Chemical Composition, Antimicrobial Activity and Potential Cytotoxic Effect of Mentha Viridis (Spearmint) Extracts from Saudi Arabia
Department of Chemistry, Science college, Albaha University, Albaha, Saudi Arabia.
Corresponding Author E-mail: nada.m@bu.edu.sa
DOI : http://dx.doi.org/10.13005/ojc/370116
Article Received on : 15-Dec-2020
Article Accepted on :
Article Published : 08 Feb 2021
Many medicinal plants have been used to treat and prevent illnesses in Saudi Arabia. The present study aimed to investigate the chemical composition of Mentha viridis obtainedfrom Albaha region of Saudi Arabiaand evaluate its antimicrobial and antiproliferative potential. The extract was obtained from plant fresh material and identified by gas chromatography-mass spectrometry (GC-MS). The antimicrobial and antiproliferative potential of the plant extract was analysed by performing four subsequent extracts: ethanol, petroleum ether, chloroform, and methanol. The GC-MS analysis showed carvone as a main component, as it comprised 64.82 % of the plant extract. In antimicrobial activity, methanol extract showed significant activityagainst Pseudomonas aeruginosa with zone of inhibition of 15 mm. The MTT assay showed thatpetroleum ether and chloroform extracts have moderate cytotoxic effect against MCF-7 breast cancer cell line with IC50 values of 193.23 μg/mL and 131.86 μg/mL, respectively. Chloroform extract also showed mild activity against HCT-116 colorectal cancer cell line with IC50value of 189.2 μg/mL. This study highlights the potential of M. viridis extracts as powerful bioactive phytochemicals with possible role in diseases and cancer therapy.
KEYWORDS:Antimicrobial; Antiproliferative; Mentha Viridis; Medicinal Plant.
Download this article as:| Copy the following to cite this article: Ali N. M. Chemical Composition, Antimicrobial Activity and Potential Cytotoxic Effect of Mentha Viridis (Spearmint) Extracts from Saudi Arabia. Orient J Chem 2021;37(1). |
| Copy the following to cite this URL: Ali N. M. Chemical Composition, Antimicrobial Activity and Potential Cytotoxic Effect of Mentha Viridis (Spearmint) Extracts from Saudi Arabia. Orient J Chem 2021;37(1). Available from: https://bit.ly/3cSiRu6 |
Introduction
Presently, the effectiveness, low cost, and fewer side effects have increased the worldwide demand of medicinal plants. Many pharmaceutical companies are engaged in large-scale pharmacologic screening of medicinal plants for developing new drugs (1). Medicinal plants are rich resources of traditional medicines and many modern medicines, including aspirin, digoxin, quinine and morphine are obtained from willow bark foxglove, cinchona bark, opium poppy, respectively (2). The effectiveness of medicinal plants has been proved on individual body systems, for example, they have profound antioxidant, anti-inflammatory, antimicrobial and immunostimulatory properties (1).
Mentha species is a member of the Lamiaceae (Labiatae) family and are mass distributed across all continents (3,4). According to latest data, Lamiaceae family is considered one of the largest families of plants that produce flowers with around 4000 species that grow worldwide (5) and is considered to have the highest number of medicinal plants (6). The use of Mentha plantsin treating many diseases, including common cold, fever, throat infection, bronchitis, ulcerative colitis, and digestive issues has been known for a long time (7,8). Moreover, its use as an antimicrobial, antioxidant, anti-motion sickness, anti-inflammatory and anticarcinogenic agent has also been reported(9–11).
Mentha viridis, commonly known as spearmint, has well known industrial importance. The leaves of the M. viridis are used as flavouring agent in culinary purposes including iced drinks and jellies (12).
The medicinal uses of M. virids are also well documented. It is considered as a relaxant, antispasmodic, and soothing agent in nausea and vomiting (13). Moreover,it is widely known as a strong stimulant and carminative (14).M. viridis extract contains various terpenes, fatty acid esters and Vitamin E, which explains its antioxidant potential(15). The essential oil from leaves of M. viridis has potent antimicrobial activity (16).
