Catalytic Studies of Complexes of Organic Compounds. Part-4: Synthesis, Characterization, Catalytic activity of Cd (II) Complex of Chiral Schiff base
Organic Research Laboratory, Smt. G. G. Khadse College, Muktainagar-425 306, MS, India.
Corresponding Author E-mail: drcjpatil@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/370111
Article Received on : 08-Dec-2020
Article Accepted on :
Article Published : 06 Feb 2021
Chiral Schiff base ligand from 3,5-Diiodo-salicylaldehyde with a chiral amine (1S,2S)- (+)-1,2-Diaminocyclohexane is synthesized, and its Cd (II) complex was synthesized. These were analysed by the physical constant, TLC, colour, UV-Vis, FTIR and 1H NMR method. Also, efforts were made to study the catalytic activity of Cd (II) chiral schiff base complex. The oxidation of benzyl alcohol was used as model reaction using acetonitrile as solvent. The present reaction system was heterogeneous system of catalysis.
KEYWORDS:Benzyl Alcohol; Cd (II) Complex; (1S,2S)-(+)-1,2-Diaminocyclohexane; Oxidation And Catalysis; O-N Donor Chiral Schiff Base;
Download this article as:| Copy the following to cite this article: Patil C. J, Salve S. B. Catalytic Studies of Complexes of Organic Compounds. Part-4: Synthesis, Characterization, Catalytic activity of Cd (II) Complex of Chiral Schiff base. Orient J Chem 2021;37(1). |
| Copy the following to cite this URL: Patil C. J, Salve S. B. Catalytic Studies of Complexes of Organic Compounds. Part-4: Synthesis, Characterization, Catalytic activity of Cd (II) Complex of Chiral Schiff base. Orient J Chem 2021;37(1). Available from: https://bit.ly/36SPELx |
Introduction
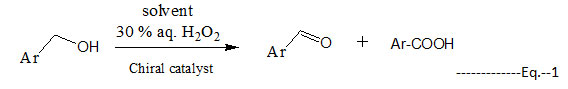
Oxidation of alcohol to the corresponding carbonyl compound (aldehyde, ketone or acid) is the key step in many organic synthetic methods. The catalytic oxidation of alcohol to aldehyde or ketone has been developed employing aqueous hydrogen peroxide (H2O2) in presence of Cd complex as catalyst is a safe process. In particular, benzyl alcohol (primary aromatic) was oxidized selectively to their corresponding aldehyde can be optimised in the present conditions. The oxidative transformation of primary alcoholic compounds to the aldehydes remains very problematic due to the oxidation of primary alcohols, the other possible product (uncontrolled oxidation process) is the analogous carboxylic acids [1-4]. Last but not the least, tandem oxidation may be achieved without the need to isolate the any intermediate or change in solvent. Schiff bases and their metal complexes are of prime importance in the field of synthetic chemistry.Literature reports that these complexes are studied in the view of organic intermediates, metal complexation [5] also voltammetric [6] reduction and oxidation processes. The potential of chiral schiff base complex as catalyst towards oxidations, ring closer, epoxidation, hydrogenations, polymerizations and various coupling reactions [7-8]. Chiral Schiff bases are the reported as a catalyst viz. in asymmetric cyclopropanation [9] and also in varied organic reactions.
The oxidative transformation of primary and secondary alcohols selectively into the respective aldehydes and ketones without forming undesired product is the most important reaction for both industrial applications and academic interest [10-11], still many processes of oxidation in use are non-environmentally friendly.
Varied reagents O2 [4,12-13], H2O2 [14] and H2O2 –TPAP doped ormosils [15] were used for oxidative catalysis of alcohol to equivalent aldehydes and ketones. A phase transfer catalyst nBu4NBr in aq. CH2Cl2, sodium periodate in presence of Mn(TNP)Cl was used for the transformation of arylalkane and cycloalkane into the related alcohols and ketones at room temperature as reported [16a] in the literature. Meanwhile a cheap protocol was reported [16b] using H2O as efficient solvent for the oxidation of aromatic alcohol and amines with H2O2 (34%) by heteropolyoxometalates. Also amechanistic approach was reported[16c] for the oxidation of alcohol with H2O2 (34 %) using simple heteropoly acids (H3PW12O40 and H3PMo12O40) as water tolerant catalyst. Recently, in the literature WO3ZnO/ Fe3O4 was prepared and was tested as nanophoto catalyst for the oxidation of benzyl alcohol and hence to form 2-substituted benzimidazoles by reacting with o-Phenylenediamine[16d]. An ecofriendly, simple and efficient method is described for the oxidation of some alcohols with 34 % hydrogen peroxide, catalysed by H3PW12O40 as simplest class of heteropolyoxometallates[16e].
Ru(III) complexes of the type [RuX2(EPh3)2(L)] (where X = Cl or Br; E = P or As; L = monobasic bidentate Schiff base ligands) have been synthesized and characterized. The catalytic efficiency of one of the ruthenium complex was determined in the case of oxidation of primary and secondary alcohols into their corresponding aldehydes and ketones in the presence of NMO(N-methylmorpholine-N-oxide) as co-oxidant[17]. An enantiopure GO (galactose oxidase) enzyme model has been used synthesized from readily available (R)‐binam and Cu(OTf)2 (TEMPO = 2,2,6,6‐Tetramethyl‐piperidin‐1‐oxyl), and has been effectively used as an efficient chiral catalyst for the oxidative kinetic resolution of secondary alcohols[18].
