Phytochemical Study and Antioxidant Activities on Extracts of the Leaves and Roots of Costus Afer Ker Gawl. (Zingiberaceae)
Coulibaly Wacothon Karime1* , Adiko N’dri Marcelline2, Benie Anoubilé3, Konan Yao Mokey Jean-Baptiste3, Bea Gouanda Thibaut3,4, James T. Titah5, Kabran Aka Faustin4, Ballo Daouda4 and Kablan Ahmont Landry Claude1
, Adiko N’dri Marcelline2, Benie Anoubilé3, Konan Yao Mokey Jean-Baptiste3, Bea Gouanda Thibaut3,4, James T. Titah5, Kabran Aka Faustin4, Ballo Daouda4 and Kablan Ahmont Landry Claude1
1UFR des Sciences Biologiques, Université Peleforo Gon Coulibaly, Côte d’Ivoire.
2Laboratoire de Pharmacognosie, Botanique, Biologie végétale et Cryptogamie, UFR des Sciences Pharmaceutiques et Biologiques, Université Félix Houphouët-Boigny, Côte d’Ivoire.
3Laboratoire de Chimie Bio-Organique et de Substances Naturelles, Université Nangui Abrogoua, Côte d’Ivoire.
4Laboratoire de Chimie Organique et de Substances Naturelles, UFR Sciences des Structures de la Matière et Technologie, Université Félix Houphouët-Boigny, Côte d’Ivoire.
5Faculty of Science, Department of Science and Mathematics, Tabor College, KS, USA.
Corresponding Author E-mail: wacothon@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360502
Article Received on : 18-06-2020
Article Accepted on : 01-10-2020
The extracts of leaves and roots of Costus afer plant have been fully investigated in an attempt to determine their phytochemical constituents and antioxidant activities. Phytochemical screening carried out using thin layer chromatography (TLC) revealed the presence of several secondary metabolites in all the selected extracts of the plant. In addition, our results showed that the leaves of C. afer are the richest in polyphenols with an average value of 3416.25 µg EAG/g MS. The total flavonoid assay revealed a highest content in the leaves (8.02 %). Furthermore, studies of the antioxidant activities using 2,2-diphenyl-1’-picrylhydrazyl(DPPH●) method showed a significant effect compared to the reference vitamin C. Ethyl acetate extract of the leaves show a higher percentage of inhibition (83 %), followed the roots (69 %).
KEYWORDS:Antioxidant; Costus Afer; DPPH; Polyphenols; Zingiberaceae
Download this article as:| Copy the following to cite this article: Karime C. W, Marcelline A. N, Anoubilé B, Baptiste K. Y. M. J, Thibaut B. G, Titah J. T, Faustin K. A, Daouda B, Claude K. A. L. Phytochemical Study and Antioxidant Activities on Extracts of the Leaves and Roots of Costus Afer Ker Gawl. (Zingiberaceae). Orient J Chem 2020;36(5). |
| Copy the following to cite this URL: Karime C. W, Marcelline A. N, Anoubilé B, Baptiste K. Y. M. J, Thibaut B. G, Titah J. T, Faustin K. A, Daouda B, Claude K. A. L. Phytochemical Study and Antioxidant Activities on Extracts of the Leaves and Roots of Costus Afer Ker Gawl. (Zingiberaceae). Orient J Chem 2020;36(5). Available from: https://bit.ly/35qvWVU |
Introduction
The number of people affected by Hemorrhoids is a public health concern and the numbers are expected to increase if nothing is done. Approximately 10 – 25 % of the tested population have this disease. This disease is commonly located around and inside the anus and rectum and can go unnoticed because it is painless. Severe situations of hemorrhoid will cause the dilation of veins around the anus [1]. However, given the increasing number of cases and the complications associated with this disease, it could have long-term socio-economic repercussions on the rural population as current drug treatments remain insufficient. Faced with this health crisis, scientists are continuously searching for ways to mitigate the disease by studying plant extracts. Plant extracts have shown tremendous therapeutic properties because they contain active agents that can act against the disease [2-6]. There is increasing need in the use of plant extracts in the developed world against many diseases. This trend is also observed in many developing countries especially Ivory Coast, where Zingiberaceae plant is currently being used as a traditional medicine against hemorrhoid [7]. In this research, we will present results on the phytochemical studies and antioxidant properties on the leaves and roots extracts of Costus afer. This work is done in an attempt to increase on the use of medicinal plants in Ivory Coast, especially Costus afer with limited literature.