Previously, no study has been reported on M. viridis cultivated in Albaha region. Its medicinal properties and safe usage have made it an ideal option for studying. In addition, plants growing in different geographical and weather conditions tend to have different phytochemical composition and different biological activities. This study aims to characterize the bioactive compounds of M. viridis from Albaha region and investigate its extracts for antimicrobial and antiproliferative potential.
Methods
Plant materials
The plant was obtained from local farmer market in Albaha region, Saudi Arabia in March 2019. Dr Haider authenticated its botanical identification. An authenticated specimen was deposited at the Botany Laboratory, Department of Biology, Albaha University.
Preparation of samples for GC-MS analysis
The leaves and stem (representing fresh aerial parts of the plant), witha weight of 25 g, was transferred to 15-ml screw test tube, mixed with 5 ml methanol, capped, vortexed for 5 min, sonicated for 30 min, mixed with about 2 g anhydrous sodium sulfate, filtered through a filtration disc of the PTFE syringe, 0.22 micron thickness. The produced filtrate is then concentrated to 1 ml using room temperature Nitrogen gas in the form of a gentle stream. The extract contains both polar and nonpolar components of the plant material. A portion from the clear extract was transferred to autosampler vial. GC-MS analysis sample was prepared by injecting 1.5 μl into the vial.
The GC- MS analysis
The GC-MS analysis of bioactive compounds from plants extract was done using Clarus 500 GC-MS (Perkin Elmer, Shelton, CT, USA). TurboMass version 5.4.2.1617 was used as a software integrator & controller. An Optima® 1 GC capillary column, Crossbond® 100% dimethyl polysiloxane (30-meter × 0.25 mm ID × 0.25 μm df), Macherey-Nagel, GMBH, Duren, Germany) was used. Al Hashmi et al., (2013)described a similar setting, but a few modifications were made. In this assay, Helium (purity 99.9999%) was used as a carrier gas, with 0.90 ml/min flow rate. Source (EI+): source was set to 215 ˚Ctemperature, GC inlet linewas set to 265 ˚C temperature, with 70 eV Electron energy, and 100V trap-emission. The oven programming went as follows: 50 °C temperatureinitially (with a 5 minutes hold), then raised to 260 ˚C (at a rate of 10 ˚C/min, with 5 minutes hold), then raised again to 280 ˚C (at a rate of 10 ˚C/min, with 2 minutes hold). Temperature of the injector was set to 265 °C, 1.0 µL was injected, and a 50:1 ratio was used for splitting. A total MS scan from 40 to 500 m/z (500 scan/sec) was applied to acquire the sample. The eluted compounds were characterized using NIST 2008, as reported in Mosbah et al., (2018).
Extraction of crude extracts
The leaves and stem (representing fresh aerial parts of the plant) were air dried at room temperature (25 ± 2º C) for about 7 days. The dried material was ground using an electric blender machine(Pulverizer HR-30B, USA).200 g powder material was macerated in 600 mL methanol with shaking for 3 days. Then they were filtered through Whatman no1 filter paper.The residue was further extracted two times by using the same fresh solvent. All filtrates were compiled for further evaporating. The resulting residue was air dried and further extracted with solvents of increasing polarity namely petroleum ether, chloroform, and ethanol by using similar procedure carried out for the methanol extraction. Finally, rotary evaporator (IKA RV-10, Germany)was used under reduced pressure and low temperatureto evaporate solvent from each filtrate extract until dryness was achieved(19).
Antimicrobial Evaluation
The antimicrobial activity of the four plant extracts (methanol, ethanol, petroleum ether, and chloroform) wastested against standard strains of four bacteria and one fungus.King Abdulaziz University Hospital, Jeddah, KSA provided the organisms through their microbiology laboratory. These strains were: Staphylococcus aureus(reference: ATCC 29213) and Bacillus subtilis (Reference:ATCC 6633), both Gram positive bacteria, as well as Escherichia coli(Reference: ATCC 35218) andPseudomonas aeruginosa(Reference: ATCC 27853), bothGram negative bacteria and finally fungus: Candida albicans (Reference:ATCC 76615).