The dilute H2O2 is an oxidant of ideal choice. It is cheap, readily available and gives water as the only by product. Many systems using aqueous H2O2 as oxidant and Ligand-based catalysts under catalytic conditions have been reported by J. F. Larrow et al., [19]. Also literature contains synthesis of ligand from (1R,2R)-(+)-1,2-Diaminocyclohexane and their complexes, However, literature reveals that there is scare or no publication on use of Cd complexes of chiral schiff bases, an asymmetric catalyst, for the oxidative transformation of benzyl alcohol. As per the literature survey done by us there is no literature available on heterogenous catalytic oxidative transformation of alcohols by these synthesized complex N,N’-(S,S)-1,2-Cyclohexylenebis-(3,5-diiodosalicylidene-iminato)cadmium (III) chloride, complex using N,O-donors along with H2O2 as oxidant.
In continuation of our reported studies [14] of complex of organic compound with respect to behaviour, here we have reported the synthesis of chiral schiff base from 3,5-Diiodo-salicylaldehyde with chiral cyclodiamine, viz. (1S,2S)-(+)-1,2-Diaminocyclohexane in 2:1 proportion. The metal complex was formed from chiral schiff base with cadmium chloride monohydrate, it was characterized and its catalytic effects were studied, on oxidation of a model compound, benzyl alcohol (Ph-CH2-OH).
Material and Methods
The chemicals used were of synthesis grades, for the present work. TLC plates of aluminium material of Merck make (silica gel 60 F254) were used with TLC grade solvents for monitoring the progress and reaction completion. Iodine chamber was used to highlight the starting and final products spot identification in TLC. The estimation of elemental halogen and cadmium in complex was estimated by reported methods [20-21]. FTIR spectral determination were made in 4000-600 cm-1 frequency on a Bruker Spectrum 2000 FT-IR spectrophotometer at SAIF, Kochi for the ligands while FTIR of complexes were recorded on Perkin FT-IR Spectrophotometer, at IISER, Bhopal in the in the range 4000-450 nm. The other instruments used for analysis are mentioned in earlier reports [14].
Experimental
Synthesis of Ligand
The ligand, L was synthesized as per previously reported work procedure [14]. TLC using mobile phase Ethyl Acetate: n-Hexane 2.5:0.5 was performed, to monitor the completion of reaction. The yellow-brown coloured crude product was filtered, dried and recrystallized from ethanol, dried and kept in vacuum desiccator containing calcium carbonate as desiccant. On further drying the yellow-brown pure product was obtained, m.p. = 123-126°C.
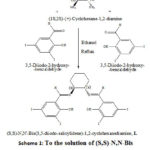 |
Scheme 1: To the solution of (S,S)-N,N-Bis (3,5-diiodo-salicylidene)-1,2-diaminocyclohexane, L |
Synthesis of Cd (II) complex of Ligand
The cadmium complex was prepared according to reported work [14,22]. To the solution of (S,S)-N,N-Bis (3,5-diiodo-salicylidene)-1,2-diaminocyclohexane, (50 mmol) in alcohol (40 ml) in 2 proportion added a solution of 1 proportion of Cadmium (II) chloride monohydrate (CdCl2.H2O), (25 mmol) in aqueous alcohol (25 ml). Bulk reaction was reflux under stirring 2-3 hours, till a homogeneous TLC is monitored in 2.5:0.5 Ethyl Acetate: n-Hexane as mobile phase. The crude brown material formed was filtered and recrystallized from ethanol. It was dried and kept in vacuum desiccator.
 |
Scheme 2: (S,S)-N,N’-Bis-(3,5-diiodo-salicylidene)-1, 2-cyclohexanediaminato cadmium (II)chloride. L (S,S)-N,N’-Bis-(3,5-diiodo-salicylidene)-1, 2-cyclohexanediaminato cadmium (II)chloride. cdl |
Cd-complex synthesized, (S,S)-N,N’-Bis-(3,5-diiodo-salicylidene)-1,2-cyclohexanediaminato cadmium (II)chloride, was tested for absence of any free ligand by taking its TLC. It is abbreviated as [(DiiodoSalcyclo)Cd (II)Cl]
Catalytic oxidation
The oxidation process of benzyl alcohol by use of CdL, used to study the catalytic parameters are as per previous report [14].
Results and Discussion
Ligand (S,S)-N,N’-Bis-(3,5-diiodosalicylidene)-1,2-diaminocyclohexane and the Cd (II) complex was synthesized in two steps. Firstly, the selected 3,5-Diiodosalicylaldehyde and the Chiral amine were condensed to get (S,S)-N,N-Bis-(3,5-diiodo-salicylidene)-1,2-diaminocyclohexane. In the second step, cadmium (II) chloride monohydrate (CdCl2.H2O) was used to prepare its Cd (II) complex i.e. [ (DiiodoSalcyclo)Cd (II)Cl]. As mentioned in our reported work [14] reports that 2nd mole may react separately after the 1st mole reacted or both moles reacts in the same step. The route of synthesis of ligand and the Cd (II) complex is depicted respectively in Scheme 1 and Scheme 2.
Table 1: The M. F., F. wt., colour and the percentage yield of Chiral Schiff bases, L and its Complex, CdL.
|
Code |
M.F. |
F. wt. (g/mol.) |
Colour |
% Yield |
|
L |
C20H18O2N2I4 |
825.96 |
Brown |
80.3 |
|
CdL |
C20H16O2N2I4Cd |
971.82 |
Light brown to yellow |
89.3 |
Synthesized chiral schiff base and its Cd (II) complex were analysed by colour, TLC, m.p., elemental (C, H, N, Cl and Cd) and spectral data viz. UV-Viz, FTIR and 1H NMR data (400 MHz, DMSO) results. The results are as displayed below.
