Removal of Heavy Metals from Chithrapuzha River Water by Iron Oxide Nanoparticles Prepared Via Green Synthesis Methods
1Research and Development Centre, Bharathiar University, Coimbatore - 641 046, Tamil Nadu, India.
2Department of Chemistry, MES College of Engineering Kuttippuram, - 679 573, Kerala, India.
3Department of Chemistry, Sri Krishna College of Engineering and Technology, Coimbatore-641 008, Tamil Nadu, India.
4Department of Chemistry, Hindusthan College of Engineering and Technology, Coimbatore-641 032, Tamil Nadu, India.
Corresponding Author E-mail: jeyaraja100@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360620
Article Received on :
Article Accepted on :
Article Published : 09 Nov 2020
On account of industrialization and increasing population, the water bodies get polluted by means of degradable and non-degradable substances. In 21st century, it is necessary to maintain a healthy environment especially water bodies for the survival of not the aquatic animals but also for healthy human life. Recent advances suggest that the issues related to water quality could be resolved by using nanoparticles and nano-filtration membrane methods from the development of nanotechnology. In this research, attempt to remove heavy metals from Chithrapuzha River water at Cochin bar mouth (S1) and Fact barge jetty (S2) using Fe2O3 prepared via green synthesis using Egg albumin and Aloe vera. Our results provoke that, the synthesis of Fe2O3 nanoparticle is cost-effective and eco-friendly and also good in nano-regime. Results of filtration studies showed that Fe2O3 nanoparticles remove heavy metals from Chithrapuzha River water and also increases the DO content which helps the survival of aquatic life.
KEYWORDS:Aloe Vera; Chithrapuzha River; Egg Albumin; Fe2O3 Nanoparticles; Heavy Metals
Download this article as:| Copy the following to cite this article: Deepa G, Jeyaraj M, Magudeswaran P. N. Removal of Heavy Metals from Chithrapuzha River Water by Iron Oxide Nanoparticles Prepared Via Green Synthesis Methods. Orient J Chem 2020;36(6). |
| Copy the following to cite this URL: Deepa G, Jeyaraj M, Magudeswaran P. N. Removal of Heavy Metals from Chithrapuzha River Water by Iron Oxide Nanoparticles Prepared Via Green Synthesis Methods. Orient J Chem 2020;36(6). Available from: https://bit.ly/3eGVlPr |
Introduction
Water has played the crucial role in the evolution of life from molecules to man. The adverse effect of water pollution is a major environmental concern on both nature and human beings. In recent years global concern for the quality and quantity of river water has increased. The water quality of river has been considerably influenced by the discharge of industrial and domestic wastewaters besides agricultural runoff. Moreover, the large quantity of various wastewaters discharged into the river not only influences the aquatic organisms and also alters the quality of the environment. As for as, 21st century is concerned, it is most urgent to take necessary steps to control water pollution to give pure water for future purpose. Various techniques like adsorption, bio-sorption precipitation, electrochemical treatments, reverse osmosis, evaporation, membrane filtration, oxidation, flotation and ion exchange processes are commonly used1-3. Among these, adsorption is an efficient and conventional method to remove toxic metal ions and bacterial pathogens from water.
Green synthesis of metal and metal oxide nanoparticles has been a highly gorgeous research area over the last decade. The development of novel and cost-effective nanomaterials for pollution detection, environmental remediation and other applications has attracted considerable attention. From the review of literature, it was found that the issues related to water quality could be resolved by using nanoparticles and nano-filtration membrane methods4-5. Because of their increased reactivity and property changes, the nanoparticles play a important role in chemical and transformation cycling. The increased reactivity of nanoparticles is mainly due to the larger surface to volume ratio as compared to the bulk materials. Nano-sized metal oxides are expected to play an important role as efficient adsorbents as they have high surface areas and a large surface to volume ratio compared with conventional sorbents.Nano-sized metal oxides are highly valued material with various applications in electrical, optical, catalysts, gas sensors, mechanical devices, sunscreens and cosmetics6-9.
Study Area
The aim of this research to determine the associations between different sized nanoparticles and toxic trace metals present in a polluted Chithrapuzha River system in Kerala. The River Chitrapuzha is one of the tributaries of Periyar River and passes through Amabalamedu, Kochi area, on the southern coast of the Indian subcontinent. The river receives a variety of effluents from refinery, fertilizer and other chemical related industries such as Fertilizers and Chemicals Travancore (FACT), Hindustan Organics Chemicals Limited (HOCL) and Kochi Refinery Limited (KRL) are located in and around Chitrapuzha River. The effluent mainly contains ammonium related compounds such as ammonium sulphate, ammonium phosphate and calcium sulphate, nitrate and heavy metals. The total effluent discharge into river is about 33,600 m3 per day.
