New Mixed Ligand Cobalt(II), Nickel(II) and Copper(II) Complexes of 2,2'-Bipyridine-3,3'-Dicarboxylic Acid (bpdc) with 2-Mercapto-5-Phenyl-1,3,4-Oxadiazole (phozSH) and Their Antioxidant Activity
Rezan Ali Saleh* , Hikmat Ali Mohammad and Salim Najm Aldin Saber
, Hikmat Ali Mohammad and Salim Najm Aldin Saber
Chemistry Department, College of Education, Salahaddin University, Erbil, Iraq
Corresponding Author E-mail : rezanali2@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360506
Article Received on : 29 July 2020
Article Accepted on :
Article Published : 07 Sep 2020
The mixing of one mole of 2,2'-bipyridine-3,3'-dicarboxylic acid (bpdc) with two mole of potassium hydroxide (KOH) in methanol were refluxed for (half h), followed by addition of one mole methanol solution of MCl2.nH2O (where M= Co, Ni or Cu). The mixture was refluxed for (2 h) to give colored complexes of the metal ions of [M(bpdc)(H2O)4]. The [M(bpdc)(H2O)4] were reacted with one mole of 2-Mercapto-5-phenyl-1,3,4-oxadiazole (phozSH) producing the colored mixed ligand complexes with general formula [M(bpdc)(phozSH)(H2O)3] in which the metal ions coordinated to the ligand through O-atoms of carboxyl group in (bpdc) and N-atom of (phozSH) ligand. The ligands and complexes are well identified by using Furrier transform infrared spectroscopy, 1H-NMR, 13C-NMR, Electronic spectroscopy, CHNS analysis, Melting point, conductivity measurement. The Antioxidant activity were screened for all the complexes by the use of 2, 2-diphenyl-1- picrylhydrazyl (DPPH) method.
KEYWORDS:Antioxidant Activity; Bipyridine; Cobalt(II); Copper(II); Nickel(II); Oxadiazole
Download this article as:| Copy the following to cite this article: Saleh R. A, Mohammad H. A, Saber S. N. A. New Mixed Ligand Cobalt(II), Nickel(II) and Copper(II) Complexes of 2,2'-Bipyridine-3,3'-Dicarboxylic Acid (bpdc) with 2-Mercapto-5-Phenyl-1,3,4-Oxadiazole (phozSH) and Their Antioxidant Activity. Orient J Chem 2020;36(5). |
| Copy the following to cite this URL: Saleh R. A, Mohammad H. A, Saber S. N. A. New Mixed Ligand Cobalt(II), Nickel(II) and Copper(II) Complexes of 2,2'-Bipyridine-3,3'-Dicarboxylic Acid (bpdc) with 2-Mercapto-5-Phenyl-1,3,4-Oxadiazole (phozSH) and Their Antioxidant Activity. Orient J Chem 2020;36(5). Available from: https://bit.ly/35cpFyF |
Introduction
Oxadiazoles have been recognized during a century, nevertheless the majority of research has focused on the symmetric 1, 2, 5- and 1, 3, 4-oxadiazoles1. 1, 3, 4-oxadiazoles and their complexes have fascinated more consideration because of their biological behavior in both agrochemicals and pharmaceuticals.2 For example, numerous derivatives of 1, 3, 4-oxadiazoles reveal analgesic,3 muscle relaxant, anti-malarial,4 anti-inflammatory,5 anti-tubercular,6,7 fungicides,8,9 anti-bacterial,10,11 insecticidal, anti-oxidant12 and anticancer.2,13
In the present work, we give an account of the preparation and identification of new mixed ligand Co(II), Ni(II) and Cu(II) complexes comprising the two ligands of 2, 2′-bipyridine-3, 3′-dicarboxylic acid (bpdc) and 2-Mercapto-5-phenyl-1,3,4-oxadiazole(phozSH). We found that bpdc ligand behave as a bidentate chelate that connected to the metal ions by oxygen atom of the carboxyl group while the phozSH ligand work as a monodentate that link to the metal ions by nitrogen atom.
