Thermodynamics Studies for 6-(4-Chlorophenyl)-1,2,3,4-Tetrahydro-4-Oxo-2-Thioxopyrimidine-5-Carbonitrile in 60% Dimethyl Sulphoxideat Different temperatures
Manoj R. Gaware
Department of Chemistry, M. V. P. Samaj’s K.P.G. Arts, Commerce and Science College, Igatpuri, Nashik-422 403,Maharashtra, India.
Corresponding Author E-mail: gawaremanoj@rediff.com
DOI : http://dx.doi.org/10.13005/ojc/360633
Article Received on : 05-09-2020
Article Accepted on :
Article Published : 30 Dec 2020
Viscometric studies for trinary mixture of6-(4-chlorophenyl)-1,2,3,4-tetrahydro-4-oxo-2-thioxopyrimidine-5-carbonitrilein 60% dimethyl sulphoxide was carried out at different temperatures ranging between 298 and 313 K over different concentration. From viscometric data ∆G, ∆S and ∆H were evaluated. The result shows that the magnitude of ∆G and ∆H are negative while ∆S is positive showing spontaneity of reaction according to thermodynamics pointing structure breaking takes place between solute and solvent. As ΔH is negative, and ΔS positive, the reaction will be spontaneous at low temperatures (decreasing the magnitude of the entropy term).The thermodynamic study reveals that as concentration increases spontaneity of reaction decreases.
KEYWORDS:Molecular Association; ∆G, ∆S, ∆H, Spontaneity; 6-(4-Chlorophenyl)-1,2,3,4-Tetrahydro-4-Oxo-2-Thioxopyrimidine-5- Carbonitrile; Viscosity
Download this article as:| Copy the following to cite this article: Gaware M. R. Thermodynamics Studies for 6-(4-Chlorophenyl)-1,2,3,4-Tetrahydro-4-Oxo-2-Thioxopyrimidine-5-Carbonitrile in 60% Dimethyl Sulphoxideat Different temperatures. Orient J Chem 2020;36(6). |
| Copy the following to cite this URL: Gaware M. R. Thermodynamics Studies for 6-(4-Chlorophenyl)-1,2,3,4-Tetrahydro-4-Oxo-2-Thioxopyrimidine-5-Carbonitrile in 60% Dimethyl Sulphoxideat Different temperatures. Orient J Chem 2020;36(6). Available from: https://bit.ly/3lOZuDP |
Introduction
Hetrocyclic compounds possessing cyclic pyrimidine ring have attracted significant important due to many biological activities1-3. On other hand, density and viscosity studies of solution plays important role in solution chemistry. By evaluating parameters like apparent molar volume, Jone Dole coefficients ‘A’ and ‘B’ different interactions such as solute-solute, solute-solvent and solvent-solvent interaction present within the solution can be studied. Such interaction provides helpful information to understand nature of solute, solvent, dielectric properties and polarity 4-6.
Thermodynamic properties plays vital role in understanding spontaneity of reaction. By measuring viscosity of solute in aqueous solution, parameters such as Gibb’s free energy, entropy and enthalpy change at different temperatures can be studied7-9.
Therefore in our present work we have systematic studied densitometric and viscometric measurement for different concentrations at temperatures range from 298 to 313 K. From these data different thermodynamic parameters are determined which gives important information about structure making or structure breaking in the solution. We have also studies the relationship between concentration of solution with the spontaneity for the reaction of ternary mixture.
Material and Methods
The compounds under study was synthesized, purified by recrystallization technique and characterized by IR and 1H NMR spectroscopy10. Different concentration of solutions ranging from 0.002 to 0.010 mol L-1 in 60 % dimethyl sulphoxide was prepared using triple distilled deionized water at room temperature. The calibration of pycknometer used to determine density was done by measuring the densities of different solvents like distilled water, acetone, toluene and carbon tetrachloride. The data obtained was compared with reported literature. The viscosity of test solutions were measured by using Ubbelohde viscometer at temperatures ranging from 298 to 313 K. Thermally controlled water bath was used and desired temperature was maintained by circulating water throughout. Digital stop watch was used to measure flow time.
Theory
Coefficient of viscosity of liquid and temperature are correlated by following equation
ηr =A. e-∆G/ RT——————- (1)
The thermodynamic parameters are evaluated from following equations
∆G = -2.303R x slope ———————— (2)
log ηr2/ ηr1 = [∆H/2.303 R] [T2-T1/T1T2] ———————- (3)
∆S = ([∆H – ∆G) / T ————————– (4)
Result and Discussion
From densitometric and viscometric studies it is observed that rise in temperature decreases the density and viscosity of the solution. The values of density and viscosity of solution at various temperatures with concentration are represented in table 1 to table 5. Gibb’s free energy are determined from the slope of graph by plotting log η Vs 1/T as shown in figure 1. It is found that compound possess negative value of Gibb’s free energy. The values of change in enthalpy in reaction were determined by using equation (3).The system showed negative values indicating the reaction is highly exothermic and spontaneous in nature. The value of change in entropy were determined from equation (4). The value of entropy change is positive which shows spontaneity in the reaction achieved by molecule flipping over each other and destruction of hydrogen bonding of compounds.