The water samples was collected from Chithrapuza River at Cochin bar mouth (S1) and Fact barge jetty (S2) during rainy and summer seasons in the year 2019 and study the effect of Fe2O3 nano particles with two different sizes on the removal of impurities from the water. The Fe2O3 based nanomaterial is used to removable of heavy metals from the contaminated water because of their significant features such as large surface area, small size and magnetic property10-15. The magnetic property of Fe2O3 nanoparticles allows easy separation of adsorbents from the system and could be reused based on the economic burden. Thus in this research focused to study the effect of different sized Fe2O3 nanoparticleson the river water collected at two different places in various seasons.
Material and Methods
The Fe2O3 nanoparticles are prepared via green synthesis technique using Egg albumin and Aloe vera plant extract via microwave assisted heating method. Analytic reagent grade FeCl3 was used as precursor along with water and Egg albumin and Aloe vera plant extract separately to prepare the base solution. The solution is kept in the microwave for about 18 minutes till the solvent get evaporated. The obtained black color precipitate was centrifuged and gets dried to collect the yield. The obtained particles were calcined at 600oC and used for water treatment. The calcined particles were characterized for structural analysis via XRD, SEM and EDAX studies. These nanoparticles were used as a filter in sand beds of height 20 cm with pH 816. The thickness of nanoparticle layer over the sand bed was maintained at 3 different thicknesses viz 2 cm, 3 cm and 4 cm. After passing the river water collected from Cochin bar mouth (S1) and Fact barge jetty (S2) during rainy and summer seasons through this filter medium was collected on a beaker placed over a magnet to remove Fe2O3 nanoparticles if any present in the filtered water. The schematic setup used in this study for the treatment of water was shown in figure 1.
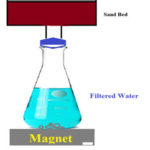 |
Figure 1: Schematic setup used for water treatment |
Results and Discussion
Analysis of Nanoparticles
The prepared nanoparticles were structurally analyzed XRD and SEM analysis. The recorded X-ray diffraction pattern of both samples (figure 2) resembles well with JCPDS card No: 24-0072 of α-Fe2O3 particles with slight variation in peak positions17. The image revealed that broadening of peaks increases with Aloe vera plant which in turn reduces the particle size. Both the samples crystallize in a cubic structure. The crystallite sizes were obtained using the Debye –Scherrer formula. The obtained values 18.56 nm and 6.75 nm for the samples A and B confirmed their existence in nano-regime.
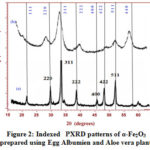 |
Figure 2: Indexed PXRD patterns of α-Fe2O3 prepared using Egg Albumien and Aloe vera plant |
The morphology of the samples plays a vital role in wastewater treatment18. SEM micrographs were recorded and depicted in figure 3. The α-Fe2O3 prepared via Egg albumin shows an irregular shape with agglomeration of different particle sizes with globular shapes showing both micro and macro pores and a few crevices and voids. Whereas the Aloe vera extract assisted particles are spherical in shape. Size distributions of both images were analyzed using image J software and from the distribution histogram, the particle size was calculated. The values obtained well matched with the size obtained from XRD.
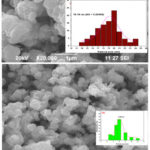 |
Figure 3: SEM micrograph with an insert of particle size histogram of α-Fe2O3 prepared using Egg albumin and Aloe vera plant. |
Table 1: Physico-chemical characteristics of water samples collected from Chithrapuzha River water at Cochin bar mouth (S1) and Fact barge jetty (S2) of during rainy and summer seasons (2019) before nanoparticles addition.