Materials and Methods
The (NiCl2.6H2O, CoCl2.6H2O, CuCl2.2H2O, KOH) materials are available in BDH and 2, 2′-bipyridine-3, 3′-dicarboxylic acid, 2-Mercapto-5-phenyl-1,3,4-oxadiazoleand 2, 2-diphenyl-1- picrylhydrazyl materials were commercially accessible from Yahoo Chem. China. The FT-IR spectra were registered on a Shimadzu IR- spectrophotometer in the range of 400-4000 cm-1 by utilizing KBr discs. (1H and 13C)-NMR spectra were performed on a Bruker 400 MHZ Ultra-shied. UV-Visible spectra were taken on a UV-Visible spectrometer, AE-UV1609 (UK) CO., LTD. The conductivity measurements were estimated on a conductivity meter type Senz µSiemen conductivity tester. CHNS analyses were executed on Euro EA 3000 Elemental Analyzer.
Synthesis of [Co(κ2-bpdc)(κ1-phozSH)(H2O)3] (1)
It was synthesized by the following stages
Stage One: Preparation of [Co(κ2-bpdc)(H2O)4]
2, 2′-bipyridine-3, 3′-dicarboxylic acid(bpdc) (0.3 millimole, 0.0732 gram) was dissolved in methanol (10 milliliter) and (5 milliliter) methanol solution of KOH (0.6 millimole, 0.0336 gram) was added. After refluxing for (30 min.), a solution of CoCl2.6H2O (0.3 millimole, 0.0713 gram) in methanol (5 milliliter) was added dropwise. The resulted solution was heated under reflux for 2 h. and filtered. The light brown precipitate was produced when the filtrate was evaporated at room temperature. (Chemical formula: C12H10CoN2O6Cl2; Yield: 0.09 gram, 65.7%; decomposition point: 296 °C; Color: light brown).
Stage two: Reaction of [Co(κ2-bpdc)(H2O)4] with (phozSH)
A warm solution of (phozSH) ligand (0.2 millimole, 0.0356 gram) in (10 milliliter) ethanol was added to a hot suspended solution of [CoCl2(κ2-bpdc)] (0.2 millimole, 0.074 gram) in CH2Cl2 (10 milliliter). The mixture was heated under reflux for 2 h. and filtered. The yellow-green precipitate was attained when the solution was evaporated at room temperature.
Yield = 0.09 gram, 81 %; Melting point: 174-176 °C; Color: yellow-green. Anal. Calc. for C20H15CoN4O7Cl2S: C, 41.03; H, 2.56; N, 9.57; S, 5.48. Found: C, 41.66; H, 2.46; N, 8.60; S, 4.57 %. IR (ν max/cm-1): ν(H2O) 3398; ν(C=O) 1718; ν(C=N) 1612; ν(C=N of bpdc) 1573; ν(N-N) 1446; ν(C-O-Co) 1076; ν(C-O-C) 1062; ν(C-S) 696; ν(M-H2O) 630; ν(Co-O) 480; ν(Co-S) 418. 1H NMR (295K, ppm, DMSO-d6): 7.65-8.9 (m, 11H, phenyl proton of bpdc and phozSH); 2.5 (s, DMSO proton); 4.14 (s, water proton in DMSO), 13C NMR (DMSO, 𝛿, 400 MHz): 175 C (carboxyl); 158 and 168 C (oxadiazole); 120.57-130.49 C (ph groups of bpdc and phozSH); 39.38 C (DMSO).
Synthesis of [Ni(κ2-bpdc)(κ1-phozSH)(H2O)3] complex (2)
It was synthesized by the following stages
Stage one: Preparation of [Ni(κ2-bpdc)( H2O)4]
A solution of KOH (0.6 millimole, 0.0336 gram) in methanol (5 milliliter) was placed to a solution of bpdc (0.3 millimole, 0.0732 gram) in methanol (10 milliliter) and refluxed for (30 minutes.). The subsequent mixture was refluxed for a further (2 h.) after addition of a methanol solution (5 milliliter) of NiCl2.6H2O (0.3 millimole, 0.0713 gram) and filtered. When the solution was evaporated at room temperature the dark-green solid was formed. (Chemical formula: C12H10NiN2O6Cl2; Yield: 0.13 gram, 94.3%; Melting point: 230-232 °C; Color: dark-green).
Stage two: Reaction of [Ni(κ2-bpdc)(H2O)4] with (PhozSH)
To a warm stirred solution of [NiCl2(κ2-bpdc)(H2O)4] (0.2 millimole, 0.0743 gram) in CH2Cl2 (10 milliliter), a hot ethanolic solution (10 milliliter) of phozSH (0.2 millimole, 0.0356 gram) was added and the resultant solution was heated under reflux for 2 h. and filtered. The green precipitate was obtained when the solution was evaporated at room temperature.