The elemental analysis data of the ligand and complex are satisfied in agreement for proposed molecular formulas. UV-Vis spectra for the Ligand, L and its Complex were recorded using ethanol as solvent. The obtained values of elemental analysis for the schiff base ligand and its metal chelates are consistent with the calculated values. The complexation of chiral Schiff base with cadmium (III) ion showed UV-Vis differences in π → π * transition was shifted from 258 nm to 265 nm, and for the n→ π * transition was changed from 343 nm to 347 nm. The UV-Vis spectra for ligand, L before and after the complex, CdL formation is depicted in Fig.1.
 |
Figure 1: UV-Vis spectra of Ligand, L (before and after complexation, CdL). |
The FTIR spectra of ligand, L before and after complex, CdL formation depicted respectively in Fig. 2A and Fig. 2B.
 |
Figure 2: (A) FTIR spectra of the Ligand, L. (B) FTIR spectra of the Complex CdL. |
The ligand exhibited a band around 2930 cm-1 weak band due to intramolecular H-bond between H of –OH group and N of C=N group, which is in agreement with earlier reports [23-24]. The stretching vibrations of phenolic OH and phenolic C ̶ O in the free ligand appear at 2930 and 1210 cm-1 respectively and the stretching bands of phenolic C ̶ O have been found at higher values (1245 cm-1) in Cd chelate confirming the coordination through phenolic oxygen (M ̶ O) [23-24]. In the FTIR spectrum of ligand, the absorption frequency at 1625 cm-1 appeared because of Schiff base which on chelation, it is shifted to lower [23-27] wave numbers upto 15 cm-1 in metal chelates indicating the participation of azomethine nitrogen in coordination with central metal ion (M-N) which is due to the reduction of double bond character in C=N bond. The complexation of Schiff base with cadmium ion understood with significant differences in FTIR frequency for >C=N and >C–O groups.
The notice of absence [28] of phenolic absorption band in the FTIR spectrum of ligand after the complex formation with cadmium ion was an evidenced for the complexation with the chiral Schiff base. The appearance of new band in FTIR spectrum as 550 nCd-O indicates complexation. The frequency of C=N shifted to lower cm-1 and disappearance of band due to –OH indicates complexation and deprotonation respectively. These results are in concurrence to earlier findings [14].
The coordination of N of the Schiff base nitrogen is indicated by appearance of a newer metal–ligand weak frequency absorption at 490 cm-1 because of nCd-N [29-30]. The extra agreement of formation of complex has been provided due to band at 550 cm-1 with weak-intensity, which is attributed to nCd-O of phenolic part of Schiff base in the complex [30]. The FTIR spectral characteristics frequencies are in concurrence with that reported for the similar compounds as reported [29-30].
The 1H NMR (in DMSO) of Chiral Schiff bases, L and its complex, CdL are depicted in Fig. 3A and Fig 3B respectively.
 |
Figure 3: (A). 1H NMR of the Chiral Schiff base Ligand, L (before complexation). (B). 1H NMR of the Chiral Schiff base complex, CdL (after complexation with Cd). |
L (C20H18O2N2I4)
Brown powder, (995 mg, 80.3 %), m.p. = 123-126 °C; Rf = 0.91 (hexane:ethyl acetate, 80:20); Anal. Calc. for [C20H18O2N2I4; FW 825.96]: C 29.08; H 2.2; N 3.39 %; Found: C 29.10; H 2.19; N 3.42 %; FTIR (in cm-1): 2915 (w), n-OH;1625, assigned to n>C=N ; 1402, n->C=C< (Ar); 3051, n>C-H; 1202, n>C-O; 1141, nC-N and 647 assigned to nC-I; 1H NMR data (400 MHz, DMSO): (d in ppm, assigned to, proton number) d = 1.35-1.75, [m, 8H (4 x CH2)] i.e 2 x -CH2-CH2-CH-N- ( proton b- to CH=N) and 2 x C-CH2-CH2-CH-N (proton g- to CH=N) in cyclohexane ring; d = 2.60 (m, 2H) -CH2-CH-N- (proton a- to CH=N) in cyclohexane ring; d8.33 (s, 2H 2 x CH=N protons); d8.08-8.31 (d, 4 Ar-H protons); d 9.88 (bs, 2H phenolic H on different rings).
LCd (C20H16O2N2I4CdCl)
Light brown to yellow powder, (434 mg, 89.3 %), m.p. = 236-239 (decompose)°C; Rf = complex at baseline (hexane: ethyl acetate, 80:20); Anal. Calc. for [C20H16O2N2I4CdCl; FW 971.82]: C 24.72; H 1.66; N 2.88; Cl 3.65; Cd 11.57 %; Found: C 24.71; H 1.65; N 2.87; Cl 3.66; Cd 11.55 %. FTIR (in cm-1): 1615, assigned to n>C=N ; 1440, n->C=C< (Ar); 2860 n>C-H; 1270 n>C-O; 1245, n-C-N; 620, nC-I; 550 nCd-O and 495 nCd-N; 1H NMR data (400 MHz, DMSO): (d in ppm, assigned to, proton number) d = 1.25-1.65, (m, 8H (4 x CH2)) 2 x C-CH2-CH2-CH-N (proton g- to CH=N) and 2 x -CH2-CH2-CH-N- (proton b- to CH=N) in cyclohexane ring; d = 2.55 (m, 2H) -CH2-CH-N- (proton a- to CH=N) in cyclohexane ring; d = 8.01 (s, 2H CH=N protons); d = 7.72-8.00 (d, 4 Ar-H protons); Ar-H).