Material and Methods
Materials
Plant material
The leaves and roots extracts of C. afer were collected in July 2017 at Aboisso (South East of Ivory Coast). This was received and identified by herbariums in the Centre National de Floristique at the University of Félix Houphouët Boigny with reference number CNF 17212. The leaves and roots of C.afer plant were thoroughly washed with water, air-dried for one month and three days and pulverized by means of an artisanal mill.
Equipment Used
Technical equipment used in this research are:a precision balance (Denver, S-234 series, Max 230 g), a Buchner, a UV lamp (254 and 366 nm), a spectrophotometer (AL8000Aquatic series), a rotary evaporator of HEIDOLPH1 type and an electric dryer. The deposits were carried out on chromatographic plates (silica gel 60 F254, aluminum, 20 × 20 cm, Merck).
Methods
Extraction of Secondary Metabolites
Hydromethanol Maceration
5.00 g of each powder was macerated in 50.00 mL of methanol (80 %) with constant stirring for 24 hours. The resulting product was obtained through suction filtration and stored between 4 – 5 ºC in a refrigerator. This operation was repeated twice, keeping the same pomace, but with a renewal of the solvent. The crude leaves and root hydromethanic extracts from C. afer were obtained using a rotary evaporator. These extracts were dried in an oven and weighed using a precision balance (Denver, S-234 series, Max 230 g) to determine the yield. These extracts were used to prepare the selective extracts for subsequent assay of polyphenols and flavonoids.
Preparation of Selective Extracts
Extracts from leaves and roots were respectively treated with the following increasing polarity solvents: hexane, chloroform, ethyl acetate and n-butanol 20.00 mL of the crude hydromethanolic extract was successively soaked with 3 x 10 mL of hexane, chloroform, ethyl acetate and n-butanol. The different fractions obtained from these solvents (hexane, chloroform, ethyl acetate and n-hutanol) were concentrated on a rotary evaporator at 50 °C and then stored in a refrigerator (4 – 5 °C) for subsequent use in phytochemical screening and antioxidant activity.
Phytochemical Screening of Selective Extracts
Choice of Phytochemical Screening Method
Phytochemical screening of the selective extracts were carried out using either thin layer chromatography (TLC) or characterization reactions in a liquid medium. In this research, we use the method of chemical screening by TLC according to reports from Békro et al., Mamyrbekova- Bekro et al., and N’gaman et al. [8, 9, 10, 11]. The different developers used for the phytochemical screening by TLC are shown in Table I.
Table 1: Reagents and revealed compounds
|
Reagents
|
Revealed compounds
|
|
Liebermann-Bürchard |
Sterols and terpenes |
|
Godin |
Sterols, terpenes and flavonoids |
|
Test on the lactone ring, KOH |
Coumarins |
|
Shinoda’s test (Cynanidin reduction test) |
Flavonoids |
|
Dragendorff |
Alkaloids |
|
Iron chloride (FeCl3) |
Tannins and polyphenols |
Detection of Sterols and Terpenes
Liebermann-Büchard’s test: In a test tube, an aliquot of the extract was diluted with 1.00 mL of acetic anhydride and 0.50 mL of concentrated H2SO4 added slowly on the walls of the test tube. The appearance of a purple color turning blue to green indicates a positive reaction for the presence of steroids and terpenes.
Detection of Flavonoids, Coumarins, Sterols and Terpenes
Godin test: The sample is prepared by mixing equal volumes of a 1 % (v / v) ethanol – vanillin solution with 3 % (v / v) perchloric acid solution. Chromatoplates were sprayed with this reagent, then with an ethanolic solution of H2SO4 at 10 % (v / v). The resulting mixture was heated to 100 °C until spots of various colors appear. The colours observed were; blue for sterols, triterpenes and coumarins; purple for sterols and polyterpenes; yellow and orangerose for flavonoids and green for triterpenes.
Detection of Flavonoids, Coumarins
KOH test: Prepare methanolic solution of KOH (5 %, m / v) by dissolving 5.00 g of KOH in 100.00 mL of methanol. After spraying the chromatographic plate, coumarins appear in the form of yellow spots at 336 nm in the visible and UV regions.
