Comparative Study of Solvation Behaviour of Oxidising Agents Like Kclo3, Kbro3 and KIO3 in Aqueous Solvent Systems at Different Temperatures
Department of Chemistry, RNC Arts, JDB Commerce, NSC Science College, Nashik Road, Nashik, Maharashtra, India.
Corresponding Author E-mail: meenakship2@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/370120
Article Received on : 04-Dec-2020
Article Accepted on :
Article Published : 12 Feb 2021
The investigation of the solvationtrend of oxidizing agents like KClO3, KBrO3 and KIO3as electrolytes in aqueous salt solution rendersthe datasuited to interpret ion–ion, solute–solvent, ion-solvent and solvent–solvent interactions and synergy. Apparent molar volumes (∅_V) and viscosity B-coefficients for KClO3, KBrO3 and KIO3solutions in aqueous 0.5 % KCl ,system have been calculated from density (ρ) and viscosity (η) measurements at 298.15 to 313.15 K using a calibrated bicapillary pycnometer and the simple, yet accurate apparatus known as Ubbelohde viscometer respectively. Jones-Dole equation,Masson’s equation, Roots equation and Moulik’s equations are implemented to analyse various interactions inter and intra ionic attractions among the ion–ion, ion–solvent, and solute–solvent. Additionallythe apparent molar volumes of transfer Δ ∅(tr) and Rate constant diffusion controlled reaction (kd)are valuated.
KEYWORDS:B-Coefficient; Density; KBrO3; KClO3; KIO3; Kd; Viscosity
Download this article as:| Copy the following to cite this article: Rathi M. V. Comparative Study of Solvation Behaviour of Oxidising Agents Like Kclo3, Kbro3 and KIO3 in Aqueous Solvent Systems at Different Temperatures. Orient J Chem 2021;37(1). |
| Copy the following to cite this URL: Rathi M. V. Comparative Study of Solvation Behaviour of Oxidising Agents Like Kclo3, Kbro3 and KIO3 in Aqueous Solvent Systems at Different Temperatures. Orient J Chem 2021;37(1). Available from: https://bit.ly/2Ngs9Fo |
Introduction
Generally, an oxidizing agent donates oxygen atoms to a reactant or substrate and hence the oxidizing agent is also called as an oxygenation reagent or oxygen-atom transfer agent [1] or it also serve as electron acceptors. Apart from oxidising agent, KBrO3 has been used as a food additive, mainly in the bread-making process, flour treatment, as a component of cold-wave hair lotions.Though potassium bromate (KBrO3) is banned for food use, it is used as a flour improver and high riser in bakery industries. It is known as a renal carcinogen and toxic effects of potassium bromate on endocrine glands was studied [2].KClO3 is used as safe animal husbandry tool[3]for economically important food animals like sheep, cattle, swine and poultry animals. Potassium chlorate is also measured in dietary supplements and flavour enhancing ingredients [4]and also in bottled drinking water or mineral water. [5].
Potassium iodate also has been used as a food additive, to prevent iodine deficiency. It may be used to protect against the health risks caused by accumulation of radioactive iodine in the thyroid by administrating and saturating the body with a stable source of iodine in the form of KIO3 prior to exposure [6].KIO3is the best alternative to potassium iodide KI, as KI has poor shelf life in humid and hot climates [7].The importance of oxidizing agents towards the medical science lead us to undertake the present study.
The extensive information on the transport, thermodynamic and physicochemicalproperties of oxidizing agents are needed and plays a vital role in multiple industries, biochemical processes, in designing and development of drugs, medicines, vaccines, dyes, marine productsand also for thermal treatment and storage of foods. For this perspective, the comparative study of solvation behaviour of oxidizing agents in aqueous solutions of 0.5 % KCl, play crucial rolenot only in generation but systematization of physicochemical information of the studied solute and solvents. The major intent of this study was to assess the effect of molar concentration of solute and temperature on the apparent molar volume, various interaction parameters and solvolysis of oxidizing agent in different solvent systems. [8-9].
