Cotoneaster Acuminatus Leaf Extract Mediated Synthesis, Characterization and in Vitro Evaluation of Antimicrobial Activity of Zinc Oxide Nanoparticles
Goutam Kumar1 , P.P. Badoni1, Arun K. Khajuria2, Mahender Singh1, Sapna Tyagi3 and Navneet Singh4*
, P.P. Badoni1, Arun K. Khajuria2, Mahender Singh1, Sapna Tyagi3 and Navneet Singh4*
1Department of Chemistry, HemvatiNandanBahugunaGarhwal University,Pauri Garhwal-246001, Uttarakhand, India.
2Department of Botany, Cluster University of Jammu, Jammu-184001, Jammu and Kashmir, India.
3Department of Chemistry, K.L.D.A.V. (P.G.) College, Roorkee- 247667, Uttarakhand, India.
4Department of Applied Science and Humanities, Roorkee College of Engineering, Roorkee-247667, Uttarakhand, India.
Corresponding Author E-mail: navneets176@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360606
Article Received on : 28-09-2020
Article Accepted on :
Article Published : 30 Dec 2020
In recent years, there is significant increase in the attention towards the green synthesis of metal oxide nanoparticles, particularly by the interaction of plant extracts and metal salts; still there is no such published evidence is available on the synthesis of ZnO nanoparticles from aqueous leaf extract of Cotoneaster acuminatus. The synthesis of ZnO nanoparticles was confirmed by using several spectroscopic techniques i.e., UV-Vis, FT-IR, powder-XRD and TEM. It was confirmed from the spectroscopic data that the synthesized nanoparticles were hexagonally orientated, size in the 16-38 nm range and encapsulated by biochemicals present in the leaf extract. These nanoparticles were further evaluated for antimicrobial activity Bacillus subtilisNCFT.583.08, Staphylococcus aureusNCFT.576.08, Pseudomonas aeruginosaNCFT.645.11, Candida albicansNCFT.1006.11 and Saccharomyces cerevisiae NCFT.1008.11strains. This research work might be considered as a successful attempt to create and evaluate medicinal properties of ZnO nanoparticles in combination with Cotoneaster acuminatus leaf extract.
KEYWORDS:Antimicrobial Activity; Cotoneaster Acuminatus; Encapsulated; Znonanoparticles
Download this article as:| Copy the following to cite this article: Kumar G, Badoni P . P, Khajuria A . K, Singh M, Tyagi S, Singh N , Cotoneaster Acuminatus Leaf Extract Mediated Synthesis, Characterization and in Vitro Evaluation of Antimicrobial Activity of Zinc Oxide Nanoparticles.Orient J Chem 2020;36(6). |
| Copy the following to cite this URL: Kumar G, Badoni P . P, Khajuria A . K, Singh M, Tyagi S, Singh N , Cotoneaster Acuminatus Leaf Extract Mediated Synthesis, Characterization and in Vitro Evaluation of Antimicrobial Activity of Zinc Oxide Nanoparticles.Orient J Chem 2020;36(6). Available from: https://bit.ly/3pSbJ4W |
Introduction
Metallic nanoparticles are of great importance because of their advanced physico-chemical and biological activities over their bulk phase. The nanoparticle having size less than 100 nm shows a higher surface to volume ratio which led to elevated surface activity. This unique property allowed them to be exploited in vast applications in many tasks of nano-biotechnology. Nano-biotechnology is the combination of nanotechnology and biotechnology for developing bio inspired and environmental skill for the synthesis of nanoparticles1,2. Previously green synthesis of ZnO nanoparticles using plant extracts have been reported using numerous medicinal plants which includes the extract of Camelliasinesis3, Calotropisprocera4, Hybanthusenneaspermus5, Mimosa pudica 6, Micrococcamercurialis7, Napheliumlappaceum 8, Nyctanthes arbor-tristis 9 etc. by different researchers and these studies indicated the presence of biochemicals such as amines, aldehydes, phenols etc. for stabilizing the metallic forms 10.