Detection of Coumarins
Test on the lactone ring: 2.00 mL of aqueous extract were added into two separate test tubes. In one of the test tubes, was added 0.50 mL of 10 % NaOH (w / v), then the test-tubes were heated in a water bath until boiling. After cooling, 4.00 mL of water were added to each test tube. If the liquid is transparent or transparently yellowish (yellow) relative to the liquid from the test tube containing no alkali, the reaction is positive. Acidifying the transparent solution with a few drops of concentrated HCl, lead to the lost of yellow color to form a cloudy suspension or a precipitate.
Detection of Flavonoids
Shinoda’s test (Cynanidin reduction test): 5 to 7 drops of concentrated HCl and 10 to 15 mg of Zn or Mg shavings were added to 2 mL of the aqueous extract. After 3 to 5 minutes, a red-orange colour was observed indicating the presence of flavonoids; coloration characterizes the flavonoids. Heating the mixture in a hot water bath for 2 to 3 minutes can increase the rate of the reaction.
Detection of Alkaloids
Dragendorff’s test: The extract was dissolved in 6 mL of ethanol and treated with 2 drops of Dragendorff’s reagent. A precipitate or orange color indicated the presence of alkaloids.
Detection of Polyphenols
FeCl3 test: To 2 mL of the aqueous extract, we added a few drops of an aqueous solution of FeCl3 2 % (w / v). A dark blue and dark green color indicate the presence of polyphenols.
Determination of Total Polyphenols
Total polyphenol content was determined using the colorimetric method proposed by Folin-Ciocalteu and used by Kabran, Kadja, N’guessan and N’Gaman [11, 12, 13, 14]. To a 1 mL solution of each extract diluted to 1 / 20 with distilled water, we added 1.5 mL of Na2CO3 (17 %, w / v) and 0.5 mL of Folin-Ciocalteu reagent (0.5 N). The whole mixture was incubated at 37 ° C for 30 minutes; the absorbance was 720 nm against a blank without extract taken as a reference.
In addition, the determination of total polyphenols was done using the linear calibration line (y = ax + b) carried out by a standard extract of gallic acid at different concentrations (0 μg / mL to 1000 μg / mL) under the same conditions as the sample. The results are expressed in microgram gallic acid equivalent per gram of the dry matter (μg EAG / g DM) of the powdered plant. The total polyphenol content (Q) was calculated using the formula:
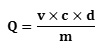
Where; v = final volume of the extract (mL); c = concentration of the extract (μg / mL), d = dilution; m= mass of dry matter of the hydrolyzed plant material (g), Q = (μg EAG / g MS)
Determination of Total Flavonoids
Total flavonoid assay was performed using the method proposed by Hariri et al., [15] and used by Kabran [12]. In this method, 2 mL of each hydro-methanolic extract was diluted to 1/ 20 with distilled water and mixed with 100 μL of Neu reagent. The absorbance was read at 404 nm and the result compared to that of quercetol taken as standard (0.05 mg / mL), diluted under the same conditions and treated with the same amount of reagent. The percentage of total flavonoids was calculated in quercetol equivalent according to the following formula below proposed by [11, 12]:

2, 2-diphényl-1-picrylhydrazyl (DPPH) radical scavenging activity
The spectrophotometric evaluation of the antioxidant activity of the selective extracts were done using the method proposed by Popovici et al., [16] and repeated by N’Gaman [11]. (2,2-diphényl-1-picrylhydrazyl) DPPH• is dissolved in absolute ethanol to obtain a concentration of 0.02 mg / mL. Similarly, solutions of the extract were prepared in absolute ethanol with varying concentrations (0.25 mg / mL, 0.5 mg / mL, 1.0 mg / mL, 1.5 mg / mL, 2.0 mg / mL and 3.0 mg / mL). In a dry and sterilized test tube, 0.5 mL of the different extract concentrations were mixed with 1.5 mL of the solution of DPPH at 30 minutes interval. The resulting mixture is thoroughly mixed and placed in the dark for 15 minutes. The absorbance of the mixture was measured using a spectrophotometer to be 517 nm (maximum wavelength of DPPH•). The absorbance was measured at 3 minutes interval for 15 minutes. This process was repeated for the different extract solutions. Ascorbic acid (vitamin C) was used as the reference positive control. Scavenging effect was calculated as:
% I= (A0-A1) × 100/A0;
Where A0 = absorbance of the control; A1 = absorbance of the extract.