Agar diffusion technique was used for the initial screening of the antibacterial and antifungal activities, as previouslydescribed(20). Briefly, Muller-Hinton agar (25 mL) containing 1 mL bacterial culture (1 × 106 CFU/mL) was used to fill Petri dishes with a capacity of 90 mm. The strains were inoculated separately. Seven holes (4 mm in diameter) were prepared in the seeded agar dishes, which were then filled with 50 μL of each extract (10 mg/mL), as well as a negative control agent (10 % dimethyl sulfoxide (DMSO)). Dishes were then incubated at 37 °C for 24 h. Success of the Inhibitory activity was marked by the absence of bacterial growth in the area surrounding the holes. Triplicates were carried out against each of the tested microorganisms. The growth inhibition zones’ diameters were measured using a calliper and averaged at the end of the incubation period. The mean values were tabulated.
Acquisition of cell lines and culture medium preparation
Human breast cancer MCF-7 and colorectal cancer HCT-116 cell lines were obtained from Dr. Thikryat Neamatallah, Pharmacology and Toxicology laboratory, Faculty of medicine, King Abdulaziz University, Jeddah, KSA. Dulbecco’s Modified Eagles Medium (DMEM)/high glucose medium was used as a culture medium, augmented with 10% fetal bovine serum (FBS), as well as 10,000 units/mL penicillin/streptomycin (Pen/Strep) and 1% glutamine). All reagents were purchased from Thermo Fisher (Thermo Fisher Scientific UK Ltd, Leicestershire, UK) except Pen/Strep, which was obtained from Sigma (Sigma-Aldrich, St. Louis, MO, USA). The cell lines were cultured in 75 cm2 flasks were used to culture the cells, and were sustained at 37 °C in a 5% CO2 humid incubator. A Class II Safety Flow Hood was used to carry out the cell culture procedure, under aseptic conditions.
MTT Assay
Both types of cells were seeded at (1 × 105 cells/mL) into a plate containing 96 wells, together with 3 duplicates. The whole assay was incubated at 37°C through the night for attachment in a humid atmosphere containing 5% CO2 as described by Mansour et al., (2016), but slightly modified. In this assay, plant extracts (methanol, ethanol, petroleum ether, and chloroform) at 7 serial dilutions (1000-10 μg/mL) were introduced in 3 identical settings (triplicates) and incubated for 72h, at a temperature of 37 °C, and 5% CO2. 0.1 % DMSO was used as a vehicle to dissolve the drugs in. Untreated cells were used as control. Afterwards, 100µl of full medium containing 10% of 3-(4,5- dimethylthiaxolyl-2)-2,5-diphenyltetrazolium bromide (MTT) (10mg/mL) was used as replacements for each well at each recorded time point. Cells were incubated again at a temperature of 37°C, and 5% CO2for 4 hours.100 µl of DMSO was added after removing the media, and incubation at a temperature of 37ºC, and 5% CO2was donefor anadditional 5 minutes. SpectraMax M3 plate reader at 570 nm was used to quantify the plates.
The following formula was used to determine the viability percentage:
Cell viability (%) = (A of treated cells/A of control cells) ×100.
Statistical analysis
Triplicates of three independent experiments were carried out and data are expressed as the mean ± SD. IC50 value was calculated by ED50 GraphPad Prism software (GraphPad Prism 5.0, GraphPad Software, Inc., CA, USA).
Results
GC-MS Analysis
The analysis showed that aerial parts (stem and leaves) ofM. viridismethanolic extract had a variety of phytochemicals, which are shown in the GC-MS chromatograms (Figure 1). Thirty-two distinct phytochemical compounds were present in M. viridismethanolicextract in different ratio and accounted for 98.6% of the total components. It was found that carvone was the most abundant phytochemical, as it was present in 64.82 % followed by eucalyptol (10.44 %), oleamide (6.34 %) and phytol (5.40 %).