The ligand under study shows a singlet at 9.88 d value indicate the phenolic proton and a singlet at 8.33 shows >CH=N- proton, multiplet signal at 8.08-8.31 d is assigned to aromatic proton. The methylene protons are absorbed as bunch of peaks in the range 1.35-1.75 d values (proton b- and g- to CH=N) of cyclohexane ring, the 2.60 d multiplet for (proton a- to CH=N) cyclohexane ring.
The Cd (II) complex shows a weak singlet at 8.01 d shows >CH=N- protons indicating involvement of N of azomethine in complexation with cadmium, multiplet signal at 7.72-8.00 d is assigned to aromatic protons. The methylene protons are absorbed as bunch of peaks in the range 1.25-1.65 d values of cyclohexane ring, the 2.55 d multiplet for cyclohexane ring. A very weak peak at 9.88 d value indicated absence of phenolic protons hence confirms the complexation with O. We have reported [14] the similar findings in earlier work and are also in concurrence with the results of Wen, Z. et al., [31].
The Cd (II) complex shows values of the molar conductivity (LM) at the concentrations of 10-3 M in in dichloromethane is 19.1 Scm2mol-1, which indicates that the present Cd (II) complex is of slight polar nature because of electrolytic behavior of halogen (chlorine). According to the molar conductance values, the schiff base complex have 1:1 metal:ligand ratio, as 1:1; consequently one chloride ion is outside the coordination sphere in the complex. Hence the chloride must be coordinated to the metal center [14,32].
The results of oxidation catalysis of benzyl alcohol by using H2O2 as oxidant and the cadmium complex of chiral schiff base as catalyst in acetonitrile to form benzaldehyde are summarized in Table 2.
Table 2: Screening conditions for oxidation [a] of primary alcohols.
|
Entry |
Alcohol |
Product |
Time (hr) |
conversion (%) |
Ref. |
|
1 |
C6H5CH2OH |
C6H5CHO |
08 |
79.00 |
2 |
|
2 |
C6H5CH2OH |
C6H5CHO |
04 |
75.02 [b] |
14 |
|
3 |
C6H5CH2OH |
C6H5CHO |
03 |
88.00 [c] |
17 |
|
4 |
C6H5CH2OH |
C6H5CHO |
22 |
71.00 |
18 |
|
5 |
C6H5CH2OH |
C6H5CHO |
04 |
51.33 [d] |
This work [d] |
[a] Reaction was performed under open-air conditions fitted with a condenser; [b] Cr (III)L complex, isolation purpose multiple size batch was conducted, alde % determined by HPLC and comparing with authentic samples; [c] achiral [RuCl2 (AsPh3)2 (DHA-ampy)] / NMO and [d] chiral catalyst Cd (II)L complex used here.
The product of oxidation catalysis obtained was dried over sodium sulphate and was concentrated in microflask under vacuum (carefully) and resulting liquid product was purified by eluting the reaction mass using hexane:ethyl acetate on silica gel column to obtain the benzylaldehyde as a colourless liquid showed FTIR (in cm-1): 1605 (s), assigned to n>C=O ; 1525, n->C=C< (Ar) ; 1222 n->C-O ; 2710 n->C-H (Ar) ; 2860, n-CHO; 1H NMR data (400 MHz, DMSO): (d in ppm, assigned to, proton number) d = 10.3 (s, 1H) -CHO; d =6.52-7.75 (m, 5 Ar-H). Similar report [14] was made with a chiral Schiff base from 3,5-Dibromosalicylaldehyde for the oxidative transformation of benzyl alcohol to benzaldehyde.
The Table 2 indicated a comparison of the present oxidation catalysis with the results available in literature which indicated that, time wise it is less time than required by other Heterogeneous catalysis and it matches with the conversion of the benzaldehyde formation [2,14,17-18]. In the present work, % conversion of oxidation of benzyl alcohol to benzaldehyde by H2O2 oxidation in presence of catalytic CdL, using solvent acetonitrile is 51.33 % which is slightly less as compared to that with the Cr (II) catalyst from chiral Schiff base with dibromo substituent which is small in size, and hence it is more efficient catalyst. As because the size of iodine makes the ligand more bulkier than when it is bromo substituent the rate of oxidation is less than the Cr (III)L catalyst recently reported [14].
Literature showed that the many ruthenium catalytic reactions were reported with a oxidants of wide variety viz., hydrogen peroxide [15], molecular oxygen [13], aqueous H2O2 [16b,c], NaIO4 [33], chloramine-T [34], iodosylbenzene [35] and benzoquinone [36], where as N-methylmorpholine-N-oxide (NMO) as oxidant is scarely available in literature. Oxidation of alcohols by complex, (g6-p-cyemene)ruthenium (II) 2- (naphthylazo)phenolate using N,O-donors by use of NMO [37].