|
Parameters |
Units |
Rainy season |
Summer season |
||
|
Cochin bar mouth (S1) |
Fact barge jetty (S2) |
Cochin bar mouth (S1) |
Fact barge jetty (S2) |
||
|
DO |
% saturation |
93 |
90 |
92 |
88 |
|
pH |
pH units |
6.7 |
7.8 |
6.9 |
9.5 |
|
BOD |
mg/l |
0.78 |
3.5 |
0.99 |
4.8 |
|
P |
mg/l |
0.02 |
1.5 |
0.09 |
2.5 |
|
N |
mg/l |
0.90 |
1.20 |
1.1 |
1.99 |
|
TURBIDITY |
NTU |
1.85 |
10.20 |
2.42 |
12.6 |
|
TDS |
mg/l |
132 |
738 |
153 |
942 |
The physico-chemical characteristics of the water samples collected from Cochin bar mouth (S1) and Fact barge jetty(S2) of Chithrapuzha River water during rainy and summer seasons in the year 2019 are given in Table-1. It is clearly indicated that the lower values of DO and higher values of BOD threaten the aquatic life of Chithrapuzha river water at S2 compared to S1. The decrease in DO values at S2 is due to the discharge of oxygen demanding waste. Like BOD the amount of nitrogen, phosphorus, turbidity and TDS increased from S1 to S2 during both rainy and summer seasons is mainly due to discharge of untreated industrial effluents located near Chithrapuzha river at S2. In order to increase the water quality of the river, the river water passed through Fe2O3 nanoparticle layer of the same three different thicknesses and analyzed the parameter after the filtration. The calculated values of physico-chemical parameters are given in Tables 2 and 3 and clearly indicate that nanoadsorbent increases the DO value whereas it decreases the BOD value. Likewise, DO value is more in the case of Aloe vera assisted Fe2O3 nanoparticles. This may be due to in addition to high specific surface areas nanoparticles also have unique adsorption properties due to different distributions of reactive surface sites and disordered surface regions. Improved values of DO are one of the insistent conditions for aquatic life. During filtration only very few particles of Fe2O3 nanoparticles were selected at the surface of the magnet. It is evident that the prepared nanoparticles have high surface binding energy and high internal diffusion resistance. The nanoparticles synthesized via green methods are non toxic and it won’t affect the water quality. It is an eco-friendly material and can be used directly to a polluted environment with less chance of secondary pollution19-20. The graphical representation of variation of BOD and DO with the thickness of nanolayer for both Fe2O3 samples during rainy and summer seasons are given in Figures 4 and 5.
Table 2: Physico-chemical characteristics of water sample collected from Chithrapuzha River at Fact barge jetty (S2) of during rainy and summer seasons (2019) after using Egg albumin assisted Fe2O3 nanoparticle filtration
|
Parameters |
|
Rainy session (S2) |
Summer session (S2) |
||||
|
Units |
1cm thick layer |
2 cm thick layer |
3 cm thick layer |
1 cm thick layer |
2 cm thick layer |
3 cm thick layer |
|
|
DO |
% saturation |
94.6 |
95.2 |
96.7 |
92.8 |
94.2 |
95.6 |
|
pH |
pH units |
7.9 |
7.8 |
7.8 |
7.6 |
7.5 |
7.3 |
|
BOD |
mg/l |
2.81 |
2.54 |
2.21 |
2.72 |
2.13 |
1.75 |
|
P |
mg/l |
1.07 |
1.0 |
0.98 |
1.08 |
1.01 |
0.93 |
|
N |
mg/l |
1.3 |
1.18 |
1.09 |
1.5 |
1.8 |
2.00 |
|
TURBIDITY |
NTU |
9.3 |
9.2 |
9.0 |
9.7 |
9.4 |
9.4 |
|
TDS |
mg/l |
235 |
223 |
215 |
230 |
219 |
212 |
Table 3: Physico-chemical characteristics of water sample collected from Chithrapuzha River at Fact barge jetty (S2) of during rainy and summer seasons (2019) after using Aloe vera assisted Fe2O3 nanoparticle filtration
|
Parameters |
|
Rainy Session (S2) |
Summer Session (S2) |
||||
|
Units |
1cm thick layer |
2 cm thick layer |
3 cm thick layer |
1 cm thick layer |
2 cm thick layer |
3 cm thick layer |
|
|
DO |
% saturation |
95.3 |
96.2 |
97.5 |
95.9 |
97.1 |
98.3 |
|
pH |
pH units |
7.8 |
7.8 |
7.4 |
7.5 |
7.3 |
7.2 |
|
BOD |
mg/l |
2.73 |
2.48 |
2.15 |
2.68 |
2.04 |
1.62 |
|
P |
mg/l |
1.03 |
0.98 |
0.95 |
1.04 |
0.97 |
0.91 |
|
N |
mg/l |
1.2 |
1.05 |
1.02 |
1.0 |
0.90 |
0.82 |
|
TURBIDITY |
NTU |
8.8 |
8.3 |
8.1 |
9.4 |
9.1 |
8.9 |
|
TDS |
mg/l |
228 |
217 |
209 |
218 |
207 |
197 |
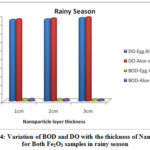 |
Figure 4: Variation of BOD and DO with the thickness of Nano layer for Both Fe2O3 samples in rainy season |
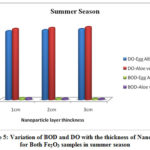 |
Figure 5: Variation of BOD and DO with the thickness of Nano layer for Both Fe2O3 samples in summer season |
Turbidity of the water decreases with nanoparticle filtration, which means that it reduces the light scattering ability of the water, which confirms that the proposed nanoparticles highly removed the clusters of fungi and algae thus it removed micro organism from water. Nitrate imposing a serious hazard to potable water provisions and producing ecological disturbances21. Children and infants are highly sensitive to the towering levels of nitrates, which causes methemoglobinemia or blue-baby syndrome and likely formation of carcinogenic nitrosamines22. In our results, we found that lowering of nitrate concentration takes place with an increase of nano layer thickness. Thus by treating with nanoparticles, the effluent water sample was converted into a good quality.