Experimental
Materials
The water used for the preparation of solutions was deionised and purified by successive distillation. The specific conductance of distilled water was found <5x 10-6 S.cm-1.The KClO3, KBrO3 and KIO3, of high purity was obtained from Sigma Aldrich, while KCl from S.D. Fine Lab were vacuum dried and used without further purification.The solutions of molarity range (6.5×10-3 to 3.65×10-2) mol.L-1 were prepared and the measurements of phytochemical properties were carried out at four different temperatures. The precision of balance used was ± 1×10-5g.
Density Measurements
Calibration of the bicapillary pycnometer was done by measuring the densities of triple distilled water. The densities of KClO3, KBrO3 and KIO3solutions in aqueous 0.5 % KCl, were measured by the same calibrated pycnometer at 298.15, 303.15, 308.15, and 313.15K temperatures. The density was measured with an accuracy of ±1.48 ×10-4 g.cm-3.
Viscosity measurements
The six different concentrations (0.0065M to 0.0365M) of solutions of KClO3, KBrO3 and KIO3were prepared in aqueous 0.5 % KCl, solvent systems. To determine the influence of temperature on viscosity, the time outflow were measured at 298.15, 303.15, 308.15, and 313.15K for all six different concentrations.by using Ubbelohde viscometer. The solution viscosities were measured with an uncertainty of ± 2.4×10-4 mPa.s and the flow time will be measured at the accuracy of± 0.01 s. Demerstat with an accuracy of ± 0.1 K is used to maintain the required temperature of thermostat.
Data Evaluation
The measured density data is used to evaluate the apparent molar volumes , using the following equation [10-13].

where ,M2, is the molar mass of the KClO3,KBrO3 and KIO3 ,C is the concentration (mol.L-1) and P and Po the densities of the solution and the solvent, respectively.
The apparent molar volumes ( ∅v ) of all the three oxidising agents were plotted against the square root of selected concentration range (C½) in accord with the Masson’s equation [14]

Values of empirical parameters ∅0v and Sv which depends on temperature and also on the nature of solute, solvent have been obtained from the linear graphs plotted between ∅v and C½The viscosity data for the KClO3,KBrO3 and KIO3 in aqueous 0.5 % KCl, were plotted in accordance with Jones-Dole equation [15]

Where ηr = (η/ηo) and η, ηo are viscosities of the solution and solvent respectively, C is the molar concentration. The B-coefficients were obtained from the linear plots using the least-square fitting method. The A- coefficient reflects solute-solute interaction [16-17] and the B-coefficient reflect the solute-solvent interactions. Since in general, A/B <<1, the Jones –Dole equation reduces to,

The relation between molar concentration and relative viscosity data of these solutions have also been fitted in Moulik equation,
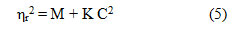
The density data of these solutions have also been used to deduce the values of R and S constants using Root’s equation,
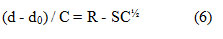
The viscosity data have been also employed to determine the diffusion controlled reaction rate constant kd[18].

Results and Discussion
The densities ( P )and viscosities (η) values of KClO3, KBrO3 and KIO3 in aqueous 0.5 % KCl, at different temperatures are reported Table-1. It is observed that densities and even viscosities increase with increase in molar concentration while it decrease with increase in temperature for all selected oxidising agents.Similar observations were previously made by [19-22] for other solutions.
Table 1: Densities and Viscosities of KClO3, KBrO3 and KIO3solution in 0.5% KCl, at different temperatures.