ZnO nanoparticles have noteworthy medicinal properties in the field of medication. Excellent bactericidal properties of these nanoparticles against microorganisms have been reported 11,12. In different field of therapeutics like antioxidant, antidiabetic, anticancer and parasitic activities of zinc oxide nanoparticles also reported in literature 13,14. ZnO nanoparticles are nontoxic, biocompatible and biosafe15 and due to its skin compatible properties it is widely used in sunscreens materials 16. The benefit of using metal oxides such as ZnO as antimicrobial nano-agents is that they include mineral nutrient essential to humans and demonstrate strong action even when administered in very small amounts. These nanoparticles show powerful antibacterial activities against various bacteria as reviewed 17. In present work, leaves of Cotoneaster acuminatuswere used for the bio-fabrication of zinc oxides nanoparticles. Itis a deciduous shrub having height upto 4m; with ovate-lanceolated leaves, base rounded, tip acuminated with flat margins and rich in medicinal properties 18. Chemicals presents in leaves of C. acuminatus are helpful in the reduction and stabilization of synthesized ZnO nanoparticles and it was predicted from already reported study that involved synthesis of silver nanoparticles 19. A study reported the antimicrobial activity of its alcoholic root extract and also assisted in investigating the biochemicals present in its extract like glucopyranoside and rhamnopyranoside along with known compounds such as glucoside, phenolic glycoside, apigenin and epicatechin20. The evaluation motive of antimicrobial properties of ZnO nanoparticles synthesized using C. acuminatus leaf extract on nanoscale was to explore their improvised medicinal properties.
 |
Image 1: Cotoneaster acuminatus (GUH20757) Click here to View image |
Materials and Methods
Chemical and Reagents
Analytical grade of zinc acetate dihydrate (Zn(OAc)2.2H2O) and sodium hydroxide (NaOH) were used for research work from Sigma-Aldrich. Experimental works were performed by using deionized distilled water.
Culture Media
Soyabean casein digest and Sabouraud’s dextrose broth of Hi Media Pvt. Bombay, India were used for antibacterial and antifungal test, respectively. Pure cultures of bacterial strains, viz. Bacillus subtilis(NCFT.583.08), Staphylococcus aureus(NCFT.576.08), Pseudomonas aeruginosa(NCFT.645.11) and fungal strains Candida albicans(NCFT.1006.11) and Saccharomyces cerevisiae (NCFT.1008.11) were used for the evaluation of antimicrobial activity.
Plant Collection and Preparation of Plant Extract
Cotoneaster acuminatus plant specimen was collected from Nag Dev Hills, Pauri (Garhwal), India and authenticated from HNB Garhwal University Herbarium under its voucher specimens no. GUH20757. C. acuminatus leaves were collected and washed several times with deionized-distilled water to remove any of the adhering dust particles and subsequently incised into very small pieces, placed in a hot air oven at 35⁰C and then meshed to the powder form using mortar-pestle. In a 250 ml Erlenmeyer conical flask, 0.5 gm of dried and finely cut C. acuminatusleaves and 100 ml deionized distilled water were heated for 25 min at 70⁰C. After cooling, leaf extract was filtered in a separate conical flask and stored for further research work.
Zinc Oxide Nanoparticles (Znonps) Synthesis
50 ml of cotoneaster acuminatus leaf extract was taken in a 250 ml of Erlenmeyer conical flask. It was heated on magnetic stirrer at 70⁰C for 10 minutes. Then 100 ml of 100 mM Zinc acetate dihydrate solution was added drop by drop to it, keeping temperature constant at 70⁰C along with adjusting pH range within 08-10 of the solution by adding few drops of 1M NaOH solution. Color change of the solution was observed from light yellow to reddish brown. Reaction conditions were maintained for 30 minutes and then cooled at room temperature. Consequently, the cooled solution was centrifuged at 5000 rpm for 10 minutes. To remove unreacted and uncoordinated material, collected ZnO nanoparticles were centrifuged again for several times with water and ethanol. ZnOnano-powder was collected by drying in the oven at 50⁰C.
Determination of Antimicrobial Activity
The methodology used for determining the diameter of zone of inhibition, minimum inhibitory and lethal concentration is according to the references 21-23 with some modifications. Soyabean casein digest broth was used to inoculate into selected bacteria at 37ºC for 18h and further suspension was ensured to provide approximately, 108 CFU/mL. The similar steps were employed for fungal strains but in this case Sabouraud′s dextrose broth was exercised for inoculation at 48-72h. Bacterial culture in Soyabean Casein Digest medium (SCDM) medium was inoculated and separately suspended in broth. 8mm diameter wells were punched into agar and loaded with nan-solution (in DMSO) and solvent blanks. Erythromycin, (1mg/mL) was concurrently employed as the positive control and DMSO as negative control. Subsequently the plates were incubated at 37ºC for 18h. The antibacterial activity was determined by quantifying the diameter of zone of inhibition. For assaying, antifungal property of nano-solution, Sabouraud′s dextrose broth medium plates were utilized and the same route as that for determination of antibacterial activity was employed and diameter of zone of inhibition was quantified after 48-72h. Fluconazole (1mg/ml) was utilized as standard. The procedures for assaying activities were repeated in triplicates to validate the readings of diameter of zone of inhibition for each of the test strain. Additionally, minimum inhibitory and minimum lethal concentration were also determined by preparing nano-solution (100µL) in sterile DMSO and sequentially diluted with N-saline (0.85% NaCl) and similar amount of bacterial or fungal suspension was poured to different test-tubes and kept for 48h incubation period.
