced Technology (IJERAT), 2018, 4, 1-6.
- Ismail, R.; Khashan, K.; Jawad, M; Mousa, A; and Mahdi, F. Materials Research Express, 2018, 5, 5-10.
CrossRef - Orabi, R.; Abo-Dief, H; and Mohamed, A. International Journal of Emerging Trends in Engineering and Development (IJETED), 2015, 5, 91-101.
- Ismail, R; Khashan, K.; and Alwan, A.Silicon, 2017, 9, 321-330.
CrossRef - Li, Y.; Ku, S.; Chen, S.; Ali, M.; AlHemaid, F. Int. J. Electrochem. Sci., 2018, 8, 1237 – 1245.
- Sinha, D; Deb, D; Goswami, D.; Ayaz, A. Materials Today: Proceedings, 2018, 5, 2056–2063.
CrossRef - Martínez, A; Estevez, M.; Vargas, S.; Quintanilla, F.; Rodríguez, R. Journal of Applied Research and Technology, 2012, 10, 38–47.
- Calogero, G.; Yum, J.; Sinopoli, A.; Marco, G.; Grätzel, M.; Nazeeruddin, M. Solar Energy, 2012, 86, 1563-1575.
CrossRef - Chang, H.; Wu, H.; Chen, T.; Huang, K; Lo, Y. Journal of Alloys and Compounds, 2010, 95, 606-610.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