|
Solute System |
Molar Conc. of Solute (C) mol/dm3 |
Temperatures |
Temperatures |
||||||
|
298.15K |
303.15K |
308.15K |
313.15K |
298.15K |
303.15K |
308.15K |
313.15K |
||
|
Density, (P ) / (g.cm-3) |
Viscosity, (η) / (mPa.s) |
||||||||
|
KClO3 |
0.0065 |
1.00362 |
1.00198 |
1.00032 |
0.99920 |
0.9143 |
0.8311 |
0.7491 |
0.6757 |
|
0.0105 |
1.00450 |
1.00284 |
1.00120 |
1.00009 |
0.9174 |
0.8351 |
0.7539 |
0.6812 |
|
|
0.0155 |
1.00560 |
1.00391 |
1.00229 |
1.00117 |
0.9213 |
0.8400 |
0.7598 |
0.6882 |
|
|
0.0215 |
1.00690 |
1.00519 |
1.00358 |
1.00249 |
0.9259 |
0.8459 |
0.7670 |
0.6966 |
|
|
0.0285 |
1.00845 |
1.00668 |
1.00513 |
1.00402 |
0.9314 |
0.8528 |
0.7754 |
0.7063 |
|
|
0.0365 |
1.01010 |
1.00854 |
1.00713 |
1.00572 |
0.9376 |
0.8607 |
0.7850 |
0.7175 |
|
|
KBrO3 |
0.0065 |
1.00431 |
1.00299 |
1.00143 |
0.99946 |
0.9180 |
0.8397 |
0.7615 |
0.6834 |
|
0.0105 |
1.00538 |
1.00407 |
1.00263 |
1.00068 |
0.9231 |
0.8457 |
0.7685 |
0.6902 |
|
|
0.0155 |
1.00676 |
1.00541 |
1.00398 |
1.00219 |
0.9294 |
0.8532 |
0.7771 |
0.7011 |
|
|
0.0215 |
1.00839 |
1.00705 |
1.00569 |
1.00402 |
0.9370 |
0.8622 |
0.7875 |
0.7128 |
|
|
0.0285 |
1.01036 |
1.00898 |
1.00764 |
1.00617 |
0.9460 |
0.8728 |
0.7996 |
0.7263 |
|
|
0.0365 |
1.01236 |
1.01107 |
1.00991 |
1.00861 |
0.9561 |
0.8848 |
0.8134 |
0.7419 |
|
|
KIO3 |
0.0065 |
1.00518 |
1.00391 |
1.00264 |
1.00099 |
0.9296 |
0.8461 |
0.7672 |
0.6834 |
|
0.0105 |
1.00666 |
1.00531 |
1.00401 |
1.00238 |
0.9349 |
0.8516 |
0.7742 |
0.6902 |
|
|
0.0155 |
1.00851 |
1.00711 |
1.00572 |
1.00407 |
0.9417 |
0.8586 |
0.7824 |
0.7011 |
|
|
0.0215 |
1.01073 |
1.00925 |
1.00775 |
1.00615 |
0.9497 |
0.8669 |
0.7924 |
0.7128 |
|
|
0.0285 |
1.01333 |
1.01176 |
1.01018 |
1.00855 |
0.9592 |
0.8767 |
0.8042 |
0.7263 |
|
|
0.0365 |
1.01649 |
1.01476 |
1.01293 |
1.01101 |
0.9699 |
0.8879 |
0.8177 |
0.7419 |
|
The values of apparent Molar Volumes ( ∅v ) and Relative Viscosities ( nr ) of KClO3, KBrO3 and KIO3 in selected solvent systems and at four different temperatures are reported in table-2. The positive values of for all three solute systems decrease with increase of concentration in KCl. Derived relative viscosities from the viscosity data are found to increase with increase in concentrations.
Table 2: Apparent molar volumes and Relative viscosities of KClO3, KBrO3 and KIO3 solution in 0.5% KCl, atdifferent temperatures.