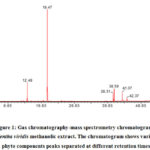 |
Figure 1: Gas chromatography-mass spectrometry chromatogram of Mentha viridis methanolic extract. The chromatogram shows various phyto components peaks separated at different retention times. |
Table 1 shows the identified components in M. viridismethanolic extract. Figure 2 illustrates the chemical structure of some active components detected in M. viridismethanolic extract.
Table 1: Chemical composition of Mentha viridis methanolic extract
|
Peak number |
Rt, mina |
Componentsb |
Area |
% Area |
|
1 |
7.99 |
2,5-Diethyltetrahydrofuran |
267132 |
0.13 |
|
2 |
9.36 |
1R-α-Pinene |
298463 |
0.14 |
|
3 |
10.62 |
β-Thujene |
407800 |
0.20 |
|
4 |
10.72 |
β-Pinene |
574268 |
0.28 |
|
5 |
11.27 |
Myrcene |
498542 |
0.24 |
|
6 |
11.41 |
n-Octan-3-ol |
751261 |
0.36 |
|
7 |
12.22 |
Benzeneacetaldehyde |
565544 |
0.27 |
|
8 |
12.48 |
Eucalyptol (1,8-Cineole) |
19610798 |
10.44 |
|
9 |
13.64 |
(E)-Sabinene hydrate |
988349 |
0.48 |
|
10 |
14.03 |
Fenchone |
72673 |
0.03 |
|
11 |
15.62 |
(-)-Camphor |
400971 |
0.19 |
|
12 |
16.29 |
3-Methyl-1,2-cyclopentanedione |
44459 |
0.02 |
|
13 |
16.49 |
Myrcenol |
44459 |
0.02 |
|
14 |
16.54 |
Borneol |
489790 |
0.24 |
|
15 |
17.15 |
Dihydrocarvone |
368367 |
0.18 |
|
16 |
17.36 |
Neodihydrocarveol |
152889 |
0.07 |
|
17 |
18.02 |
cis-Carveol |
200112 |
0.10 |
|
18 |
18.47 |
Carvone |
130497712 |
64.82 |
|
19 |
19.28 |
cis-Carvone oxide |
117097 |
0.06 |
|
20 |
22.72 |
β-Bourbonene |
594923 |
0.29 |
|
21 |
22.86 |
β-Elemene |
317625 |
0.15 |
|
22 |
23.57 |
Caryophyllene |
583306 |
0.28 |
|
23 |
24.71 |
(+)-Epi-bicyclosesquiphellandrene |
355040 |
0.17 |
|
24 |
25.28 |
Germacrene D |
459971 |
0.22 |
|
25 |
25.81 |
1,5-Heptadiene, 2,5-dimethyl-3-methylene- |
203301 |
0.10 |
|
26 |
30.00 |
Copaene |
78219 |
0.04 |
|
27 |
31.03 |
α-Cadinol |
53101 |
0.03 |
|
28 |
36.14 |
Tridecanoic acid, methyl ester |
520004 |
0.25 |
|
29 |
38.31 |
Linolenic acid, methyl ester |
6695976 |
3.22 |
|
30 |
38.59 |
Phytol |
11224343 |
5.40 |
|
31 |
41.07 |
Oleamide |
13174507 |
6.34 |
|
32 |
42.37 |
Limonen-6-ol, pivalate |
8055191 |
3.88 |
|
|
|
Total |
|
98.64 |
aRetention time (as minutes).
bCompounds listed in order of elution from a column.
 |
Figure 2: Chemical structures of some bioactive constituents of Mentha viridis extract. |
Antimicrobial Activities
The in vitro antifungal andantibacterial bioassays were carried out using the extracts from M. viridis.A variety of bacteria (both Gram-positive and Gram-negative) as well as fungal strains were used as test strains and agar diffusion assays were carried out to analyse the antimicrobial properties of M. viridis. The results were determined on the basis of formation of zones of inhibition (measured in mm) around the extract on the agar plates which were seeded with test microbial strains. The methanolic extract showed significant antibacterial activity against P. aeruginosawith zone of inhibition of 15 mm while ethanol, chloroform and petroleum ether extracts did not show any antimicrobial activity against the tested microorganisms. The results are given in Table 2.