A standardized graph of the extract concentration versus the scavenging activity was plotted to extrapolate the concentration efficiency of the plant extract at 50 % reduction of 2,2-diphényl-1-picrylhydrazyl (DPPH) (IC50) [12].
Results and Discussion
Phytochemical Screening
Extracts with Hexane
For the detection of secondary metabolites, we used the solvent system: Hexane / AcFor the detection of secondary metabolites, we used the solvent system: Hexane / AcOEt (8: 2; v / v). Before spraying, we observed three spots for the leaf extract and one spot for the root extract. In the UV region at 254 nm, we observed three spots for the leaf extract and five spots for the root extract. In addition, at 365 nm, we observed ten spots for the leaf extract and four spots for the root extract.OEt (8: 2; v / v). Before spraying, we observed three spots for the leaf extract and one spot for the root extract. In the UV region at 254 nm, we observed three spots for the leaf extract and five spots for the root extract. In addition, at 365 nm, we observed ten spots for the leaf extract and four spots for the root extract.
In this method, family-specific developers were used to determine large families of secondary metabolites.
With the Liberman-Bürchard reagent, we observed nine spots for the leaf extract and eight spots for the root extract. With Godin’s reagent, we identified six spots for the leaf extract and twelve spots for the root extract. The use of sulfuric Vanillin solvent gave eleven spots for the leaf extract and twelve spots for the root extract.
Potassium hydroxide (KOH) specific to coumarins, gave us at 365 nm in the UV region four spots for the leaf extract and three spots for the root extract.
In short, we would note that the plant organs would contain sterols, flavonoids and coumarins (blue or light blue, purple or Brown, purple or light purple, yellow, red, orange, gray spots). Thus, by comparing the Rf of the spots observed, the leaf extracts with hexane would contain six spots which have been identified as sterols, two spots identified as terpenes; four spots identified as coumarins and five spots identified as flavonoids. In addition, with the root extract, we had two spots identified as sterols; four spots identified as terpenes; three spots identified as coumarins and seven spots identified as flavonoids. Previous reports isolated these metabolites from the leaf extract with hexane in C. afer. [17].
Table 2: Screening of the leaf and root extracts of C. aferwith hexane
| Secondary metabolites |
Leaves |
Roots |
|
Sterols and terpenes |
++ |
++ |
|
Coumarins |
++ |
++ |
|
Flavonoids |
++ |
++ |
(++) = abundance
Extracts with Chloroform
The solvent system: CHCl3 / AcOEt / Hexane (5: 6 : 2.5 ; v / v / v) was used to determine the secondary metabolites.
Before spraying with the solvent system, no spot were visible.
In the UV region at 254 nm, the chromatograms presented four gray spots for the leaf and then three gray spots for the root extracts. On the other hand, in the UV region at 365 nm, we observed four spots for the leaf and two spots for the root extracts.
After treatment with Neu’s reagent, we observed six spots for the leaves and four spots for the roots. Further treatment of the extracts with AlCl3 gave six spots for the leaves and six spots for the roots.
These results would show that our compounds are indeed flavonoids (blue or light blue, purple or light purple, yellow, green, orange spots). Thus, by comparing the Rf of the spots, the chloroform extract would contain five spots, which have been identified as flavonoids (three at the level of the leaves “Rf = 0.17; Rf = 0.24; Rf = 0.31″ and two at the level of the roots “Rf = 0.15; Rf = 0.24″).
Table 3: Screening of extracts with chloroformon the leaves and roots of C. afer
| Secondary metabolites |
Leaves |
roots |
|
Flavonoids |
++ |
++ |
(++) = Abundance of compound
Extracts with Ethyl Acetate
In the method of identification of secondary metabolites in ethyl acetate extracts, the solvent system CHCl3 / AcOEt / CH3CHOOH (6.5 : 3 : 0.5 ; v / v / v) has been used.
In the visible we observed two yellow spots for the leaves and three yellow spots for the roots.
In the UV region at 254 nm, we observed five spots for the leaves and twelve spots for the roots for the ethyl acetate extract. On the other hand, in the UV region at 365 nm, we observed four spots for the leaves and seven spots for the roots.
