Synthesis of Carbon Quantum Dot from Water Hyacinth Stalk by Radiation Processing
Kanokorn Wechakorn1, Panida Sangangam1, Nattamon Puengposop1, Pattra Lertsarawut2 and Tanagorn Kwamman2*
1Department of Chemistry, Faculty of Science and Technology, Rajamangala University of Technology Thanyaburi, Pathum Thani 12110, Thailand
2Research and Development Division, Thailand Institute of Nuclear Technology (Public Organization), Nakorn Nayok 26120, Thailand
Corresponding Author E-mail: tanagorn@tint.or.th
DOI : http://dx.doi.org/10.13005/ojc/360514
Article Received on : 10-09-2020
Article Accepted on : 11-10-2020
Article Published : 19 Oct 2020
Water hyacinth is a severe issue, resulting in river blocking in high flood risk areas like Pathum Thani province, Thailand. To reduce the amount of invasive water hyacinths, their stalks were used as carbon precursors for carbon dots (CDs) by one-pot gamma irradiation, which is a promising method for high-quality and large-scale production. Furthermore, this method was compared with the conventional hydrothermal method. This finding proved that the optical properties of as-prepared CDs from both methods were no significant differences. The CD solution had a pale yellow color and exhibited tuneable fluorescence emission. They are strongly absorbed in the UV region of 250-300 nm. An effect of ethanol pretreatment was also studied. It was found that the ethanol pretreatment has no substantial effect on the photophysical and chemical properties of as-prepared CDs, whereas it was crucially affected by the pH stability of CDs. The maximum fluorescence emission of CDs with (ECD-G) and without (CD-G) ethanol pretreatment were 443 nm (~2.5% of quantum yields) and 440 nm (~2.0% of quantum yields) with the excitation at 360 nm, respectively. The carboxyl groups were the primary functional group of CD-G and ECD-G, confirmed by the FTIR spectroscopy.
KEYWORDS:Fluorescent Materials; Quantum Dots; Radiation Processing; Water Hyacinth
Download this article as:| Copy the following to cite this article: Wechakorn K, Sangangam P, Puengposop N, Lertsarawut P, Kwamman T. Synthesis of Carbon Quantum Dot from Water Hyacinth Stalk by Radiation Processing. Orient J Chem 2020;36(5). |
| Copy the following to cite this URL: Wechakorn K, Sangangam P, Puengposop N, Lertsarawut P, Kwamman T. Synthesis of Carbon Quantum Dot from Water Hyacinth Stalk by Radiation Processing. Orient J Chem 2020;36(5).Available from: https://bit.ly/3o7YyM6 |
Introduction
Water hyacinth (Eichornia crassipes) is classified as abundant aquatic wastes leading to oxygen depletion and water flow reduction. Thick mats of water hyacinth seriously hindered the water flow; thus, its mechanical harvesting is required to control the amount of water hyacinth. Nonetheless, water hyacinth contains rich natural carbon sources: cellulose (~30%), hemicellulose (~29%), and lignin (19%) as well as protein, up to 22%.1 Considering using water hyacinth for carbon dots synthesis could be an alternative solution. Carbon dots (CDs) are the family of carbon-based fluorescence nanoparticles. The extensive attention of CDs has grown remarkably since the first was discovered in 2004.2-4 Their unique properties: nontoxicity, high quantum yields (QY) with a tunable band-gap, photo-stability, low cost, biocompatibility, and chemical inertness, have drawn the attention to several applications such as sensors,5-11 bio-imaging12-13 and light emitting diode.14-16 Photophysical properties of CDs are district characteristics originating from the combination of poly-aromatic hydrocarbon scaffolds and surface states.17-18 Carbon nanodots based sensors are growing interesting because the fluorescence intensity and the emission shift are crucially sensitive to the surface environment. Surface functionalization is essential to control the photophysical properties for specific analytes.6,19-20 For example, hydrogen sulfide (H2S) can be promptly detected by green emission of the CDs via a reduction of azide moiety to amine groups.21 Luminescence quenching mechanisms of CDs are remarkable useful for sensor applications.22-23 Luminescence of electron-rich amine group on CDs is entirely off after the absorption of nitrogen dioxide gas (NO2).18
Several abundant carbon biomasses have been reported as carbon precursors for CD synthesis, such as natural biomasses included rice husk,24 sugarcane bagasse,25 wood charcoal,26 and coffee ground.27 However, cellulose and lignin are water-insoluble due to the rigid natural structures. The combination of strong acid and high temperature is required for breaking bundles of lignocellulose to the small polysaccharide.28 According to previous studies, lignocellulose biomass was successfully used for CD fabrication by pyrolysis29 and carbonization.30 Recently, a few developments of CDs from water hyacinth were synthesized as the fluorescent sensors. In 2019, Prathumsuwan
et al., reported the CD based fluorescent sensor for borax detection from water hyacinth leave via acid-treated pyrolysis.31 Deka et al., also synthesized the CDs from water hyacinth by carbonization with phosphoric acid pretreatment.8 Nevertheless, the drawbacks of conventional methods are the toxic chemical usages and multistep synthesis. Therefore, green synthesis or non-chemical treatment of water hyacinth is incredibly beneficial and challenging. Radiation processing is straightforward, cost-effective, and environmentally friendly. Water radiolysis creates reactive radicals (e-, HO•, H•, H2, and H2O2), causing degradation, polymerization,
or/and crosslinking during irradiation, depending on the energy and the dose of the ionizing radiation.32-34
In this study, the one-pot gamma irradiation synthesis was used to fabricate the carbon dots from water hyacinth collected from Pathum Thani province. No toxic chemical and high temperature are used in the fabrication process. The chemical, physical and optical properties of as-prepared CDs were investigated compared with the CDs synthesized by the hydrothermal method. An effect of ethanol pretreatment was also investigated.