Table 2: Antibacterial and antifungal activities of Mentha viridis extracts.
|
Extracts |
Zone diameter (mm) |
||||
|
Gram-positive bacteria |
Gram-negative bacteria |
Fungus |
|||
|
Staphylococcus aureus |
Bacillus subtilis |
Escherichia coli |
Pseudomonas aeruginosa |
Candida albicans |
|
|
Chloroform |
– |
– |
– |
– |
– |
|
Methanol |
– |
– |
– |
15± 0.04 |
– |
|
Ethanol |
– |
– |
– |
– |
– |
|
Petroleum ether |
– |
– |
– |
– |
– |
aThe diameters of the inhibition zones. Values are the means ± SD of three cultures.
MTT (IC50) Assay
The antiproliferative effect of the extract of M. viridis on human cells’ viability was analysed using MTT assay. Two cancer cell lines, human breast cancer (MCF-7) and colorectal cancer (HCT-116) cell lines were selected and tested against M. viridis extracts. Readings were taken using spectrophotometer SpectraMax M3 and are given in Table 3. Petroleum ether and chloroform extracts showed moderate cytotoxic effectsagainst MCF-7 with IC50 values 193.23 μg/mL and 131.86 μg/mL, respectively. Chloroform extract also showed mild activity against HCT-116 with IC50value of 189.2 μg/mL. Ethanol and methanol extracts showed IC50 higher than 200 μg/mL against the tested cell lines, which is indicative of no cytotoxicity.
Table 3: The IC50 of Mentha viridis extracts against tested human cancer cell lines.
|
Compound |
IC50* (μg/mL) MCF-7 |
IC50*(μg/mL) HCT-116 |
|
Standard drug (paclitaxel) |
0.23 ± 2.2 ´ 10-6 |
0.32 ± 5.7 ´ 10-6 |
|
Chloroform extract |
131.86 ± 4.2×10-5 |
189.2 ± 1.13×10-4 |
|
Petroleum ether extract |
193.23 ± 5.9×10-5 |
>200 |
|
Ethanol extract |
>200 |
>200 |
|
Methanol extract |
>200 |
>200 |
* IC50 is the half maximal inhibitory concentration (μg/mL). Values are the means ± SD of three cultures.
Discussion
Various types of infectious bacteria and cancers are a constant threat for human health and are the leading cause of morbidity and mortality worldwide. This continuous risk calls for the need of exploring and finding new cost-effective therapies with better effectiveness. Medicinal plants have proved to be a significant source of novel therapeutic substances. Today, various plant-derived potent chemicals are being extensively used and studied for human therapeutic purposes. The present study aimed to analyse the phytochemical contents of M. viridis extract and evaluate its antimicrobial and anticancer properties.
GC-MS Analysis
The methanolic extract of different parts of M. viridiswere subjected to GC-MS analysis to determine its complete phytochemical composition and to determine the ratio of each component in the respective plants. The peaks in the GC-MS chromatogram showed that about thirty distinct phytochemicals were present in the M. viridis extract. The most abundant component was carvone which has the highest ratio, i.e., 64.82 %. Few other components were present in high quantities, including eucalyptol (1,8-cineole) (10.44 %), oleamide (6.34 %) and phytol (5.40 %). Various studies have reported carvone as the most abundant component of M. viridis (22).For example, according to the findings of Verma et al. (2010), the analysisofM. viridis sample collectedfrom India (specifically from the mid-hills of Himalayan region)at different stages of crop growth showed that carvone is the most prevalent phytochemical with percentages ranging between of 59.6-72.4 % in different stages of sampling. Moreover, phytochemical analysis of M. viridis collected from farms in Al-Kadaro region of Sudan showed carvone (64.63%) as a major component (16). Furthermore, another study reported that carvone (50.47%) followed by 1,8-cineole (9.14%), limonene (4.87%), camphor (3.68%), and β-caryophyllene (3%) were the key component of Tunisian M. viridis (23).