Spectroscopic Characterization
Elite-UV-visible spectrophotometer, X’PERT-PRO Diffractometer, PanAlytical; CuKα radiation (λmax =1.54Å), Transmission Electron Microscope (JEOL JEM 1011, 100kva) and FTIR Spectrophotometer Perkin Elmer Model RZX has been used to characterize ZnO nanoparticles.
Results And Discussion
Characterization
The formation of ZnO nanoparticles was sensibly confirmed by UV-Vis spectrophotometer within the 250–700nm range. The Electronic absorption spectrum of biosynthesized ZnO nanoparticles represented a characteristic peak at 352nm (Figure 1). The calculated direct band gap (Eg) was 3.52 eV and it was calculated by the following equation i.e.:

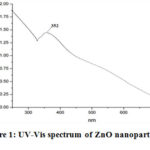 |
Figure 1: UV-Vis spectrum of ZnO nanoparticles |
XRD (2 θ) values with (hkl) 30.58o (100), 33.22o (002), 35.06o (101), 46.38o (102), 55.40o (110) and 61.63o (103) were observed and pattern displayed the formation of hexagonal phase ZnO nanostructures (Figure 2).
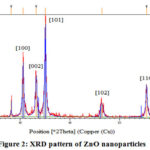 |
Figure 2: XRD pattern of ZnO nanoparticles |
TEM measurements confirmed that ZnO nanoparticles were nearly hexagonal in shape and nanoparticles size was found in the range of 16-38 nm having average diameter 27nm (Figure 3).
 |
Figure 3: TEM image of ZnO nanoparticles |
In FT-IR spectrum, a broad and overlapped peak is present between 3000-3550 cm-1 which is centered at 3394 cm-1 corresponding to O-H stretching of hydroxyl group (intermolecular hydrogen bonded) along with other groups having exchangeable protons (amine, amide, carboxylic acid groups) are predictable. The absorptions at 1787.61 cm−1 and 1763.21 cm−1 were expected for C=O stretching vibrations, peak at 1593cm-1indicates N-H bending of amine, absorption peak at 1384 cm-1 is related with O-H bending (alcohol, phenol and carboxylic acid groups),absorption at 1069 cm-1 is due to C-N stretching (amine), absorptions at 902, 886 cm-1 and 835 cm-1 are related with – C=C- bending, absorption at 541cm-1 is due to Zn-O bending (Figure 4).
 |
Figure 4: FTIR spectrum of ZnO nanoparticles |
From obtained results, C. acuminatus may be considered as a remarkable supply for the future nano-medicine and this property is supposed to be due to their active bio components that played an important role in the development of ZnO nanoparticleswhich is further supported by the fact that the plant extracts are very useful agents in the synthesis of metallic nanoparticles 24. Based on the capability of plants to reduce metal ions and the participation of energetic bioreductants and stabilizers, the synthesis of ZnO nanoparticlescould be expected. Various studies suggested that ZnO nanoparticles reveal a characteristic broad absorption peak in the 330–460 nm range 25, peaking at 352 nm confirms the fabrication of ZnO nanoparticles using active bio components in the leaf extract. The existence of leaf extract bio-components such as flavonoids, and other polyphenols that remained around zinc ions even after repeated washing with water and ethanol, were indicated by different peaks present in theFTIR spectrum. These hydroxyl group bearing bio compounds assisted in the reduction of zinc ions to ZnO nanoparticles and could be accounted for their interaction with the zinc surface; forming a coating around the nanoparticles and preventing their further agglomeration 26.The absorption in the UV region and its band gap implies its utility in the medical field due to catalytic action against microbial systems 27. XRD diffraction pattern of the nanopowder revealed the formation of hexagonal phase ZnO nanostructures. Hexagonal structure of the synthesized ZnO nanoparticles was confirmed by the TEM images.