Materials and Methods
Materials and Instrumentations
Water hyacinth stalks were collected from a pond in Rajamangala University of Technology Thanyaburi, Pathum Thani. All chemical reagents were used as an analytical grade without further purification. Ethanol was purchased from RCI-Lab Scan. Quinine hemisulfate was acquired from Sigma-Aldrich. The 0.2-micron filter was purchased from Chromplus.
A dialysis bag (1,000 Da) was purchased from CelluSep H1, Membrane filtration productions, Inc.
UV-Vis absorption spectra were performed on a Perkin Elmer Lambda 35 UV/Visible spectrometer. Fluorescence emission spectra were carried out on Hitachi F-4600 fluorescence spectrophotometer. Fourier-transformed infrared spectroscopy (FTIR) spectra were measured on Thermoscientific Nicolet is5/iD7 ATR in the range of 4,000-500 cm-1.
Synthesis of CDs
The water hyacinth stems were washed with water and dried under sunlight for 72 hours followed by drying in the oven at 80oC for 24 hours. They were ground by electric motor and mortar to provide a green powder (Virgin powder). The chlorophyll effect was investigated by the removal of chlorophyll from the pretreatment of stalk powders by soaking in ethanol for 72 hours and dried in the oven at 80oC for 24 hours. After that, the 50 mg of virgin powders and pre-treated powders were mixed with deionized water (25 mL) in the vial and irradiated by gamma rays (Co-60 source) at the dosage of 80 kGy (30, 366 Ci) at the ambient condition, named the sample as CD-G and ECD-G, respectively. The as-received yellow solution was filtered by the Buchner funnel and 0.2-micron membrane filter, followed by dialyzed in deionized water by a dialysis bag (1,000 MWCO) for 48 hours. After that, the resulting solution was centrifuged at 10,000 rpm for 30 min to separate the agglomerated particles. Finally, CDs as a yellow solid was freeze-dried for further analysis.
Furthermore, the comparison between the novel one-pot gamma irradiation and the conventional hydrothermal method were studied. The 20 mg of virgin powders and pre-treated powders were mixed with deionized water (15 mL), followed by heating at 180oC for 3 hours. The samples are named as CD-H and ECD-H, respectively.
Quantum Yield Measurement
For the standard reference, the absorbance of quinine hemisulfate in 0.1 M H2SO4 was performed less than 0.1 to prevent the self-quenching effect. The following equation determined the quantum yields of the CDs:
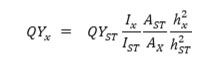
Where “QY” was the fluorescence quantum yield, “A” was the absorbance, “I” was the integrated fluorescence emission intensity, and “h” was the refractive index. The subscript “x” refers to the sample, and “ST” refers to the standard.