Removal of Heavy Metals
Generally heavy metals are considered to be a hazard toward ecosystems and humans because of their high-potential toxicity23-27. Heavy metals such as copper, chromium, zinc, manganese and iron in very small quantities are necessary for the function of human body. But, if the metals present to a certain quantity, they can cause poisoning, resulting in severe implications to human health. Like plastics, heavy metals are non-biodegradable materials. The existence of heavy metals even at trace level is believed to be a threat to the human health and ecological environment28.
The metal analysis of Chithrapuzha River water at Cochin bar mouth (S1) and Fact barge jetty (S2) during rainy and summer seasons after using Egg albumin and Aloe vera assisted Fe2O3 nanoparticle filtration are given in the tables from 4 to 7 and also metal analysis of Chithrapuzha River water at S1 and S2 during rainy and summer seasons before nano-particles addition is given in the table 8. It is clearly indicates that Fe2O3 nanoparticle have received increasing attention in removing heavy metals from water. This study exhibited that the prepared Egg albumin and Aloe vera assisted Fe2O3 nanoparticles can be used as an alternate to the traditional adsorbents methods for the removal of heavy metal ions from Chithrapuzha River water at S1 and S2 with high removal efficiency within a very short time. The removal of heavy metals like cadmium, copper, lead, zinc, chromium and iron as typical metal ions present in wastewater, by adsorption onto Fe2O3 nanoparticles was successfully accomplished. Adsorption was very fast and equilibrium was attained within 15-20 minutes. It also indicated that adsorption was highly dependent on the initial concentration of heavy metals present in the water samples.
Table 4: Metal analysis of Chithrapuzha River water at Cochin bar mouth (S1) during rainy and summer seasons-2019 after using Egg albumin assisted Fe2O3 nanoparticle filtration
|
Metals analyzed in mg/l |
Rainy Session (S1) |
Summer Session (S1) |
||||
|
1cm thick layer |
2cm thick layer |
3 cm thick layer |
1cm thick layer |
2cm thick layer |
3 cm thick layer |
|
|
Cadmium |
0.39 |
0.32 |
0.29 |
0.76 |
0.72 |
0.68 |
|
Copper |
0.11 |
0.09 |
0.06 |
0.30 |
0.29 |
0.26 |
|
Lead |
2.47 |
2.41 |
2.39 |
1.81 |
1.72 |
1.52 |
|
Zinc |
4.89 |
4.81 |
4.72 |
2.18 |
2.06 |
1.72 |
|
Chromium |
1.19 |
1.11 |
1.03 |
0.42 |
0.38 |
0.32 |
|
Iron |
0.72 |
0.68 |
0.52 |
0.46 |
0.23 |
0.1 |
Table 5: Metal analysis of Chithrapuzha River water at Cochin bar mouth (S1) during rainy and summer seasons-2019 after using Aloe vera assisted Fe2O3 nanoparticle filtration
|
Metals analyzed in mg/l |
Rainy Session (S1) |
Summer Session (S1) |
||||
|
1cm thick layer |
2cm thick layer |
3 cm thick layer |
1cm thick layer |
2 cm thick layer |
3 cm thick layer |
|
|
Cadmium |
0.47 |
0.46 |
0.45 |
0.8 |
0.78 |
0.77 |
|
Copper |
0.17 |
0.16 |
0.14 |
0.31 |
0.30 |
0.29 |
|
Lead |
2.50 |
2.49 |
2.47 |
1.82 |
1.80 |
1.79 |
|
Zinc |
4.90 |
4.88 |
4.83 |
2.34 |
2.31 |
2.29 |
|
Chromium |