GC-MS profiling of our study also indicated the presence of fatty acid methyl esters(tridecanoic acid,linolenic acid) terpenoids and terpenoid alcohol(eucalyptol, phytol). Most of the plant terpenoids and their derivatives are biologically active and are used extensively as traditional herbal remedies for many diseases(24). They are also used worldwide in food, cosmetics and pharmaceuticalindustries(25).
Antimicrobial Activities
The significant antimicrobial activity was only shown by methanol extract whose concentration was 10 mg/ mL, forming an inhibition zone as of 15 mm when interacting withP. aeruginosa. However, no antimicrobial activity was noted by other extracts (10 mg/ mL) obtained from M. viridis against the tested microorganisms. The results are similar to those byMkaddem et al., (2009) who stated thatno antimicrobial activity of essential oil extract (15 µl/ mL) from Tunisian M. viridis was observed against E. coli and S. aureus. Referring to Silva et al., (2015), the essential oil from M. viridis showed antimicrobial activity against E. coli (a gram –ve bacterium) and S.aureus(a gram +ve bacterium)forming zones of inhibition of 6 mm and 8 mm, respectively, when the minimal inhibitory concentration (MIC)was62.5 µl/ mL. Other study showed that MIC of 12.5 mg/mL is the lowest concentration at which all the tested microorganisms are inhibited (16).Our study found different results, which may be associated with differences in the concentration of essential oil and crude extracts of M. viridis used against the tested microorganisms.In addition, the disagreement was probably due tothe difference in amounts and the nature of thecomponents that presented in the essential oil and crude extracts of M. viridis, because Silva et al., (2015)found linalool (40.70%), carvone (13.52%) and α-terpinene (8.56%) as the chief components.
MTT (IC50) Assay
The antiproliferative effects of M. viridis extracts (10 µg/mL)on human breast cancer (MCF-7) and colorectal cancer (HCT-116) cell lines, were analysed using the MTT (IC50) assay. Chloroform extract showed moderate antiproliferative activity and had IC50 (μg/mL) values less than 200 against both the MCF-7 and HCT-116 cell lines. Petroleum ether extract showed inhibition effect only against MCF-7 with IC50 values 193.23 μg/mL. However, no cytotoxic activity was observed in methanol and ethanol extracts against the tested cell lines. Sharma et al., (2014), evaluated the anticancer potential of methanolic and aqueous extracts of whole plant of M. viridis against MCF-7 and HCT-116 in vitro,using a 100 µg/mL concentration, and sulforhodamine Blue (SRB) assay. Methanolic extract exhibited cytotoxicity against MCF-7 while aqueous extract was found active against HCT-116 (27). Our study found different results, whichcan be attributed to the difference in doses concentration applied against the tested cancer cell lines.
In this study, it is noticed that the non-polar solvents (petroleum ether and chloroform) used for the plant extraction exhibited more cytotoxic effects against tested cancer cells. These effects are most likely due to the presence of more bioactive terpenoids and sesquiterpenes in these solvents. It has been reported that carvone and its derivatives do not exhibit cytotoxic effects against cancer cells (28) while many of the plant terpenoids inhibit different human cancer cells and are used as anticancer drugs (25,29,30).