Antimicrobial Activity of Znonps
Metal based nanoparticles shows potential anti-microbial activity in comparison to their bulky forms, and it may be due to large surface area of nanoparticles 28. Suresh D, et al., 2015 developed multifunctional ZnO nanoparticles using Cassia fistula extract and confirmed their potential antibacterial behavior 29. Bala N, et al., 2015 in another study reported the ZnO nanoparticles through Hibiscus subdariffa extract and this study showed prominent antibacterial effects of synthesized nanoparticles 30.Furthermore, in last few years, various researchers have confirmed boosted antimicrobial behavior of plant extract mediated synthesized metallic nanoparticles. In this research work, antibacterial and antifungal activities of C. acuminatusleaf extract mediated synthesized ZnO nanoparticles were investigated by using standard methods with some modifications.Antibacterial activity of ZnO sample was examined against B. subtilis, S. aureus and P. aeruginosa strains (Table 1). The nano-agents displayed beneficiary diameter of zone of inhibition against B. subtilisand S. aureus at 35mm and 32mm, respectively and their MIC and MLC (Table 2) values further confirmed the biocidal effects on selected microbial pathogens.
Table 1: Antibacterial activity of ZnONps in terms of zone of inhibition (mm)
|
Sample |
Diameter of zone of inhibition (mm) |
||
|
P. aeruginosa |
S. aureus |
B. subtilis |
|
|
ZnONps (100μl) |
15 |
32 |
35 |
|
Erythromycin |
45 |
35 |
38 |
Table 2: Antibacterial activity of ZnONps in terms of MIC and MLC values (μL)
|
P. aeruginosa |
S. aureus |
B. subtilis |
|||
|
MIC |
MLC |
MIC |
MLC |
MIC |
MLC |
|
5 |
10 |
22 |
28 |
5 |
10 |
*MIC- Minimum inhibitory concentration and
MLC- Minimum lethal concentration
Jamdagni P, et al. (2018) in his study reported that using flower extract it is possible to develop active antifungal ZnOnano agents (Jamdagni 2018). In our case, antifungal activity of ZnO nanoparticles has been tested against C. albicansand S. cerevisiae and their diameter of zone of inhibition (mm) were sensibly measured at 10mm and 15mm, respectively (Table 3). Furthermore, the MIC and MLC values (Table 4) of nanosamples showed their effectiveness against the selected fungal strains.
Table 3: Antifungal activity of ZnONps in terms of zone of inhibition (mm)
|
Sample |
Diameter of zone of inhibition (mm) |
|
|
C. albicans |
S. cerevisiae |
|
|
ZnONps (100μl) |
10 |
15 |
|
Fluconazole |
28 |
27 |
Table 4: Antifungal activity of ZnONps in terms of MIC and MLC ((µL)
|
C. albicans |
S. cerevisiae |
||
|
MIC |
MLC |
MIC |
MLC |
|
03 |
07 |
06 |
10 |
Thus from this section of antibacterial and antifungal activities, it’s not surprising if we assume that nanoparticles with such antibacterial and antifungal potential against the selected strains may result benefits for the clinicians or experimenters on further investigating their action against other drug resistant pathogenic strains. This study may also motivate them for their large scale production.
Conclusion
The above research work provides a sustainable pathway for the fabrication of ZnO nanoparticles using aqueous leaf extract of C. acuminatus. This sustainable pathway helps in the useless destruction of the biodiversity as it demands a very small amount of the plant material and other supporting materials. The leaf extract of the C. acuminatusproved to be very efficient for the fabrication and stability of the zinc ions. The characterization part confirmed that the fabricated materials are in the nanometer range and encapsulated by bio components present in the leaf extract. Apart from this, evaluation of antimicrobial activity against selected microbes showed very good results. Such antimicrobial potential in synthesized ZnO nanoparticles might be due to pooled effect of biocidalproperty of zinc ions and C. acuminatus leaf metabolites. Therefore, antimicrobial results of synthesized ZnO nanoparticles seem motivating for the creation of other metal based nanoparticles through C. acuminatus extract for potential applications in various fields of science and technology.
Acknowledgement
The authors acknowledged University Grant Commission for the financial assistance through the Junior Research Fellowship and NCFT, New Delhi for performing antimicrobial tests.
Conflict of Interest
Contributing authors have no conflict of interest regarding the publication of this research work.