Possible mechanism for the Benzyl alcohol to Benzaldehyde oxidation reaction
A possible route of oxidation mechanism based on the above discussed data can be proposed as below,
 |
Scheme 3: The mechanistic route proposed for oxidative transformation of the benzyl alcohol in presence of aq. H2O2 (aqueous) solution catalyzed by chiral schiff base Cd (II) complex. |
It is marked, presence of coordinated chiral ligand in Cd (II) complex (Scheme 3) may form Cd (II) intermediate-I within the reaction because of the reaction of oxidant with the complex catalyst as reported [38-42]. Specifically, the detection of intermediate Cd (II) species from reaction intermediate-II has occurred by monitoring the successive spectral observation of complex in the oxidation process (above Eq.-1). Strength of peculiar absorption band maximum is changed little during transformation, which is dependent on oxidative addition. It is because of formation of positive species in the oxidative transformation to oxide intermediate-II via intermediate-I in coordination of oxidant to the central metal ion [43,44]. Several reports have been found in literature that the oxo-cadmium (II) complex is probably an intermediate-III in the oxidative transformation process of organic reaction catalyzed by Cd (II) complex [39-42].
Thus, Cd (II)−−O is formed through the oxygen transfer from oxidant molecule to give potential intermediate-III. The modification in the colour of the reaction at the start of the oxidative catalytic reaction of the solution from light yellow to slight brown is in support for oxygen atom transfer from H2O2 (oxident) to the central metal ion (Cd (II) to oxo-cadmium (II) intermediate-III. When an aqueous H2O2 was mixed to solution, colour turned to brown and marked the change is because of the formation of Cd (II)–O moiety, which explains reaction of peroxido function of Cd (II)-oxidant and the presence of strong transition (charge transfer) in intermediate-III. The active oxo-cadmium (II) intermediate could oxidatively transfer benzyl alcohol (substrate) through the complex with benzyl alcohol to potential oxo-cadmium (II) intermediate IV to regenerate oxidized catalyst complex (intermediate-I) and yield chemo selective product (i.e., benzaldehyde). Conceptually, above sequence can be written in the form of Scheme 4 as below…
 |
Scheme 4: General scheme for the benzyl alcohol oxidative transformation to benzaldehyde. |
Typical mechanism of catalytic reaction for Cd2+-moiety is discussed here. But, on the basis of colour change through catalytic system of reaction and depending on mechanisms assigned for reactions of similar nature [39-42,45], one could predict that important process is oxo-cadmium (II) moiety presence in catalytic transformation through alcohols oxidation, as depicted in Scheme 3, which is scarely discussed.
Conclusion
The chiral Schiff base was synthesized from chiral amine and its mononuclear Cd (II) complex was synthesized. The prepared Cd (II) complex was characterized by different analytical and spectral methods. Cd (II)-complex was tested as catalysts for one pot conversion of benzyl alcohol to aldehyde by the oxidation by use of H2O2 in mild conditions. The possible oxidized products of benzyl alcohol were benzaldehyde and or an impurity, benzoic acid. It is heterogeneous system of catalysis. In the visible region, complex exhibited a peculiar absorption band responsible for the metal-to-ligand charge transfer (MLCT). The prepared CdL is a promising catalyst in oxidation of alcohol using hydrogen peroxide as terminal oxidant. The main benefit of this catalytic system are the easy synthesis of ligand and its Cd complex and also modification of ligand is easy process. In present work, % conversion (oxidation) benzyl alcohol to benzaldehyde by H2O2 oxidation in presence of catalytic CdL, using solvent acetonitrile is less than the Cr (III)L catalyst with comparatively less bulkier ligand (substituent) recently reported [19]. This work leads to the development of new class of catalyst system for oxidative transformation of benzyl alcohol to benzaldehyde and related work is now underway.
Scope
In future this and similar complex were used in the oxidation and other type of the catalysis of organic compounds.
Acknowledgement
Authors are thankful to the Management of Smt. G. G. Khadse College, Muktainagar for providing required facilities. Authors are thankful for recording UV-Vis spectra and HPLC analysis by Shree Reliable’s Industrial laboratories, Jalgaon (MS). We are also thankful to SAIF, Kochi, Kerla for FTIR and 1H NMR spectra recording.