|
Solute System |
Molar Conc. of Solute (C) mol/dm3
|
Temperatures |
Temperatures |
||||||
|
298.15K |
303.15K |
308.15K |
313.15K |
298.15K |
303.15K |
308.15K |
313.15K |
||
|
Apparent molar volumes, ( ∅v) /cm3.mol-1 |
Relative viscosities, ( nr) |
||||||||
|
KClO3 |
0.0065 |
121.43 |
121.61 |
121.76 |
121.91 |
1.0188 |
1.0095 |
1.0099 |
1.0177 |
|
0.0105 |
121.34 |
121.54 |
121.70 |
121.84 |
1.0223 |
1.0131 |
1.0163 |
1.0261 |
|
|
0.0155 |
121.25 |
121.45 |
121.62 |
121.74 |
1.0266 |
1.0179 |
1.0244 |
1.0366 |
|
|
0.0215 |
121.16 |
121.37 |
121.52 |
121.67 |
1.0318 |
1.0239 |
1.0341 |
1.0492 |
|
|
0.0285 |
121.06 |
121.28 |
121.41 |
121.57 |
1.0379 |
1.0312 |
1.0454 |
1.0639 |
|
|
0.0365 |
120.94 |
121.14 |
121.30 |
121.47 |
1.0448 |
1.0396 |
1.0583 |
1.0807 |
|
|
KBrO3 |
0.0065 |
165.59 |
165.81 |
166.06 |
166.40 |
1.0231 |
1.0236 |
1.0267 |
1.0293 |
|
0.0105 |
165.39 |
165.62 |
165.89 |
166.20 |
1.0286 |
1.0309 |
1.0360 |
1.0396 |
|
|
0.0155 |
165.23 |
165.44 |
165.71 |
165.98 |
1.0356 |
1.0401 |
1.0477 |
1.056 |
|
|
0.0215 |
165.03 |
165.25 |
165.48 |
165.74 |
1.0441 |
1.0510 |
1.0617 |
1.0735 |
|
|
0.0285 |
164.85 |
165.08 |
165.29 |
165.53 |
1.0541 |
1.0639 |
1.0782 |
1.0939 |
|
|
0.0365 |
164.62 |
164.88 |
165.09 |
165.29 |
1.0655 |
1.0785 |
1.0966 |
1.1175 |
|
|
KIO3 |
0.0065 |
212.39 |
212.67 |
213.00 |
213.32 |
1.0358 |
1.0312 |
1.0344 |
1.0293 |
|
0.0105 |
212.14 |
212.43 |
212.74 |
213.07 |
1.0418 |
1.0381 |
1.0434 |
1.0396 |
|
|
0.0155 |
211.89 |
212.17 |
212.48 |
212.82 |
1.0494 |
1.0466 |
1.0548 |
1.0560 |
|
|
0.0215 |
211.60 |
211.88 |
212.20 |
212.52 |
1.0583 |
1.0567 |
1.0683 |
1.0735 |
|
|
0.0285 |
211.27 |
211.60 |
211.89 |
212.24 |
1.0688 |
1.0687 |
1.0843 |
1.0939 |
|
|
0.0365 |
210.99 |
211.26 |
211.54 |
211.96 |
1.0808 |
1.0824 |
1.1024 |
1.1175 |
|
The apparent molar volumes at infinite dilution
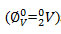
and slopes Sv , calculated using Masson equation (2) are reported in table-3. The ∅V0 values of KClO3, KBrO3 and KIO3 under investigation in KCl, are large and positive suggests presence of strong solute-solvent interactions promotes structure making effect [23].
Table 3: Masson,Moulik, Jone-Dole and Roots parameters of KClO3, KBrO3 and KIO3solution in 0.5% KCl at different temperatures.
|
Parameters |
Temperature (K) |
KClO3 |
KBrO3 |
KIO3 |
|
Masson’s Parameters |
||||
| ∅V0 |
298.15 |
121.7 |
166.2 |
213.4 |
|
303.15 |
121.9 |
166.4 |
213.7 |
|
|
308.15 |
122.1 |
166.8 |
214.1 |
|
|
313.15 |
122.2 |
167.2 |
214.3 |