After spraying, the chromatoplates showed various stains with the Neu reagent. We observed seven spots for the leaves and eight spots for the roots. With AlCl3 at 365 nm in the UV region, we observed three spots for the leaves and seven spots for the roots. With FeCl3, five spots were observed for the leaves and six spots for the roots.
These results would reflect the actual presence of flavonoids and tannins (blue or light blue, purple or light purple, yellow, green, black or gray spots). Thus, by comparing the Rf of the spots, the ethyl acetate extract would contain a total of nine spots which have been identified as flavonoids: five in the leaves of Rf = 0.04; Rf = 0.10; Rf = 0.19; Rf = 0.36 and Rf = 0.56 and four in the roots of Rf = 0.15; Rf = 0.36; Rf = 0.51; Rf = 0.77. Then four spots identified as tannins: one in the leaves of Rf = 0.07 and three in the roots of Rf = 0.20; Rf = 0.43 and Rf = 0.58. These metabolites have been isolated from ethyl acetate extracts from the leaves and roots of a variety of C. afer from Cameroon by other authors [18].
Table 4: Screening of extracts with ethyl acetate on the leaves and roots of C. afer
| Secondary metabolites |
Leaves |
roots |
|
Flavonoids |
++ |
++ |
|
Tannins |
+ |
++ |
(+) = presence, (++) = Abundance of compound
Extracts with n-butanol
In the phytochemical screening of the extracts with n-butanolic, we used the AcOEt / MeOH / H2O / CH3CHOOH system (6 : 0.75 : 0.7 : 0.25; v / v / v / v) as eluent.
Before spraying, four spots and one spot on extracts of the leaves and roots respectively.
In the UV region at 254 nm, five spots were seen on extracts of the leaves and seven spots extracts of the roots. In addition, at 365 nm we observed seven spots on the extracts of the leaves and six spots on extracts on the roots.
After spraying with Neu’s reagent, we observed nine spots on extracts of the leaves and ten spots on extracts of the roots. Furthermore, when AlCl3 reagent was used at 365 nm in the UV region, five and seven spots were respectively observed on the extracts of the leaves and roots. Further analysis with FeCl3 reagent gave tow brown spots each on the extracts of the leaves and roots.
Using the potassium hydroxide reagent (KOH) at 365 nm, we were able to observe five spots on extracts of the leaves and seven spots on extracts of the roots. Using specific developers of the alkaloids together with Dragendorff reagent gave seven spots on extracts of the leaves and no spots on extracts of the roots.
The results on the extracts reveal the presence of flavonoids, tannins, coumarins and alkaloids (blue, fluorescent blue, yellow, black, Orange, red and gray spots). Comparing the retention factors, Rfs on the spots, extracts with n-butanol had twelve spots in total, which were identified as flavonoids seven from extracts of the leaves (Rf = 0.11; Rf = 0.16; Rf = 0.24; Rf = 0.33; Rf = 0.77; Rf = 0.83; Rf = 0.91) and five from extracts of the roots (Rf = 0.08; Rf = 0.13; Rf = 0.16; Rf = 0.71; Rf = 0.77). In addition, four spots were identified as tannins: two spots from the extracts of the leaves (Rf = 0.69 and Rf = 0.91) and two spots from extracts of the roots (Rf = 0.65 and Rf = 0.75). Six spots were identified as belonging to coumarins: four spots from extracts of the leaves with Rf = 0.08; Rf = 0.12; Rf = 0.20; Rf = 0.77 and two spots from extracts of the roots with Rf = 0.06; and Rf = 0.77. The seven spots identified as alkaloids were all from extracts of the leaves and none for the roots. These results also confirm previous report by Godwill et al. on C. afer from Nigeria using extracts with n-butanol [20].
Table 5: Screening of extracts with n-butanolon the leaves and roots of C. afer
| Secondary metabolites |
Leaves |
roots |
|
Coumarins |
++ |
++ |
|
Flavonoids |
++ |
++ |
|
Tannins and polyphenols |
++ |
++ |
|
alkaloids |
++ |
– |
(+) = Presence; (++) = Abundance of compound, (-) = Absence
Total Polyphenol Content
The total phenolic compounds in extracts of the leaves and roots of C. afer extracted with hydromethanolic are presented in Figure 1. From the results, it is observed that the extract from dry leaves gave higher total polyphenol content (3416.25 μg EAG / g MS) with abundance in flavonoids, coumarins and tannins. In addition, the total polyphenol content in extracts of the roots is 3017.50 μg EAG / g MS.