References
- Schmidt, A. K. C.; Stark, C. B. W. TPAP-Catalysed direct oxidation of primary alcohol to carboxylic acid through stabilized aldehyde hydrates, Org. Lett., 2011, 13 (16) 4164-4167 htpps://doi.org/10.1021/ol2014335
CrossRef - Biradar, A. V.; Dongare, M. K.; Umbarkar, S. B. Selective oxidation of aromatic primary alcohols to aldehydes using molybdenum acetylide oxo-peroxo complex as catalyst. Tetra. Lett., 2009, 50 (24) 2885-2888. htpps://doi.org/10.1016/j.tetlet.2009.03.178
CrossRef - Zhao, M. M.; Li, J.; Mano, E.; Song, Z. J.; Tschaen, D. M. Oxidation of primary alcohol to carboxylic acids with sodium chlorite catalyzed by TEMPO and bleach: 4-Methoxy-phenyl acetic acid. Org. Synth., 2005, 81, 195-203. htpps://doi.org/10.15227/orgsyn.081.0195
CrossRef - Mizugaki, H.; Ji, T.; Ebitani, K.; Kaneda, K. Highly efficient oxidation of alcohols to carbonyl compounds in the presence of molecular oxygen using a novel heterogeneous ruthenium catalyst. Tetra. Lett., 2002, 43 (40) 7179-7183. htpps://doi.org/10.1016/S0040-4039 (02)01678-7
CrossRef - a) Madhava, A. S.; Patil, C. J.; Ramachandraiah, G.; Vyas, D. N. Electrochemical Studies of Schiff Base Complexes: Part-I. Electrochemical Studies of Ni (II) Schiff Base Complexes. Bull. Electrochem., 1995, 11 (9) 442, ISSN: 0256-1654, b) Madhava, A. S.; Patil, C. J.; Ramachandraiah, G.; Vyas, D. N. Electrochemical Studies of Schiff Base Complexes: Part-II. Electrochemical studies of some Cd (II) Schiff base complexes. Bull. Electrochem., 1996, 12, 355, ISSN:0256-1654
- a) Patil, C. J.; Madhava, A. S.; Ramachandraiah, G.; Vyas, D. N., Electrochemical Studies of Schiff Bases: Part-1. Electrochemical Studies of Schiff Bases Bull. Electrochem., 1991, 7 (6) 283 – 285, ISSN: 0256-1654, b) Patil, C. J.; Madhava, A. S.; Ramachandraiah, G.; Vyas, D. N., Electrochemical Studies of Schiff Bases: Part-4. Voltammetric studies of Schiff bases-2-hydroxy-naphthalidene-aniline and its-CH3 and-OCH3 derivatives, Bull. Electrochem., 1995, 11 (3) 159, ISSN:0256-1654
- Gupta, K. C.; Sutar, A. K. Catalytic activities of Schiff base transition metal complexes, Coordin. Chem. Rev., 2008, 252 (12-14) 1420-1450, htpps://doi.org/10.1016/j.ccr.2007.09.005
CrossRef - Gupta, K. C.; Sutar, A. K.; Lin, C. C. Polymer-supported Schiff base complexes in oxidation reactions, Coordin. Chem. Rev., 2009, 253 (13-14) 1926-1946, htpps://doi.org/10.1016/j.ccr.2009.03.019
CrossRef - Li, Z. N.; Zheng, Z.; Chen, H. Highly efficient and enantioselective cyclopropanation of styrene with diazoacetates using a new copper (Schiff bases) catalyst, Tetrahedron: Asymm., 2000, 11 (5) 1157-1163. htpps://doi.org/10.1016/S0957-4166 (00)00037-9
CrossRef - Vannucci, A. K.; Hull, J. F.; Chen, Z.; Binstead, R. A.; Concepcion, J. J.; Meyer, T. J. Water Oxidation Intermediates Applied to Catalysis: Benzyl Alcohol Oxidation, J. Am. Chem. Soc., 2012, 134 (9) 3972-3975. htpps://doi.org/10.1021/ja210718u
CrossRef - Lee, A. F.; Ellis, C. V.; Naughton, J. N.; Newton, M. A.; Parlett, C. M. A.; Wilson, K. Reaction-Driven Surface Restructuring and Selectivity Control in Allylic Alcohol Catalytic Aerobic Oxidation over Pd. J. Am. Chem. Soc., 2011, 133 (15) 5724-5727, htpps://doi.org/10.1021/ja200684f
CrossRef - Tang, R., Diamond, S. E.; Neary, N.; Mares, F. Homogeneous catalytic oxidation of amines and secondary alcohols by molecular oxygen, J. Chem. Soc. Chem. Commun., 1978, 13, 562-562. htpps://doi.org/10.1039/C39780000562
CrossRef - Bilgrien, C.; Davis, S.; Drago, R. S. The selective oxidation of primary alcohols to aldehydes by oxygen employing a trinuclear ruthenium carboxylate catalyst. J. Am. Chem. Soc., 1987, 109 (12) 3786-3787, DOI: 10.1021/ja00246a049
CrossRef - Salve, S. B.; Patil, C. J. Catalytic Studies of Complexes of Organic Compounds. Part-3: Synthesis of Chiral Schiff base, Cr (III) Complex and its Characterization. Int. J. Res. Anal. Rev., 2019, 6 (2) 331-343
- Campestrini, S.; Carraro, M.; Tonellato, U.; Pagliaro, M.; Ciriminna, R. Alcohols oxidation with hydrogen peroxide promoted by TPAP-doped ormosils. Tetra. Lett., 2004, 45 (39) 7283-7286, htpps://doi.org/10.1016/j.tetlet.2004.08.020
CrossRef - a) Mohajer, D.; Tayebee, R.; Goudarziafshar, H. Oxidation of Cycloalkanes and Arylalkanes with Sodium Periodate Catalysed by Manganese Porphyrins, J. Chem. Research (S), 1998, 822-823, DOI: 10.1039/A804080J; b) Tayebee, R.; Alizadeh, M. H. Water as an Efficient Solvent for Oxygenation Transformations with 34 % Hydrogen Peroxide Catalyzed by some Heteropolyoxometalates, Monatsh. Chemie, 2007, 138, 763–769, DOI 10.1007/s00706-007-0660-z; c) Li, B.; Tayebee, R.; Esmaeili-Shahri, E.; Namaghi, M. S. Selective photocatalytic oxidation of aromatic alcohols to aldehydes with air by magnetic WO3ZnO/Fe3O4. In situ photochemical synthesis of 2-substituted benzimidazoles, RSC Adv., 2020, 10, 40725-40738, DOI: 10.1039/d0ra08403d, https://www.jstor.org/stable/24099296; d) Tayebee, R.; Alizadeh, M. H. Environmentally benign oxidation of some alcohols with 34% hydrogen peroxide catalysed by H₃PW₁₂O₄₀Curr. Sci., 2007, 93(2) 133-135, https://www.jstor.org/stable/24099296.