|
|
Sv |
298.15 |
-4.05 |
-8.62 |
-12.8 |
|
303.15 |
-4.03 |
-8.34 |
-12.64 |
|
|
308.15 |
-3.89 |
-8.93 |
-13.06 |
|
|
313.15 |
-3.16 |
-10.07 |
-12.42 |
|
|
Moulik Parameters |
||||
|
K |
298.15 |
57.27 |
66.49 |
71.27 |
|
303.15 |
68.16 |
86.46 |
80.91 |
|
|
308.15 |
64.00 |
112.20 |
108.80 |
|
|
313.15 |
86.68 |
142.70 |
142.70 |
|
|
M |
298.15 |
1.03 |
1.05 |
1.08 |
|
303.15 |
1.02 |
1.06 |
1.07 |
|
|
308.15 |
1.03 |
1.06 |
1.08 |
|
|
313.15 |
1.03 |
1.07 |
1.07 |
|
|
Jone-Dole Parameters |
||||
|
A |
298.15 |
0.03 |
0.21 |
0.28 |
|
303.15 |
0.05 |
0.19 |
0.30 |
|
|
308.15 |
-0.03 |
0.19 |
0.28 |
|
|
313.15 |
0.03 |
0.17 |
0.29 |
|
|
B |
298.15 |
0.35 |
0.71 |
0.54 |
|
303.15 |
0.93 |
1.15 |
0.47 |
|
|
308.15 |
1.58 |
1.60 |
1.14 |
|
|
313.15 |
1.79 |
2.28 |
1.16 |
|
|
Roots Parameters |
||||
|
R |
298.15 |
1.45 |
0.75 |
0.74 |
|
303.15 |
1.46 |
0.73 |
0.73 |
|
|
308.15 |
1.50 |
0.73 |
0.73 |
|
|
313.15 |
1.39 |
0.59 |
0.59 |
|
|
S |
298.15 |
-5.17 |
-2.36 |
-2.36 |
|
303.15 |
-5.21 |
-2.31 |
-2.31 |
|
|
308.15 |
-5.50 |
-2.21 |
-2.21 |
|
|
313.15 |
-4.86 |
-1.41 |
-2.36 |
|
The Diffusion reaction rate constant (kd) evaluated by equation (7) and are reported in table-4.The apparent molar volumes of transfer Δ ∅(tr) of KClO3, KBrO3 and KIO3 and are obtained from the following relation and are included in table-5
Table 4: Diffusion reaction rate constant kd (L mol-1 s-1) values of KClO3, KBrO3 and KIO3 solution in 0.5% KCl solution .
|
Solvent System |
Molar Conc. of (C) mol/dm3 |
Temperatures |
|||
|
298.15K |
303.15K |
308.15K |
313.15K |
||
|
Diffusion reaction rate constant kd (L mol-1 s-1 ) x 1010 |
|||||
|
KClO3 |
0.0065 |
7.23 |
8.09 |
9.12 |
10.28 |
|
0.0105 |
7.21 |
8.05 |
9.06 |
10.19 |
|
|
0.0155 |
7.18 |
8.00 |
8.99 |
10.09 |
|
|
0.0215 |
7.14 |
7.95 |
8.91 |
9.97 |
|
|
0.0285 |
7.10 |
7.88 |
8.81 |
9.83 |
|
|
0.0365 |
7.05 |
7.81 |
8.70 |
9.68 |
|
|
KBrO3 |
0.0065 |
7.20 |
8.00 |
8.97 |
10.16 |
|
0.0105 |
7.16 |
7.95 |
8.89 |
10.06 |
|
|
0.0155 |
7.11 |
7.88 |
8.79 |
9.90 |
|
|
0.0215 |
7.05 |
7.79 |
8.68 |
9.74 |
|
|
0.0285 |
6.99 |
7.70 |
8.54 |
9.56 |
|
|
0.0365 |
6.91 |
7.60 |
8.40 |
9.36 |
|
|
KIO3 |
0.0065 |
7.11 |
7.94 |
8.90 |
10.16 |
|
0.0105 |
7.07 |
7.89 |
8.83 |
10.06 |
|
|
0.0155 |
7.02 |
7.83 |
8.73 |
9.90 |
|
|
0.0215 |
6.96 |
7.75 |
8.62 |
9.74 |
|
|
0.0285 |
6.89 |
7.67 |
8.49 |
9.56 |
|
|
0.0365 |
6.82 |
7.57 |
8.36 |
9.36 |
|
Table 5: The apparent molar volumes of transfer Δ (tr) of KClO3, KBrO3 and KIO3 solution in 0.5% KCl solution .