Yield = 0.08 gram, 80 %; decomposition point: 210-212 °C; Color: green. Anal. Calc. for C20H17NiN4O8Cl2S: C, 39.82; H, 2.82; N, 9.29; S, 5.31. Found: C, 39.15; H, 2.27; N, 8.33; S, 4.58 %. IR (ν max/cm-1):ν(H2O) 3421;ν(C=O) 1724; ν(C=N) 1610; ν(C=N of bpdc) 1573, ν(N-N) 1446; ν(C-O-Ni) 1093; ν(C-O-C) 1076; ν(C-S) 696; ν(Ni-H2O) 651; ν(Ni-O) 480; ν(Ni-S) 420. 1H NMR (295K, ppm, DMSO-d6): 8.4-9.5 (m, 11H, phenyl roton of bpdc and phozSH); 2.5 (s, DMSO proton); 3.49 (s, water in DMSO), 13C NMR (DMSO, 𝛿, 400 MHz): 172 C (carboxyl); 150 and 160 C (oxadiazole); 123.96-130.18 C (ph groups of bpdc and phozSH); 39.32 C (DMSO).
Synthesis of [Cu(κ2-bpdc)(κ1-phozS)(H2O)3]Cl2 complex (3)
It was synthesized by the following stages
Stage One: Preparation of [Cu(κ2-bpdc)( H2O)4]
To a methanol solution (10 ml) of bpdc (0.3 millimole, 0.0732 gram), a methanol solution (5 milliliter) of KOH (0.6 millimole, 0.0336 gram) was added and refluxed for (30 min.) then a solution of CuCl2.2H2O (0.3 millimole, 0.0514 gram) dissolved in (5 milliliter) methanol was added and the resultant solution was refluxed for a further 2h. and filtered. The green solid was achieved when the solvent was evaporated at room temperature. (Chemical formula: C12H10CuN2O6Cl2; Yield: 0.07 gram, 61.9 %; Melting point: 236-238 °C; Color: green).
Stage two: Reaction of [Cu(κ2-bpdc)(H2O)4] with (PhozSH)
To a hot suspended solution of [Cu(κ2-bpdc)(H2O)4] (0.2 millimole, 0.0752 gram) in dichloro methane (10 milliliter), a hot solution of phozSH (0.2 millimole, 0.0356 gram) in ethanol (10 milliliter) was added and heated under reflux for 2 h. then filtered. After the solvent was evaporated at normal temperature the light-green product was achieved.
Yield = 0.09 gram, 81 %; Melting point: 174-176 °C; Color: yellow-green. Anal. Calc. for C20H18CoN4O8S: C, 45.02; H, 3.37; N, 10.50; S, 6.01. Found: C, 45.66; H, 4.06; N, 10.10; S, 5.47 %. IR (ν max/cm-1): ν(H2O) 3398; ν(SH) 2953; ν(C=O) 1718; ν(C=N) 1612; ν(C=N of bpdc) 1573; ν(N-N) 1446; ν(C-O-Co) 1076; ν(C-O-C) 1062; ν(C-S) 696; ν(M-H2O) 630; ν(Co-O) 480; ν(Co-N) 443. 1H NMR (295K, ppm, DMSO-d6): 7.65-8.9 (m, 11H, phenyl proton of bpdc and phozSH); 2.59 (s, SH proton); 4.14 (s, water proton in DMSO), 13C NMR (DMSO, 𝛿, 400 MHz): 175 C (carboxyl); 158 and 168 C (oxadiazole); 120.57-130.49 C (ph groups of bpdc and phozSH); 39.38 C (DMSO).
![Scheme 1: Synthesis of [M(κ2-bpdc)(κ1-phozSH)(H2O)3], where M= Cobalt, Nickel or Copper(II).](http://www.orientjchem.org/wp-content/uploads/2020/09/Vol36No5_New_Reza_Sch1-150x150.jpg) |
Scheme 1: Synthesis of [M(κ2-bpdc)(κ1-phozSH)(H2O)3], where M= Cobalt, Nickel or Copper(II). |
Results and Discussion
FT-IR Spectra for the Synthesized Complexes
The IR spectra of Cobalt(II), Nickel(II) and Copper(II) complexes, contain broad band at (3398 and 3421, 3088) cm-1 and occurrence of a new weak peaks at (630 and 651 and 638) cm-1 were ascribed to ѵ(O-H) of coordinated water molecule to the metal ions14. The (SH) stretching band at (2567) cm-1 of (phozSH) was shifted to a higher frequency to (2953) cm-1 in complex 1 and (2852) cm-1 in complex 2 and 315-18. A new weak band was appeared at (443, 513, 542) cm-1 in the spectra of Cobalt(II), Nickel(II) and Copper(II) complexes were respectively indicated to the linkage of oxadiazole nitrogen to the metal ions. The broad band of (O-H) group of (bpdc) at (2576) cm-1 was disappeared in all complexes and a new weak peak was observed at (480, 450 and 420) cm-1 were indicated to O-coordination of (bpdc) ligand to (Cobalt(II), Nickel(II) and Copper(II)) metals correspondingly19-22.