From the current findings we can safely conclude that extracts from M. viridis can be ideal candidates for novel therapeutic research. The difference observed in the antibacterial and antiproliferative properties of M.viridis between our results and other reported findings is certainly due to the chemical composition, methods used, strains tested, dose concentrations applied, growing conditions and regions. The emergence of novel infectious diseases and development of bacteria resistance against the available antibiotics have made it inevitable that medicinal plants, especially from novel environments, should be explored for their therapeutic potentials. The present study is among the very first studies that have investigated and explored the M. viridis species of Albaha Region, KSA, and it highlights the need and importance of similar studies.
Acknowledgements
The principal investigator of this study gratefully acknowledges the Deanship of Scientific Research, Albaha University, Albaha, KSA for supporting by grant #17–1439.
Conflict of Interest
There are no conflicts of interest to declare.
References
- Dar RA, Shahnawaz M, Qazi PH. General overview of medicinal plants: A review. J Phytopharm. 2017;6(6):349–51.
- Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2(2):303–36.
CrossRef - Salehi B, Stojanović-Radić Z, Matejić J, Sharopov F, Antolak H, Kręgiel D, et al. Plants of genus Mentha: From farm to food factory. Plants. 2018;7(3):70.
CrossRef - Mamadalieva NZ, Akramov DK, Ovidi E, Tiezzi A, Nahar L, Azimova SS, et al. Aromatic medicinal plants of the Lamiaceae family from Uzbekistan: ethnopharmacology, essential oils composition, and biological activities. Medicines. 2017;4(1):8.
CrossRef - Sadeghi Z, Akaberi M, Valizadeh J. Otostegia persica (Lamiaceae): A review on its ethnopharmacology, phytochemistry, and pharmacology. Avicenna J phytomedicine. 2014;4(2):79.
- Ouakouak H, Chohra M, Denane M. Chemical composition, antioxidant activities of the essential oil of Mentha pulegium L, South East of Algeria. Int Lett Nat Sci. 2015;39.
CrossRef - Eissa TAF, Palomino OM, Carretero ME, Gómez-Serranillos MP. Ethnopharmacological study of medicinal plants used in the treatment of CNS disorders in Sinai Peninsula, Egypt. J Ethnopharmacol. 2014;151(1):317–32.
CrossRef - Darwish RM, Aburjai TA. Effect of ethnomedicinal plants used in folklore medicine in Jordan as antibiotic resistant inhibitors on Escherichia coli. BMC Complement Altern Med. 2010;10(1):9.
CrossRef - Benzaid C, Tichati L, Djeribi R, Rouabhia M. Evaluation of the Chemical Composition, the Antioxidant and Antimicrobial Activities of Mentha× piperita Essential Oil against Microbial Growth and Biofilm Formation. J Essent Oil Bear Plants. 2019;22(2):335–46.
CrossRef - Shaikh S, Yaacob H Bin, Rahim ZHA. Prospective role in treatment of major illnesses and potential benefits as a safe insecticide and natural food preservative of mint (Mentha spp.): a Review. Asian J Biomed Pharm Sci. 2014;4:1–12.
CrossRef - Jain D, Pathak N, Khan S, Raghuram GV, Bhargava A, Samarth R, et al. Evaluation of cytotoxicity and anticarcinogenic potential of Mentha leaf extracts. Int J Toxicol. 2011;30(2):225–36.
CrossRef - Verma RS, Padalia RC, Chauhan A. Chemical profiling of Mentha spicata L. var.‘viridis’ and Mentha citrata L. cultivars at different stages from the Kumaon region of western Himalaya. Med Arom Plant Sci Biotechnol. 2010;4:73–6.
- Aziz MM, Saqib NU, Akhtar N, Asif HM, Jamshaid M, Sultana S, et al. Phytochemical screening and evaluation of the diuretic activity of aqueous methanol extract from aerial parts of mentha viridis linn (labiatae) in albino rats. Trop J Pharm Res. 2014;13(7):1121–5.
CrossRef - Grieve M. A modern herbal. Vol. 2. Courier Corporation; 2013.