CrossRef - Kannan, S.; Ramesh, R. Synthesis, characterization, catalytic oxidation and biological activity of ruthenium (III) Schiff base complexes derived from 3-acetyl-6-methyl-2H-pyran-2,4 (3H)-dione. Polyhedron, 2006, 25 (16) 3095–3103. https://doi.org/10.1016/j.poly.2006.05.042
CrossRef - Alamsetti, S. K.; Mannam, S.; Mutupandi P.; Sekar, G. Galactose Oxidase Model: Biomimetic Enantiomer-Differentiating Oxidation of Alcohols by a Chiral Copper Complex. Chem. – A Eur. J., 2009, 15(5) 1086-1090, https://doi.org/10.1002/chem.2008064
CrossRef - Larrow, J. F.; Jacobssen, E. N.; Gao, Y.; Hong, Y.; Nie, X.; Zepp, C. M. A practical method for the Large-Scale Preparation of [N, N’-Bis (3,5-di-tertbutylsalicylidene)-1,2-cyclohexanediaminato manganese (III) chloride, a Highly Enantioselective epoxidation catalyst. J. Org. Chem., 1994, 59 (7) 1939-1942, htpps://doi.org/10.1021/jo00086a062
CrossRef - Szekeres, L. Determination of cadmium by EDTA Titration. Microchem. J., 1972, 17, 360-363, htpps://doi.org/10.1016/0026-265X (72)90075-6
CrossRef - Vogel, A. I., A Text Book of Quantitative Inorganic Analysis, Third Ed.; Longman, London, 1978
- Saghatforoush, L. A.; Khalilnezhad, R.; Ershad, S.; Ghammamy, S.; Hasanzadeh, M. Synthesis, Characterization and Electrochemical Properties of m-Oxalato Copper (II) and Nickel (II) Complexes of Anthranilic Acid Schiff Base Ligands. Asian J. Chem., 2009, 21 (8) 6326-6334
- Raman, N.; Baskaran, T.; Selvan A.; Jeyamurugan, R. DNA interaction and antimicrobial studies of novel copper (II) complex having ternary Schiff base. J. Iran. Chem. Res., 2008, 1, 129-139
- Kovcic, J. E. The C=N stretching frequency in the infrared spectra of Schiff’s base complexes-I. Copper complexes of salicylidene anilines. Spectrochim. Acta., 1967, 23A (1), 183-187. htpps://doi.org/10.1016/0584-8539 (67)80219-8
CrossRef - Abdel-Latif, S. A.; Hassib, H. B.; Issa, Y. M. Studies on some salicylaldehyde Schiff base derivatives and their complexes with Cr (III), Mn (II), Fe (III), Ni (II) and Cu (II). Spectrochim. Acta. Part A: Mol. Biomol. Spectro., 2007, 67 (3-4) 950-7. htpps://doi.org/10.1016/j.saa.2006.09.013
CrossRef - Amer, S.; El-Wakiel, N.; El-Ghamry, H. Synthesis, spectral, antitumor and antimicrobial studies on Cu (II) complexes of purine and triazole Schiff base derivatives. J. Mol. Stru., 2013, 1049, 326-335. htpps://doi.org/10.1016/j.molstruc.2013.06.059
CrossRef - Naik, V. M.; Patil, S. K.; Tallur, S. B.; Mallur, N. B. Synthesis, spectral and thermal studies of tin (IV) complexes using 2-benzimuidazolyl mercaptoaceto hydrazone type of ligands. J. Ind. Chem. Soc., 2008, 85, 22-25
CrossRef - Kapadia, M. A.; Patel, M. M.; Patel, G. P.; Joshi, J. D. Microbial screening of Thorium (IV) and Dioxouranium (VI) chelates with oxine and phenols. J. Ind. Chem. Soc., 2007, 84 (7) 637-639.
CrossRef - a) Abo-Aly, M. M.; Salem, A. M.; Sayed, M. A.; Abdel Aziz, A. A., Spectroscopic and structural studies of the Schiff base 3-methoxy-N-salicylidene-o-amino phenol complexes with some transition metal ions and their antibacterial, antifungal activities, Spectrochim. Acta A 2015, 136B, 993–1000, DOI: 10.1016/j.saa.2014.09.122; b) Patil, C. J.; Salve, S. B., Partly presented the work in Avishkar-2018, Jalgaon District level entry. Catalytic Studies of Complexes of Organic Compounds. Part-1. Synthesis, Characterization of Aldimines and Evaluation of their Biological activity, 2018, Poster No. AV-1095; c) Aboaly, M. M.; Khalil, M. M. H., Synthesis and Spectroscopic Study of Cu(II), Ni(II), and Co(II) Complexes of The Ligand Salicylidene-2-Amino-thiophenol, Spectro.. Lett., 2001, 34, 495–504, 10.1081/SL-100105095.