 |
Figure 1: IR spectrum of bpdc ligand |
 |
Figure 2: IR spectrum of phozSH ligand |
![Figure 3: IR spectrum of [Co(κ2-bpydc)( κ1-phozSH)(H2O)3]Cl2 complex](http://www.orientjchem.org/wp-content/uploads/2020/09/Vol36No5_New_Rez_Fig3-150x150.jpg) |
Figure 3: IR spectrum of [Co(κ2-bpdc)( κ1-phozSH)(H2O)3]Cl2 complex |
![Figure 4: IR spectrum of [Ni(κ2-bpydc)( κ1-phozSH)(H2O)3]Cl2 complex](http://www.orientjchem.org/wp-content/uploads/2020/09/Vol36No5_New_Rez_Fig4-150x150.jpg) |
Figure 4: IR spectrum of [Ni(κ2-bpdc)( κ1-phozSH)(H2O)3] |
![Figure 5: IR spectrum of [Cu(κ2-bpydc)(κ1-phozSH)(H2O)3]Cl2 complex](http://www.orientjchem.org/wp-content/uploads/2020/09/Vol36No5_New_Rez_Fig5-150x150.jpg) |
Figure 5: IR spectrum of [Cu(κ2-bpdc)( κ1-phozSH)(H2O)3] |
1H-NMR Spectra for the Synthesized Complexes
The 1H-NMR spectra of (1, 2) complexes have been recorded in DMSO-d6 and complex (3) in CDCl3 solvent. The assignment of all signals have been attained by comparing the 1H-NMR spectra of the ligands registered in literature. Each sort of signal has a particular chemical shift range that can be applied for initial assignment.
The 1H-NMR spectra of complexes (1-3) displayed an unresolved multiplet signals in the region 𝛿(7.65-8.9), (8.4-9.5) and (7.67-8.4) ppm, were respectively ascribed to aromatic phenyl protons of both (bpdc) and (phozSH) ligands.
Furthermore, disappearance of the singlet carboxyl protons of (bpbc) ligand at (11.0) ppm indicated the coordination of O-atom of bpdc to Cobalt(II), Nickel(II) and Copper(II) metals. By contrast, the thiol proton of (phozSH) ligand was shifted to a higher chemical shift and appeared at (2.59, 3.49, 3.70) ppmwereassigned to N-bound coordination of oxadiazole nitrogen to Co, Ni and Cu(II) metals, respectively23-26.
![Figure 6: 1H-NMR of [Co(κ2-bpydc)(κ1-phozS)(H2O)3]Cl2](http://www.orientjchem.org/wp-content/uploads/2020/09/Vol36No5_New_Rez_Fig6-150x150.jpg) |
Figure 6: 1H-NMR of [Co(κ2–bpdc)(κ1-phozS)(H2O)3]Cl2 |
![Figure 7: 1H-NMR of [Ni(κ2-bpydc)( κ1-phozS)(H2O)3]Cl2](http://www.orientjchem.org/wp-content/uploads/2020/09/Vol36No5_New_Rez_Fig7-150x150.jpg) |
Figure 7: 1H-NMR of [Ni(κ2-bpdc)( κ1-phozSH)(H2O)3] |
![Figure 8: 1H-NMR of [Cu(κ2-bypdc)( κ1-phozS)(H2O)3]Cl2](http://www.orientjchem.org/wp-content/uploads/2020/09/Vol36No5_New_Rez_Fig8-150x150.jpg) |
Figure 8: 1H-NMR of [Cu(κ2-bpdc)( κ1-phozSH)(H2O)3] |
13C-NMR Spectra for the Synthesized Complexes
The 13C-NMR spectral data of the prepared complexes were estimated in DMSO-d6 solvent. In the spectra of (1-3) complexes, the carboxyl carbon atoms of (bpdc) ligand observed in the regions (175.53, 172 and 167) ppm respectively, while the two oxadiazole carbon atoms of (phozSH) ligand were occurred at 𝛿(158 and 168), (150 and 160) and (152 and 162) ppm respectively. The aromatic phenyl carbon atoms of both bpdc and phozSH correspondingly occurred within (120.57-130.49), (123.96-130.18) and (122-132.08) ppm.25-27