Table 1: Relative viscosity for different concentrations at various temperatures.
|
Temp |
1/T x 10-3 |
0.002 mol L-1 |
0.004 mol L-1 |
0.006 mol L-1 |
0.008 mol L-1 |
0.010 mol L-1 |
|||||
|
ηr |
log ηr |
ηr |
log ηr |
ηr |
log ηr |
ηr |
log ηr |
ηr |
log ηr |
||
|
298 |
3.356 |
1.1001 |
0.04143 |
1.1018 |
0.04210 |
1.1039 |
0.04293 |
1.1053 |
0.04348 |
1.1073 |
0.04427 |
|
303 |
3.3003 |
1.0321 |
0.01372 |
1.0342 |
0.01460 |
1.0362 |
0.01544 |
1.0380 |
0.01620 |
1.0401 |
0.01708 |
|
308 |
3.2468 |
1.0290 |
0.01242 |
1.0325 |
0.01389 |
1.0360 |
0.01536 |
1.0367 |
0.01565 |
1.0375 |
0.01599 |
|
313 |
3.1949 |
1.0257 |
0.01102 |
1.0284 |
0.01216 |
1.0315 |
0.01347 |
1.0342 |
0.01461 |
1.0371 |
0.01582 |
Table 2: Values of thermodynamic parameters at different concentrations.
|
Conc.(mol L-1) |
∆G (J mol-1 K-1) |
∆H (J mol-1 K-1) |
∆S (J K-1) |
|
0.002 |
-3333.90 |
-500.41 |
9.35 |
|
0.004 |
-3261.91 |
-496.61 |
9.13 |
|
0.006 |
-3188.00 |
-496.43 |
8.88 |
|
0.008 |
-3142.43 |
-492.64 |
8.75 |
|
0.010 |
-3117.54 |
-491.02 |
8.67 |
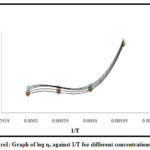 |
Figure 1: Graph of log ηr against 1/T for different concentrations |
Conclusion
From the above data the viscous flow fordifferent compounds in 60% aqueous dimethyl sulphoxide at different temperatures are thermodynamically spontaneous and exothermic. We found decrease in ∆G, ∆H and ∆S with increase in concentration. The magnitude of these thermodynamic parameters clearly indicates that the reaction is spontaneous according to thermodynamics. The study reveals that there is structure breaking between solute and solventalong with decrease in spontaneity with concentration. This shows strong molecular interaction between the molecules and solvent i.e. solute-solvent interaction is high rather than solute-solute and solvent-solvent interactions.
Acknowledgement
The author thanks Dr. P.R Bhabad and Prof. (Dr) J. S. Aher for guidance to carry out the research work.
Conflict of Interest
There is no conflict of interest.
References
- Clark, J.; Shahhet,M., Korakas,D., Varvounis, G.Hetero. Chem. 1993,30, 1065-1072
CrossRef - Ogowva,K., Yamawaki, I., Matsusita, Y., Nomura, N., Kador, P., Kinoshita.Eur. J Med Chem.1993, 28, 769-781.
CrossRef - Tozkoparan, B., Ertan,M., Kelicen,P., Demirdar,R.Farmaco,1999, 54,588-593.
CrossRef - Aher,J. S., Gaware,M.R., Sawant,A. B., Lokhande,D.D., Patil,S.V.Res. World, 2016, 4(4),120-124.
- Aher,J. S., Gaware,M.R.,Lokhande,D.D.Inter. J. Eng. Res. Gen. Sci.,2017,5(4), 2017, 13- 19
- Gaware,M.R., Aher,J. S. Inter. J. Chem. Phy. Sci.,.2018,7(Spl Issue), 89-96.
- Sonar,A. N. Ultra Chem.,2012, 8(1), 101-104
CrossRef - Dhake,R. B. Glo. J. Res.Ana.,2016, 5(2), 2016, 178-179
CrossRef - Sonar, A. N., Pawar, N. S.J. Chem. Pharm. Res.,2011, 3( 1),
- Gaware,M.R., Aher,J. S., Lokhande,D. D., Tambade,P. J., Bhagare,A. M.Ind. J. Chem.- B, 2017, 56 (B) 09, 997-999

This work is licensed under a Creative Commons Attribution 4.0 International License.