1.26 |
1.23 |
1.20 |
0.47 |
0.45 |
0.44 |
|
Iron |
0.86 |
0.84 |
0.78 |
0.45 |
0.43 |
0.42 |
Table 6: Metal analysis of Chithrapuzha River water at Fact barge jetty (S2) during rainy and summer seasons-2019 after using Egg Albumin assisted Fe2O3 nanoparticle filtration
|
Metals analyzed in mg/l |
Rainy Session (S2) |
Summer Session (S2) |
||||
|
1cm thick layer |
2cm thick layer |
3 cm thick layer |
1cm thick layer |
2cm thick layer |
3 cm thick layer |
|
|
Cadmium |
0.88 |
0.85 |
0.80 |
1.75 |
1.70 |
1.68 |
|
Copper |
1.68 |
1.61 |
1.59 |
3.89 |
3.82 |
3.79 |
|
Lead |
6.47 |
6.39 |
6.29 |
5.6 |
5.52 |
5.42 |
|
Zinc |
10.01 |
9.4 |
8.9 |
7.75 |
7.69 |
7.51 |
|
Chromium |
2.39 |
2.32 |
2.29 |
0.88 |
0.82 |
0.78 |
|
Iron |
2.6 |
2.56 |
2.50 |
1.89 |
1.83 |
1.80 |
Table 7: Metal analysis of Chithrapuzha River water at Fact barge jetty (S2) during rainy and summer seasons-2019 after using Aloe vera assisted Fe2O3 nanoparticle filtration
|
Metals analyzed in mg/l |
Rainy Session (S2) |
Summer Session (S2) |
||||
|
1cm thick layer |
2cm thick layer |
3 cm thick layer |
1cm thick layer |
2cm thick layer |
3 cm thick layer |
|
|
Cadmium |
0.90 |
0.88 |
0.82 |
1.78 |
1.74 |
1.7 |
|
Copper |
1.70 |
1.68 |
1.65 |
3.90 |
3.88 |
3.83 |
|
Lead |
6.45 |
6.40 |
6.39 |
5.62 |
5.54 |
5.5 |
|
Zinc |
10.5 |
10.1 |
9.8 |
7.80 |
7.78 |
7.63 |
|
Chromium |
2.40 |
2.37 |
2.30 |
0.89 |
0.82 |
0.78 |
|
Iron |
2.6 |
2.56 |
2.5 |
1.89 |
1.81 |
1.76 |
Table 8: Metal analysis of Chithrapuzha River water before nanoparticles addition
|
Metals analyzed in mg/l
|
Rainy Session |
Summer Session |
||
|
Cochin bar mouth (S1) |
Fact barge jetty (S2) |
Cochin bar mouth (S1) |
Fact barge jetty (S2) |
|
|
Cadmium |
0.48 |
0.92 |
0.82 |
1.80 |
|
Copper |
0.18 |
1.80 |
0.32 |
3.90 |
|
Lead |
2.51 |
6.50 |
1.84 |
5.68 |
|
Zinc |
4.92 |
10.8 |
2.38 |
7.82 |
|
Chromium |
1.28 |
3.62 |
1.60 |
4.85 |
|
Iron |
0.88 |
2.68 |
0.46 |
1.93 |
Conclusion
The water samples collected from Chithrapuzha River at Fact barge jetty (S1)was highly contaminated when compared to Cochin bar mouth (S1) during both rainy and summer seasons. Nanomaterials have been extensively used to remove heavy metals in water due to their unique properties. In this work, Fe2O3 nano-particles via green synthesis using Egg albumin and Aloe vera which reduces the toxicity of prepared particles. The results showed that the improved values of DO and reduced values of BOD confirm the high adsorption of the prepared Fe2O3 nanoparticles. Likewise the iron oxide nanoparticles used for the removal of heavy metals from the water systems developed as well efficient and cost-effective nanoadsorbent. These nanomaterials exhibit great advantages as adsorbents not only towards heavy metals and also due to less particle size, Egg albumin and Aloe vera assisted Fe2O3 nanoparticles showed many improvements in water quality parameters on both seasons.
Acknowledgement
None.
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Bakkaloglu, I.; Butter, T. J.; Evison, L. M.; Holland, I. C. Water Sci. Technol.1998, 38, 268-277.