Results and Discussion
Optical Properties
The UV-Vis absorption and fluorescence spectra of the CDs solution synthesized by hydrothermal and gamma irradiation were elucidated in deionized water. All sample solution appeared a pale-yellow. Fig. 1 represents the UV-Vis absorption spectra of as-prepared CDs, that strongly absorbed in the UV region. For the gamma irradiation method, the intense absorption peaks showed in the range of 250-400 nm, corresponding to p→p* transition of sp2 carbon and n→p* transition of C=O existed. On the other hand, the hydrothermal synthesis (CD-H and ECD-H), the absorption peak shifted to lower wavelengths. Notably, an effect of chlorophyll can be seen in that higher UV adsorption intensities were observed in the CDs prepared from the powders with EtOH pretreatment rather than the virgin powders. The strong absorption in the UV region indicates the characteristic properties of carbon dots.35-36
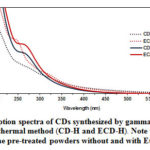 |
Figure 1: UV-vis absorption spectra of CDs synthesized by gamma irradiation (CD–G and ECD–G) and by hydrothermal method (CD–H and ECD–H). Note that CD and ECD were prepared from the pre–treated powders without and with EtOH, respectively. Click here to View figure |
UV-Vis and fluorescence spectra are generally employed to prove the optical properties of prepared-carbon dots.2,4,18,35,37 As shown in Fig. 2, the CDs solution was excited with the increasing wavelength from 300 to 420 nm, the emission intensity exhibited red shifts with decreasing intensity, suggesting that the carbon dots had tunable fluorescence emission properties. Therefore, it could be confirmed that ECD-G, ECD-H, CD-G, and CD-H are carbon dots based on their unique optical properties, corresponding to the previous literature.2 In this work, the emission of as-prepared CDs strongly depends on the excitation wavelengths. For the gamma irradiation, no significant differences in emission wavelength regarding the same excitation were observed in ECD-G (Fig. 2a) and CD-G (Fig. 2b). With EtOH pretreatment (ECD-G), the maximal emission displayed at 443 nm, while without pretreatment (CD-G), the maximum wavelength displayed at 440 nm at an excitation wavelength of 360 nm. However, their fluorescence intensities were slightly different, which would be an effect of Chlorophyll in CD-G. It is seen that both ECD-G and CD-G exhibited red shift as excited from 320 nm to 500 nm.
For the hydrothermal method, the maximal emission intensities of ECD-H (Fig. 2c) and CD-H (Fig. 2d) showed at 403 nm and 398 nm (λex = 360), respectively. Similar to gamma irradiation, the pretreatment with EtOH enhanced the maximum fluorescence intensity compared with the CDs synthesized from the virgin powder. The fluorescence emission wavelength was also dependent on the excitation wavelength, corresponding to the CDs prepared by the gamma irradiation. These results indicated that the fluorescence behavior of carbon dots synthesized by gamma irradiation had similar properties as the hydrothermal method. According to the optical properties, since the gamma irradiation is a promising method for the synthesis of CDs with several advantages; the reaction occurs without catalysts, organic solvents, and toxic chemicals at ambient temperature, only the CDs prepared by the gamma irradiation was further studied. However, complex radiation chemistry involved in CD synthesis would be suggested to study further to get the optimal irradiation condition.
Moreover, the quantum yield of CDs based on gamma radiation was determined in deionized water using quinine hemisulfate salt monohydrate as a standard reference (F = 0.54). The quantum yields of CD-G and ECD-G were 2.5 ± 0.2% and 2.0 ± 0.1%, respectively.
Chemical Properties of CDs
The functional groups on the surface of CDs synthesized by gamma irradiation were determined by FTIR. As shown in Fig. 3, the IR peaks appeared at 3292 cm-1, 1405 cm-1, and 1573 cm-1 are assigned to O-H stretching, O-H bending and C=O stretching vibration, respectively. CD-G and ECD-G exhibited an intense peak at 1579 cm-1, corresponding to C=O stretching vibration conjugated with the C=C bond. The C-H stretching vibration of ECD-H also appeared at 2924 cm-1. These results indicated that the carboxylic acid conjugated with sp2 hybridization of the C=C bond was the functional group on their surfaces. Notably, CDs’ functional group is different from the lignocellulose, suggesting that the core structures and chemical properties change during the gamma irradiation.38
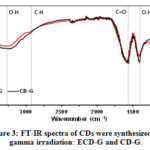 |
Figure 3: FT–IR spectra of CDs were synthesized by gamma irradiation: ECD–G and CD–G. Click here to View figure |
PH Stability of CDs
The pH stability test of CDs synthesized by gamma irradiation was studied in the pH range of 1-13. Their emission intensities changed according to pH, as shown in Fig. 4. The fluorescence intensities of ECD-G gradually dropped regarding an increase in pH. In contrast, for CD-G, over the pH range of 1-3, the fluorescence intensities reached maximal and dramatically dropped over the pH range of 4-13. It can be explained from the fact that the aggregation of CDs without EtOH pretreatment, as mention in the particle size analysis was higher than of with EtOH pretreatment. It had been reported that the fluorescence of carbon dots strongly depends on their size.18 It could be the reason that ECD-G and CD-G responded to pH differently. This pH stability also differs from the previous report of CDs derived from water hyacinth by pyrolysis at 250oC in that the fluorescence intensity increase as a function of pH.31 It would suggest that the preparation method also affects the CDs’ optical properties despite using the same carbon precursor. In addition, chlorophyll is confirmed that plays a crucial role in photoluminescence quenching mechanisms of QDs. Therefore, to get high-quality CDs from water hyacinth stalks, chlorophyll is needed to be extracted as much as possible.