- A Hassan H, S Kabbashi A, Abedallah A, D Wagh V, Abdalla Ahmed Hamdi O. Chemical Composition, Antioxidant Activity and Cytotoxicity of Essential Oil of Mentha viridis. Acta Sci Med Sci. 2019;3(8):200–5.
CrossRef - Balla OY, Ali MM, Garbi MI, Kabbashi AS. Chemical composition and antimicrobial activity of essential oil of Mentha viridis. Biochem Mol Biol. 2017;2(5):60–6.
CrossRef - Al Hashmi LS, Hossain MA, Weli AM, Al-Riyami Q, AlSabahi JN. Gas chromatography–mass spectrometry analysis of different organic crude extracts from the local medicinal plant of Thymus vulgaris L. Asian Pac J Trop Biomed. 2013;3(1):69–73.
CrossRef - Mosbah H, Louati H, Boujbiha MA, Chahdoura H, Snoussi M, Flamini G, et al. Phytochemical characterization, antioxidant, antimicrobial and pharmacological activities of Feijoa sellowiana leaves growing in Tunisia. Ind Crops Prod. 2018;112:521–31.
CrossRef - Jeyaseelan EC, Jenothiny S, Pathmanathan MK, Jeyadevan JP. Antibacterial activity of sequentially extracted organic solvent extracts of fruits, flowers and leaves of Lawsonia inermis L. from Jaffna. Asian Pac J Trop Biomed. 2012;2(10):798–802.
CrossRef - Kizil S, Hasimi N, Tolan V, Kilinc E, Yuksel U. Mineral content, essential oil components and biological activity of two mentha species (M. piperita L., M. spicata L.). Turkish J F Crop. 2010;15(2):148–53.
- Mansour R Ben, Jilani IBH, Bouaziz M, Gargouri B, Elloumi N, Attia H, et al. Phenolic contents and antioxidant activity of ethanolic extract of Capparis spinosa. Cytotechnology. 2016;68(1):135–42.
CrossRef - Bouyahya A, Lagrouh F, El Omari N, Bourais I, El Jemli M, Marmouzi I, et al. Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocatal Agric Biotechnol. 2020;23:101471.
CrossRef - Mkaddem M, Bouajila J, Ennajar M, Lebrihi A, Mathieu F, Romdhane M. Chemical composition and antimicrobial and antioxidant activities of Mentha (longifolia L. and viridis) essential oils. J Food Sci. 2009;74(7):M358–63.
CrossRef - Yadav N, Yadav R, Goyal A. Chemistry of terpenoids. Int J Pharm Sci Rev Res. 2014;27(2):272–8.
- Perveen S, Al-Taweel A. Introductory chapter: terpenes and terpenoids. Terpenes and Terpenoids. 2018;1–12.
CrossRef - Silva LF, das Graças Cardoso M, Batista LR, de Souza Gomes M, Rodrigues LMA, Rezende DA de CS, et al. Chemical characterization, antibacterial and antioxidant activities of essential oils of Mentha viridis L. and Mentha pulegium L.(L). Am J Plant Sci. 2015;6(05):666.
CrossRef - Sharma V, Hussain S, Gupta M, Saxena AK. In vitro anticancer activity of extracts of Mentha spp. against human cancer cells.Indian J Biochem. Biophys.2014;
- Moro IJ, Gondo GDGA, Pierri EG, Pietro RCLR, Soares CP, Sousa DP de, et al. Evaluation of antimicrobial, cytotoxic and chemopreventive activities of carvone and its derivatives . Vol. 53, Brazilian Journal of Pharmaceutical Sciences . scielo ; 2017.
CrossRef - Prakash V. Terpenoids as cytotoxic compounds: A perspective. Pharmacogn Rev. 2018;12(24).
CrossRef - Sánchez M, Mazzuca M, Veloso MJ, Fernández LR, Siless G, Puricelli L, et al. Cytotoxic terpenoids from Nardophyllum bryoides. Phytochemistry. 2010;71(11–12):1395–9.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