CrossRef - Gunatilake, S. K. Multidiscip. Eng. Sci. Stud. 2015, 1(1), 12-18.
- Sörme, L.; Lagerkvist, R. Total Environ. 2002, 298(1), 131-145.
- Savage, N.; Diallo, M. S. Nanopart. Res. 2005, 7, 331–342.
CrossRef - Aorkas, M.; Tsiourvas, D.; Paleos, C. M. Mater. 2003, 15(14), 2844–2847.
CrossRef - Lu, H.; Wang, J.; Stoller, M.; Wang, T.; Bao, Y.; Hao, H. Mater. Sci. Eng. 2016, http://dx.doi.org/10.1155/2016/4964828.
CrossRef - Sun, Y. F.; Liu, S. B.; Meng, F. L.; Liu, J. Y.; Jin, Z.; Kong, L. T.; Liu, J. H. Sensors. 2012, 12(3), 2610-2631.
CrossRef - Wang, X.; Cao, R.; Zhang, S.; Hou, P.; Han, ; Shao, M.; Xu, X. J. Mater. Chem. A. 2017, 45, 23999-24010.
CrossRef - Lu, P. J.; Fang, S.W.; Cheng, W. L.; Huang, S. C.; Huang, M. C.; Cheng, H. F. Food. Drug. Anal. 2018, 26, 1193-1200.
CrossRef - Pragnesh, N. D.; Chopda, L. V. Nanotechnol. 2014, 1, 1-14. http://dx.doi.org/10.1155/2014/398569.
CrossRef - Xu, P.; Zeng, G. M.; Huang, D. L.; Feng, C. L.; Hu, S.; Zhao, M. H.; Liu, Z. F. Total. Environ. 2012, 424, 1–10.
CrossRef - Warner, C. L.; Chouyyok, W.; Mackie, K. E.; Neinar, D.; Saraf, L. V.; Droubay, T. C.; Warner, M. G.; Addlemen, R. S. Langmuir. 2012, 28(8), 3931–3937.
CrossRef - Karatapanis, A. E.; Petrakis, D. E.; Stalikas, C. D. Chim. Acta. 2012, 726, 22–27.
CrossRef - Yang, H.; Tian, Z.; Wang, J.; Yang, S. Sensor Actuat B-Chem. 2012, 161(1), 429–433.
CrossRef - Teja, A. S.; Koh, P. Y. Cryst. Growth. Ch. 2009, 55, 22–45.
CrossRef - Subha, V.; Divya, K.; Gayathri, S.; Jagan Mohan, E.; Keerthanaa, N.; Vinitha, M.; Kirubanandan, S.; Renganathan, S. Drug Des. Dev. Ther. 2018, 2(5), 178-184.
CrossRef - Joya1, M. R.; Bar´on-Jaimez, J.; Barba-Ortega, J. Phys. Conf. Ser. 2013, 466, 012004.
CrossRef - Naidoo, S.; Olaniran, A. O. J. Environ. Res. Public Health. 2014, 11(1), 249–270.
CrossRef - Liu, Y.; Chen, X.; Li, J.; Burda, Chemosphere,2005, 61(1), 11-18.
CrossRef - Liu, Y.; Wang, X.; Yang, F.; Yang, Micropor. Mesopor. Mat. 2008, 114(1), 431-439.
CrossRef - Nasreen, S.; Rafique, U. J. Chem. Environ. Eng. 2012, 3(5), 355-361.
- Majumdar, D.; Gupta, N. Indian J. Environ. Health, 2000, 42, 28–39.
CrossRef - Zhou, D.; Zhang, L.; Zhou, J.; Guo, S. Water Res. 2004, 38(11), 2643-2650.
CrossRef - Karri, R. R.; Sahu, J. N. J. Envi. Manage. 2018, 206, 178-191.
CrossRef - Wang, J.; Zheng, S.; Shao, Y.; Liu, J.; Xu, Z.; Zhu, D. J. Colloid Interface Sci. 2010, 349(1), 293-299.
CrossRef - Lingamdinne L. P.; Koduru, J. R.; Chang, Y. Y.; Karri, R. R. J. Mol. Liq. 2018, 250, 202-211.
CrossRef - Karri, R. R.; Sahu, J. N. J. Mol. Liq. 2018, 265, 592-602.
CrossRef - Bhattacharya, K.; Parasar, D.; Mondal, B.; Ded, P. Sci. Rep. 2015, 5(1), 1-9. http://dx.doi.org/10.1038/srep17072.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