|
Solvent System |
Molar Conc. of KBrO3 (C) mol/dm3 |
Temperatures |
|||
|
298.15K |
303.15K |
308.15K |
313.15K |
||
|
Δ (tr) /cm3.mol-1 |
|||||
|
KClO3 |
0.0065 |
-0.06 |
-0.10 |
-0.12 |
-0.03 |
|
0.0105 |
-0.11 |
-0.13 |
-0.16 |
-0.07 |
|
|
0.0155 |
-0.12 |
-0.13 |
-0.19 |
-0.12 |
|
|
0.0215 |
-0.15 |
-0.17 |
-0.22 |
-0.18 |
|
|
0.0285 |
-0.24 |
-0.15 |
-0.27 |
-0.19 |
|
|
0.0365 |
-0.20 |
-0.20 |
-0.29 |
-0.28 |
|
|
KBrO3 |
0.0065 |
-0.65 |
-0.76 |
-0.82 |
-0.93 |
|
0.0105 |
-0.66 |
-0.65 |
-0.68 |
-0.77 |
|
|
0.0155 |
-0.68 |
-0.64 |
-0.6 |
-0.67 |
|
|
0.0215 |
-0.75 |
-0.6 |
-0.51 |
-0.49 |
|
|
0.0285 |
-0.80 |
-0.6 |
-0.42 |
-0.37 |
|
|
0.0365 |
-0.83 |
-0.57 |
-0.37 |
-0.21 |
|
|
KIO3 |
0.0065 |
-0.17 |
-0.23 |
-0.08 |
-0.13 |
|
0.0105 |
-0.23 |
-0.24 |
-0.13 |
-0.17 |
|
|
0.0155 |
-0.24 |
-0.24 |
-0.17 |
-0.26 |
|
|
0.0215 |
-0.29 |
-0.27 |
-0.22 |
-0.29 |
|
|
0.0285 |
-0.36 |
-0.27 |
-0.25 |
-0.36 |
|
|
0.0365 |
-0.40 |
-0.3 |
-0.39 |
-0.42 |
|
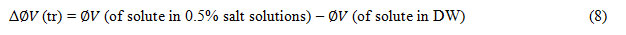
Figure-1 is the plot of ∅v (cm3.mol-1)Versus C½ (mol1/2.dm-3/2) for KClO3 solution in 0.5% KCl at T=298.15 to 313.15K. KBrO3 and KIO3 solution in 0.5% KCl also gave the similar linear plots. It is clear that for all the three solutes i.e. KClO3, KBrO3 and KIO3 in 0.5 % KCl solution, the values of ∅v (cm3.mol-1) are positive while the listed slope Sv is negative. Since extent of solute-solute interactions are interpreted from the slope Sv,here Sv is negative [24-25], which indicate the strong interaction amongst solute-solute. Secondly there is no specific trend with Sv values either with temperature or concentration, it proposes that the solute-solute interactions are unaffected to change in temperature.
 |
Figure 1: Plots of (cm3.mol-1) versus C½ (mol1/2.dm-3/2) for KClO3, KBrO3, KIO3 in 0.5 % KCl at T=298.15 to 313.15K. |
Plots of (ηr – l)/C1/2 vs C1/2 for KClO3,KBrO3 and KIO3solution in 0.5% KCl at different temperatures are shown in figure 2. The linear plots of (ηr – l)/C1/2 vs C1/2 are obtained at all temperatures with regression coefficients higher than 0.99. The slopes of the plots are positive.
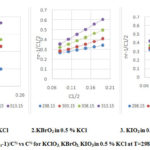 |
Figure 2: Plots of (ηr-1)/C½ vs C½ for KClO3, KBrO3, KIO3 in 0.5 % KCl at T=298.15 to 313.15K. |
Sharma, Rani, R., Kumar, A., & Bamezai,[26]observed that during ionic-ionic interactions, co-sphere of two ionic species overlaps and it adds to increase the volume of solution while decrease in volume is noted due to overlapping of co-sphereof hydrophilic-hydrophobic groups or ion hydrophobic groups The negative values of Δ∅v (tr) have been reported in terms of weaker ion-ion and ion-hydrophilic group interactions than the ion-hydrophobic interaction. It results in an decrease in volume. Co-sphere overlap model developed by Gurney [27]is utilised for the better understanding of various inter ionic attractions. It is also interpreted that the properties of solvent molecule in the hydration co-sphere depend on the nature of solute species [28-29].