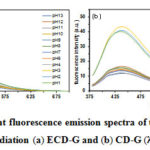 |
Figure 4: The pH–dependent fluorescence emission spectra of the CD solution (0.1 mg/ml) synthesized by gamma irradiation (a) ECD–G and (b) CD–G (λex = 360 nm) |
From this finding, one-pot gamma irradiation is a comparable method with the hydrothermal method. This method is considered as a high potential to use in the CD large-scale production. However, suitable irradiation conditions are needed to be optimized along with the radiation chemistry of CDs formation for future works. Interestingly, surface passivation and modification of CDs synthesized from gamma irradiation would be easy due to their carboxyl group on their surface. Therefore, they can potentially use in bio- and chemical- sensor applications by surface modification.
Conclusions
We successfully synthesized carbon dots from water hyacinth stems by gamma irradiation. The carbon dot had the carboxylic acid as the functional group on the surface, confirmed by FTIR. The optical properties of the carbon dot were studied by using UV-Vis absorption and fluorescence spectroscopic properties. The carbon dot maximum absorbed in the UV region and emitted in the visible region. The tunable fluorescence emission of carbon dot exhibited by excitation at a different wavelength. The quantum yield of carbon dot was found to be 2.5 ± 0.2% compared to that of with pretreatment of ethanol at 2.0 ± 0.1%, suggesting that chlorophyll may affect the fluorescence emission. In addition, pH conditions influenced to the fluorescence intensity of carbon dot. The carbon dot synthesized from gamma radiation has similar optical properties as the hydrothermal method based on the spectroscopic results.
Acknowledgments
This work was supported by RMUTT research foundation scholarship. We wish to thank the Department of Chemistry, Faculty of Science and Technology, Rajamamgala University of Technology Thanyaburi and Thailand Institute of Nuclear Technology (Public Organization) for kindly providing research facilities. We would like to thank the central instrumentation laboratory of Rajamangala University of Technology Thanyaburi for FTIR analysis and fluorescence spectrophotometer. In addition, we would like to thank Nuatawan Thamrongsiripak for technical supports.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Carlini, M.; Castellucci, S.; Mennuni, A., Energy Procedia., 2018, 148, 431-438.
CrossRef - Ðordevic, L.; Arcudi, F.; Prato, M., Nature Protocols., 2019, 14, 2931–2953.
CrossRef - Huang, C.-C.; Hung, Y.-S.; Weng, Y.-M.; Chen, W.; Lai, Y.-S., Trends in Food Science & Technology., 2019, 86, 144-152.
CrossRef - Lee, S., Journal of Industrial and Engineering Chemistry., 2019, 77, 365-370.
CrossRef - Liang, X.-C.; Chen, S.; Gao, J.; Zhang, H.; Wang, Y.; Wang, J.-H.; Feng, L., Sensors and Actuators B: Chemical., 2018, 265, 293-301.
CrossRef - D, P.; Saini, S.; Thakur, A.; Kumar, B.; Tyagi, S.; Nayak, M. K., Journal of Hazardous Materials., 2017, 328, 117-126.
CrossRef - Ngoc Anh, N. T.; Chang, P.-Y.; Doong, R.-A., RSC Advances., 2019, 9(46), 26588-26597.
CrossRef - Deka, M. J.; Dutta, P.; Sarma, S.; Medhi, O. K.; Talukdar, N. C.; Chowdhury, D., Heliyon., 2019, 5(6), e01985.
CrossRef - Shehab, M.; Ebrahim, S.; Soliman, M., Journal of Luminescence., 2017, 184, 110-116.
CrossRef - Ansi, V. A.; Renuka, N. K., Sensors and Actuators B: Chemical., 2018, 264, 67-75.
CrossRef - Qu, F.; Guo, X.; Liu, D.; Chen, G.; You, J., Sensors and Actuators B: Chemical., 2016, 233, 320-327.