References
- Geonmonond, R. S.; Silva A. G. D.; Camargo, P. H. An. Acad. Bras. Cienc.2018, 90, 719–744.
CrossRef - Sobha, K.; Surendranath, K.; Meena, V.; Jwala, T. K.; Swetha, N.; Latha, K. S. M.Biotechnol. Mol. Biol. Rev.2010, 5(1), 1–12.
- Shah, R. K.; Boruah, F.; Parween, N. Int. J. Curr. Microbiol. Appl. Sci.2015, 4(8), 444-450.
- Poovizhi J.; Krishnaveni B. Int. J. Pharm. Sci. Drug. Res.2015, 7(5), 25-431.
- Shekhawat, M. S.; Ravindran, C. P.; Manokari M. Trop. Plant Res.2014, 1(2) 48-59.
- Fatimah, I.; Pradita, R. Y.; Nurfalinda, A. Procedia Eng.2016, 148, 43-48.
CrossRef - Manokari, M.; Ravindran, C. P.; Shekhawat M. S. WSN2016, 30, 117-128.
- Yuvakumar, R.; Suresh, J.; Hong, S. I. Adv. Mat. Res.2014, 952, 137-140.
CrossRef - Jamdagni, P.; Khatri, P.; Rana, J. S. J. King Saud Univ. Sci. 2018, 30(2), 168-175.
CrossRef - Sharma, D.; Kanchi, S.; Bisetty, K. Arab. J. Chem.2019, 12(8), 3576-3600.
CrossRef - Sangeetha, G.; Rajeshwaria, S.; Venckatesh, R. Mater. Res. Bullet.2011; 46, 2560–2566.
CrossRef - Sawai, J. J. Microbiol. Methods2003, 54, 177–182.
CrossRef - Ahmed, S.; Annu,;Chaudhry, S. A.; Ikram, S. J. Photochem. Photobiol. B2017, 166, 272–284.
CrossRef - Yamamoto, O. Int. J. Inorg. Mater2001, 3, 643–646.
- Hameed, A. S. H.; Karthikeyan, C.; Ahamed, A. P.; Thajuddin, N.; Alharbi, N. S.; Alharbi, S. A.; Ravi, G. Sci. Rep.2016, 6(1), 1-11.
CrossRef - Smijs, T. G.; Pavel, S. Nanotechnol. Sci. Appl.2011, 4, 95-112.
CrossRef - Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N. H. M.; Ann, L. C.; Bakhori, S. K. M.; Mohamad, D. Nanomicro. Lett2015, 7(3), 219-242.
CrossRef - Gaur, R. D. Flora of the District Garhwal, North West Himalaya. Transmedia1999.
- Kumar, G.; Badoni, P. P.; Singh, M. Int. J. Adv. Res. Sci. Eng. Technol.2018, 7, 135-139.
- Sati, S. C.; Sati, M.; Sharma, A.; Joshi, M. Int. J. Pharm.Pharm. Sci.2010, 2, 58-60.
- Perez, C.; Anesini, C. J. Ethnopharmacol.1994, 44, 41-46.
CrossRef - Usman H.; Abdulrahman, F. I.; Ladan, A. H. Res. J. Biol. Sci.2007, 2(3), 244-247.
CrossRef - Vollekova, A.; Kostalova, D.; Sochorova, R. Microbiology,2001, 46(2), 107-111.
CrossRef - Makarov, V. V.; Love, A. J.; Sinitsyna, O. V.; Makarova, S. S.; Yaminsky, I. V.; Taliansky, M. E.; Kalinina, N. O. ActaNaturae2014, 6(1), 35-44.
CrossRef - Dobrucka, R.; Dlugaszewska, J.; Kaczmarek, M. Biomed. Microdevices2018, 20(1), 5–18.
CrossRef - Datta, A.; Patra, C.; Bharadwaj, H.; Kaur, S.; Dimri, N.; Khajuria, R.J. Biotechnol. Biomater2017, 7(3), 271–276.
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.; Wang, X.; Liao, Y.P.; Wang, M.ACS Nano2012, 6(5), 4349–4368.
CrossRef - MubarakAli, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Colloids Surfaces B2011, 85, 360–365.
CrossRef - Suresh, D.; Nethravathi, P. C.; Rajanaika, H.; Nagabhushana, H.; Sharma, S. C. Mater SciSemicond Process2015, 31, 446-454.
CrossRef - Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P.RSC Adv.2015, 5, 4993-5003.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