Conclusions
In the present research article, solvolysis and transport properties of KClO3, KBrO3 and KIO3solutions in 0.5% salt solutions at different temperatures and concentrations are methodically reported. he effect of temperature on the ∅0v has been reported in terms of ion-solvent interactions. It has been concluded that in all the three solute systems, there exist strong solute– solvent interactions in these systems. The ∅0v values reported in present study are found to be positive suggest presence of ion-solvent interactions. Thelarge and positive ∅0v values for all the three solute systems has been employed to predict the presence of strong solute-solvent interactions promotes structure making effect . The Moulik, Roots and Jones-Dole reduced equation are verified for KClO3, KBrO3, and KIO3 solutions in these solvent systems. The positive Kd value interprets that solvolysis ofKClO3, KBrO3, and KIO3in 0.5% salt solutions at different temperatures and concentrations is diffusion controlled process rather than activated controlled process.
Acknowledgement
I would like to sincerely acknowledge the guidance and help provided by Dr. Arun B. Nikumbh for compilation of this research report.
Conflict of Interest
There is no conflick of interest.
References
- Smith, M. B., & March, J. (6th ed.). New York: Wiley-Interscience.2007
- Stasiak, M. L.- Endokrynologia Polska,2009, 60(1), 40-50.
- Anderson, R. J. (2007). Journal of food protection. 2007, 70, 308-315.
CrossRef - Snyder SA, P. R. Anal Chim Acta.,2006, 567(1), 26-32.
CrossRef - Sorlini S, G. F. Water Res.,2014, 54, 44-52.
CrossRef - Astbury, J., Horsley, S., & Gent, N. Journal of Public Health.1999, 21(4), 412-414.
CrossRef - Pahuja, D., Rajan, M., Borkar, A., & Samuel, A.. Health physics, 2008,65(5), 545-549.
CrossRef - Nikumbh, A. B. Int. J. Technical Res. Appl,2014, 2(6), 116-122.
- Nikumbh, A. B. International Journal of Applied Chemistry (SSRG-IJAC),2016, 3(3), 1-6.
- Hu, B. H. Journal of Chemical & Engineering Data,2016, 61(10), 3618-3626.
CrossRef - Shinde, S. P. Journal of Solution Chemistry,2018, 47(6), 1060-1078.
CrossRef - Santos, C. I. Journal of Molecular Liquids,2016, 223, 209-216.
CrossRef - Caro, R. H. Journal of Chemical & Engineering Data,2020, 65(7), 3735-3743.
CrossRef - Bhujbal, R. C. (2019). Current Pharma Research,2019, 9(2), 2824-2830.
CrossRef - Jones, G. &. Journal of the American Chemical Society,1929, 51(10), 2950-2964.
CrossRef - H. Falkenhagen, M. D. (1929). Zeitschrift Für Physik.1929, 30, 611-616.
- Nain, A. K. Journal of Molecular Liquids, 2012.165, 154-160.
CrossRef - Chiorboli, C. I. The Journal of Physical Chemistry,1988, 92(1), 156-163.
CrossRef - Pérez-Durán, G. &.-S. Journal of Chemical & Engineering Data,2019, 64(5), 1999-2010.
- Guo, H. D. Journal of Molecular Liquids,2020, 299, 112191.
CrossRef - Banipal, P. K. Journal of Chemical & Engineering Data.2016 61(5), 1756-1776.
CrossRef - Rathi,M.V.Nikumbh, A. B. Journal of Emerging Technologies and Innovative Research,2019, 6(1), 167-175.
- Lomesh, S. K. Journal of Molecular Liquids, 2019,284, 241-251.
CrossRef - Gaware, M. R. Bulletin of Pure & Applied Sciences-Chemistry,2018, 37(2), 76-81.
CrossRef - Shakeel, M. &. Journal of the Chinese Chemical Society, 2020, 67(9), 1552-1562.
CrossRef - Sharma, T. R. Journal of Molecular Liquids,2020, 300, 111985.
CrossRef - Gurney R.W.,. Ionic processes in solution. New York: (McGraw Hill , 1953).
- Nain, A. K. Journal of Molecular Liquids,2020, 298, 112006.
CrossRef - Gupta, J. &. Journal of Molecular Liquids,2019,293,111547.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