If we compare our results with those obtained from other plants with higher phenolic compounds (5660 μg EAG / g) [21], grape seeds (7500 μg EAG / g) [22], parsley (2802 μg EAG / g), Brussels sprouts (2571 μg EAG / g), lychee (2223 μg EAG / g), broccoli (989 μg EAG / g) and celery (847 μg EAG / g) [23], we can conclude that our extracts are also rich in phenolic compounds.
 |
Figure 1 : Total Phenols content of different parts of BF: Leaves and BR: Roots |
Total Flavonoid Content
The total flavonoids content in C. afer are presented in Figure 2. From the results, extracts of the leaves have higher flavonoids (08.02 %) compared to extracts of the roots (04.79 %). Previous reports on the phytochemical screening by TLC also confirm this quantitative experiment of flavonoids in the leaves. The high therapeutic properties of extracts of the leaves are probably due to the high flavonoids content [18].
 |
Figure 2: Total flavonoids content on different extracts ofC. afer |
Antioxidant Activity of C. afer using spectrophotometry
2,2-diphényl-1-picrylhydrazyl (DPPH) radical scavenging activity from extracts with n-butanol.
The inhibition percentage of 2,2-diphényl-1-picrylhydrazyl (DPPH) radical using extracts with ethyl acetate were done at different concentrations (0.025; 0.05; 1.00; 1.50; 2.00; 3 mg / mL). The results are presented in Figure 3. It is observed that extracts from the leaves have a higher inhibition (3 – 83 %) compared to extracts from the roots (5 – 69 %). Our results have lower inhibition percentages compared to the reference, vitamin C.
 |
Figure 3: 2,2-diphényl-1-picrylhydrazyl(DPPH)radical scavenging activity using extracts with ethyl acetate extracts (Leaves and roots) |
Determination of Inhibition Concentrations (IC50)
The 50 % growth inhibitory concentration of cells (IC50) was determined graphically from regression curves from the percentage inhibition of 2,2-diphényl-1-picrylhydrazyl (DPPH) as a function of the concentration of the leaf and root extracts. The results are presented in Table 6. From the table, extracts from the leaves have a lower IC50 compared to the roots. The IC50 of the extracts from the leaves and roots of C. afer using ethyl acetate are higher compared to vitamin C. Similarly, the IC50 is less than 5 mg / mL [19].
Table 6: IC50 values for the extracts with ethyl acetate and vitamin C
|
|
t = 3min |
t = 6min |
t = 9min |
|
ethyl acetate Leaves |
1.16 |
1.17 |
1.15 |
|
ethyl acetate roots |
2.92 |
2.88 |
2.93 |
|
Vit. C |
0.58 |
0.59 |
0.61 |
Conclusion
In this research, we have successfully presented results on the phytochemical, chromatographic, and antioxidant activities on the medicinal plant C. afer. Extracts of the leaves and roots of this plant obtained with different solvents and/or solvent systems revealed the presence of secondary metabolites and antioxidant activities. .
It is seen that, both extracts (leaves and roots) obtained with hexane contain flavonoids, coumarins, tannins, steroids (sterols) and terpenes. In addition, alkaloids were present only in the leaves and not in the roots. Furthermore, extracts of the leaves and roots obtained with chloroform show an abundance of flavonoids. Extracts obtained with ethyl acetate and n-butanol reveal the presence of flavonoids and tannins, polyphenols and coumarins. The results obtained showed high contents of phenolic compounds in the leaves.
The antioxidant activity obtained using spectrophotometric technique with 2,2-diphényl-1-picrylhydrazyl (DPPH) radical indicate that both extracts of the leaves and roots have good antioxidant activity, especially with those containing high content of phenolic compounds.
Acknowledgement
The authors are highly indebted to herbariums in the Centre National de Floristique, Botanical Department of University of Félix Houphouet Boigny, for identifying the plant material. In addition, we wish to thank the Ministry of Research of the République de Côte d’Ivoire for the enormous financial support.
References
- Patrick A. ; Pierre B. Hémorroïdes et maladie hémorroïdaire 2004.
- Delaveau P. ; Albin M. Histoire et renouveau des plantes médicinales 1982, 383.