CrossRef - a) Mahmoud, W. H.; Deghadi, R. G., Mohamed, G. G., J. Therm. Anal. Calorim., 2016, 120, 893-903, DOI 10.1007/s10973-016-5826-7, Preparation, geometric structure, molecular docking thermal and spectroscopic characterization of novel Schiff base ligand and its metal chelates Screening their anticancer and antimicrobial activities; b) Bellamy, L. J., The Infrared Spectrum of complex molecules, Third Edn., Chapman Hall Ltd., London, 1975; c) Shafaatian, B.; Ozbakzaei, Z.; Notash, B.; Rezvani, S. A., Synthesis, characterization, single crystal X-ray determination, fluorescence and electrochemical studies of new dinuclear nickel(II) and oxovanadium(IV) complexes containing double Schiff base ligands, Spectrochim. Acta., A, 2014, 5, 248-255, DOI: 10.1016/j.saa.2014.11.108,
CrossRef - Wen, Z.; Li, D.; Qi, J.; Chen, X.; Jiang, Y.; Chen, Li.; Gao, B.; Cui, Y.; Dean Q. Effect of the phenyl ring substituent on stereoselectivity in the ring opening polymerization of the raclactide initiated by salen aluminum complexes. Colloid Poly. Sci., 2015, 293 (12) 3449-3457. htpps://doi.org/10.1007/s00396-015-3720-7
CrossRef - a) Çapan, A.; Uruş, S.; Sönmez, M. Ru (III), Cr (III), Fe (III) Complexes of Schiff Base Ligands Bearing Phenoxy Groups: Application as Catalysts in The Synthesis of Vitamin K3, J. Saudi Chem. Soc., 2017,doi: https://doi.org/10.1016/j.jscs.2017.12.007 b) Geary, W. J. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coordin. Chem. Rev., 1971, 7, 81-122. htpps://doi.org/10.1016/S0010-8545 (00)80009-0
- Trahanosky, W. S. Oxidation of Organic Chemistry, Part B, Academic Press, 1973
- Sharpless, K. B.; Akashi, K.; Oshima, K. Ruthenium catalyzed oxidation of alcohols to aldehydes and ketones by amine-n-oxides, and references therein. Tetra. Lett., 1976, 17 (29) 2503-2506, htpps://doi.org/10.1016/S0040-4039 (00)78130-5
CrossRef - Muller P.; Godoy, J. Catalyzed oxidation of alcohols and aldehydes with iodosylbenzene. Tetra. Lett., 1981, 22 (25) 2361-2364, DOI: 10.1016/S0040-4039 (01)82906-3
CrossRef - Backvall, J. E.; Chowdhury, R. L.; Karlsson, U. Ruthenium-catalysed aerobic oxidation of alcohols via multistep electron transfer, J. Chem. Soc., Chem. Commun., 1991, 473. htpps://doi.org/10.1039/C39910000473
CrossRef - Kumar, K. N.; Venkatachalam, G.; Ramesh, R.; Liu, Y. Half-sandwich para-cymene ruthenium (II) naphthylazophenolato complexes: Synthesis, molecular structure, light emission, redox behaviour and catalytic oxidation properties. Polyhedron, 2008, 27 157–166, doi:10.1016/j.poly.2007.08.037
CrossRef - Abdel-Rahman, L. H.; Abu-Dief, A. M.; Adam, M. S. S.; Hamdan, S. K. Some new nano-sized mononuclear Cu (II) Schiff base complexes: Design, characterization, molecular modelling and catalytic potentials in benzyl alcohol oxidation, Catal. Lett., 2016, 146(8) 1373–1396, DOI: 10.1007/s10562-016-1755-0,
CrossRef - Jarupinthusophon, S.; Thong-In, U.; Chavasiri, W. Catalytic oxidative cleavage of terminal olefins by chromium (III) stearate, J. Mol. Catal. A Chem., 2007, 270, 289–294, DOI: 10.1016/j.molcata.2007.02.007.
CrossRef - Bandini, M.; Cozzi, P. G.; Umani-Ronchi, A.; [Cr (Salen)] as a ‘bridge’ between asymmetric catalysis, Lewis acids and redox processes, Chem. Commun., 2002, 919–927, 10.1039/B109945K.
CrossRef - Kerrigan, N. J.; Langan, I. J.; Dalton, C. T.; Daly, A. M.; Bousquet, C.; Gilheany, D. G. Asymmetric alkene epoxidation with chromium oxo salen complexes. Effect of added phosphoryl ligands, Tetra. Lett., 2002, 43(11) 2107–2110, 10.1016/S0040-4039(02)00193-4.
CrossRef - Samnani, P. B.; Bhattacharya, P. K.; Ganeshpure, P. A.; Koshy, V. J.; Satish, S. Mixed ligand complexes of chromium (III) and iron (III): synthesis and evaluation as catalysts for oxidation of olefins, J. Mol. Catal. A Chem., 1996, 16, 110, 89–94, 10.1016/1381-1169(95)00299-5.
CrossRef - Abd El-Lateef, H. M.; Adam, M. S. S.; Khalaf, M. M. Synthesis of polar unique 3d metal-imine complexes of salicylidene anthranilate sodium salt. Homogeneous catalytic and corrosion inhibition performance, J. Taiwan Inst. Chem. Eng., 2018, 88, 286–304, 10.1016/j.jtice.2018.04.024.
CrossRef - Adam, M. S. S.; Mohamad, A. D. M. Catalytic epoxidation and corrosion inhibition potentials of CuII and CoII pyridinylimino phenolate complexes. Polyhedron, 2018, 151, 118–130, DOI: 10.1016/j.poly.2018.05.035.
CrossRef - Wu, G.; Wang, X.; Li, J.; Zhao, N.; Wei, W.; Sun, Y. A new route to synthesis of sulphonato-salen-chromium (III) hydrotalcites: Highly selective catalysts for oxidation of benzyl alcohol to benzaldehyde, Catal. Today, 2008, 131, 402–407, DOI: 10.1016/j.cattod.2007.10.085.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