CrossRef - Jiang, K.; Sun, S.; Zhang, L.; Wang, Y.; Cai, C.; Lin, H., ACS Applied Materials & Interfaces., 2015, 7(41), 23231-23238.
CrossRef - Wang, L.; Li, W.; Wu, B.; Li, Z.; Pan, D.; Wu, M., Chemical Engineering Journal., 2017, 309, 374-380.
CrossRef - Xiao, L.; Sun, H., Nanoscale Horizons., 2018, 3(6), 565-597.
CrossRef - Li, Y.; Wang, C.; Huang, L.; Zhang, F.; Wang, H.; Wang, Q.; Yang, L.; Xie, M.; Gan, Y.; Li, H., Journal of Alloys and Compounds., 2020, 836, 155410.
CrossRef - Qiao, G.; Chen, G.; Wen, Q.; Liu, W.; Gao, J.; Yu, Z.; Wang, Q., Journal of Colloid and Interface Science., 2020, 580, 88-98.
CrossRef - Jiang, X.; Kang, Z.; Guo, X.; Zhuang, H., Wiley., 2019.
- Jelinek, R., Springer International Publishing., 2016.
- Blanco, E., Blanco, G., Gonzalez-Leal, J.M. J Nanopart Res., 2015, 17, 214.
CrossRef - Hou, Y.; Lu, Q.; Deng, J.; Li, H.; Zhang, Y., Analytica Chimica Acta., 2015, 866, 69-74.
CrossRef - Yu, C.; Li, X.; Zeng, F.; Zheng, F.; Wu, S., Chemical Communications., 2013, 49(4), 403-405
CrossRef - Tejwan, N.; Saha, S. K.; Das, J., Advances in Colloid and Interface Science., 2020, 275, 102046.
CrossRef - Qu, J.-H.; Wei, Q.; Sun, D.-W., Critical Reviews in Food Science and Nutrition., 2018, 58(14), 2466-2475.
CrossRef - Wang, Z.; Yu, J.; Zhang, X.; Li, N.; Liu, B.; Li, Y.; Wang, Y.; Wang, W.; Li, Y.; Zhang, L.; Dissanayake, S.; Suib, S. L.; Sun, L., ACS Applied Materials & Interfaces., 2016, 8(2), 1434-1439.
CrossRef - Sangjan, A.; Ngamsiri, P.; Klomkliang, N.; Wu, K. C. W.; Matsagar, B. M.; Ratchahat, S.; Liu, C.-G.; Laosiripojana, N.; Sakdaronnarong, C., Renewable Energy., 2020, 154, 1204-1217.
CrossRef - Nirala, N. R.; Khandelwal, G.; Kumar, B.; Vinita; Prakash, R.; Kumar, V., Talanta., 2017, 173, 36-43.
CrossRef - Wang, L.; Li, W.; Wu, B.; Li, Z.; Wang, S.; Liu, Y.; Pan, D.; Wu, M., Chemical Engineering Journal., 2016, 300, 75-82.
CrossRef - Den, W.; Sharma, V. K.; Lee, M.; Nadadur, G.; Varma, R. S., Frontiers in Chemistry., 2018, 6, 141.
CrossRef - Ye, Q.; Yan, F.; Luo, Y.; Wang, Y.; Zhou, X.; Chen, L., Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy., 2017, 173, 854-862.
CrossRef - Wu, Q.; Li, W.; Tan, J.; Wu, Y.; Liu, S., Chemical Engineering Journal., 2015, 266, 112-120.
CrossRef - Prathumsuwan, T.; Jaiyong, P.; In, I.; Paoprasert, P., Sensors and Actuators B: Chemical., 2019, 299, 126936.
CrossRef - Choppin, G.; Rydberg, J.; Liljenzin, J. O., Elsevier Science., 2001.
- M mozumder, A., Elsevier Science., 1999.
- Saphwan Al-Assaf, X. C., Khairul Zaman Haji Mohd Dahlan, Murat Sen, Piotr Ulanski, international atomic energy agency: Vienna., 2016.
- Liu, M. L.; Chen, B. B.; Li, C. M.; Huang, C. Z., Green Chemistry., 2019, 21(3), 449-471.
CrossRef - Liu, W.; Li, C.; Ren, Y.; Sun, X.; Pan, W.; Li, Y.; Wang, J.; Wang, W., Journal of Materials Chemistry B., 2016, 4(35), 5772-5788.
CrossRef - Abbas, A.; Mariana, L. T.; Phan, A. N., Carbon., 2018, 140, 77-99.
CrossRef - Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G., Renewable and Sustainable Energy Reviews., 2018, 90, 223-247.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