- Anderson J. W.; Baird P.; Davis, R. H. Jr.; et al. Nutr. Rev. 2009, 67, 188-205.
CrossRef - Park Y.; Hunter D. J.; Spiegelman D.; et al. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies JAMA, 2005, 294, 2849-57.
- Martinez M. J. ; Bonfill X. ; Moreno R. M. ; et al. Phlebotonics for venous insufficiency Cochrane Database Syst. 2005, 3, 003229.
- Jepson R. G. ; Craig J. C. Mol. Nutr. Food Res. 2007, 51, 738-745.
CrossRef - Loder P.; Kamm M.; Nicholls R. L. Br. J. Surg. 1994, 81, 946-954.
CrossRef - Benkiki N. Ruta Montana, Matricaria pubescens et Hypericum perfoliatum. Thèse de Doctorat d’état, Faculté des Sciences, Département de chimie, Université El-Hadj Lakhdar Batna 2006, 198
- Békro Y-A. ; Mamyrbekova-Békro J. Y-A. ; Boua B. B. ; Tra Bi F. ; Ehilé E. Sciences et Nature, 2007, 4, 217-225.
CrossRef - Mamyrbékova-Békro J.Y-A. ; Konan M. ; Békro Y-A. ; Djié Bi M. ; Zomi Bi T. ; Mambo V. ; Boua B. B. European Journal of scientific Research 2008, 24, 219-228
- N’gaman K. C. C. ; Békro Y-A. ; Mamyrbékova-Békro J. ; Bénié A. ; Gooré B. S. European Journal of Scientific Research, 2009, 36, 161-171
- Kabran G. R. M. Etude chimique et cytotoxique de dix plantes de Côte d’Ivoire, utilisées dans le traitement traditionnel du cancer du sein. Thèse de Doctorat, Université Nangui Abrogoua 2014, 125, 265
- Kadja A. B. Sept plantes africaines utilisées comme cure-dents : compositions minérale, phénolique et activités biologiques. Thèse de doctorat. Université Nangui Abrogoua, Abidjan 2014, 208, 125
- N’Guessan A. H. O. Composition qualitative, quantitative et activités anti oxydante et anti prolifération de dix plantes médicinales de Côte d’Ivoire. Thèse de doctorat. Université Nangui Abrogoua, Abidjan 2014, 188, 191
- Hariri E. B. ; Sallé G. ; Andary C. Involvement of flavonoids in the resistance of two poplar cultivars to mistletoe (Viscum album L.). Protoplasma, 1991, 16, 20-26.
CrossRef - Popovici C. ; Saykova I. ; Tylkowski B. Evaluation de l’activité antioxydante des composés phénoliques par la réactivité avec le radical libre DPPH. Revue de génie industriel 2009, 4, 25-39
- Anyasor G. N.; Onajobi F.; Osilesi O., Adebawo O.; Martins O. E. Journal of Ethnopharmacology 2014, 1-33
- Armelle D. T.; Lauve R. Y. T.; Protus A. T.; Jules R. K.; Gabriel A. A. Costus afer Possesses Carbohydrate Hydrolyzing Enzymes Inhibitory Activity and Antioxidant Capacity In Vitro 2015, 1-11.
CrossRef - Godswill N. A.; Funmilayo D. O.; Odutola O.; And O. A. Hematological and lipid profile evaluation of a hexane fraction of Costus afer leaves in arthritic rats 2015, 1-9
- Godswill N. A.; Onajobi F., O.O.; Adebawo O.; Efere M.O. Chemical constituents in n-butanol fractions of Costus afer ker Gawl leaf and stem 2013, 1-7
- Bessas A. Dosage biochimique des composés phénoliques dans les dattes et le miel recoltés dans le sud algérien. Mémoire de fin d’étude, Université Djillali Liabes – Sidi Bel Abbes 2008, 57
- Cristina P. ; Ilonka S. ; Bartek T. Evaluation de l’activité antioxydante des composés phénoliques par la réactivité avec le radical libre DPPH; Université technique de Moldova, Kisinew, Moldavie ; Université de technologie chimique et de métallurgie, Sofia, Bulgarie. Revue de génie industriel 2009, 4, 25-39
- Akowah G. ; Zhari I. ; Norgyati I, Sadikun A. ; Khamsah S. Food chemistry, 2004, 87, 559-566.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