![Figure 9: 13C-NMR of [Co(κ2-bpydc)(κ1-phozS)(H2O)3]Cl2 complex](http://www.orientjchem.org/wp-content/uploads/2020/09/Vol36No5_New_Rez_Fig9-150x150.jpg) |
Figure 9: 13C-NMR of [Co(κ2-bpdc)( κ1-phozSH)(H2O)3] |
![Figure 10: 13C-NMR of [Ni(κ2-bpydc)( κ1-phozS)(H2O)3]Cl2 complex](http://www.orientjchem.org/wp-content/uploads/2020/09/Vol36No5_New_Rez_Fig10-150x150.jpg) |
Figure 10: 13C-NMR of [Ni(κ2-bpdc)( κ1-phozSH)(H2O)3] |
![Figure 11: 13C-NMR of [Cu(κ2-bpydc)(κ1-phozS)(H2O)3]Cl2 complex](http://www.orientjchem.org/wp-content/uploads/2020/09/Vol36No5_New_Rez_Fig11-150x150.jpg) |
Figure 11: 13C-NMR of [Cu(κ2-bpdc)( κ1-phozSH)(H2O)3] |
Elemental Analysis for the Synthesized Complexes
The elemental analysis (C, H, N, S) data for all the synthesized complexes are listed in table 1. These numbers are coherent with the proposed stoicheometries. Other physical properties such as colors, molecular weight (M.Wt.) and melting points (M. P.) of the synthesized complexes are also written.
Table 1: Colors, M.Wt. , M.P. and (CHNS) analysis for the synthesized complexes
|
Synthesized compounds |
Colour |
M.Wt |
M.P. |
(Calculated) Found % |
|||
|
g/mol |
(°C) |
C H N S |
|||||
|
1 |
yellow-green |
533 |
174-176 |
(45.02) |
(3.37) |
(10.50) |
6.01 |
|
45.66 |
4.06 |
10.10 |
5.47 |
||||
|
2 |
Green |
532.75 |
d.p. 210-212 |
(45.04) |
(3.37) |
(10.51) |
(6.01) |
|
45.15 |
3.27 |
10.33 |
4.58 |
||||
|
3 |
light-green |
537.60 |
d.p. 217-219 |
(44.64) |
3.34 |
(10.41) |
(5.96) |
|
44.20 |
3.40 |
8.51 |
5.51 |
||||
Electronic Spectra for the Prepared Complexes
The electronic spectra of (bpdc and phozSH) ligands were performed in ethanol and their synthesized complexes in DMSO solvent. The UV.-Vis. spectra of bpdc and phozSH ligands displayed two absorption peaks at (41666, 37037) and (40000, 33333) cm-1, these transitions were correspondingly ascribed to π- π* and n- π* transitions. The spectrum of Co(II) complex (1), showed three bands in the UV and visible region at 14925, 16393 and 28571 cm-1 , were due to 4T1g(F) → 4T2g , 4T1g(F) → 4A2g and 4T1g(F) → 4T2g(P) transitions, respectively.
The spectrum of Ni(II) complex (2) exhibited two d-d transitions at 23809 and 30303 cm-1 were respectively attributed to 1A1g → 1A2g and 1A1g → 1B1g , and the Cu(II) complex also exhibit two transition bands at 23809 and 29411 cm-1 were due to 2B1g → 2A1g and 2B1g → 2B2g transitions. The electronic transitions for all metal complexes indicate an octahedral geometry (Table 2)28,29.
Table 2: Electronic spectral bands of the ligands and their metal complexes
|
Compounds |
Absorption band |
Assignment Transition |
|
|
Nm |
cm-1 |
||
|
bpdc |
240 |
41666 |
π → π* |
|
270 |
37037 |
n → π* |
|
|
phozSH |
250 |
40000 |
π → π* |
|
300 |
33333 |
n → π* |
|
|
1 |
350 |
28571 (ѵ3) |
4T1g(F) → 4T2g(P) |
|
610 |
16393 (ѵ2) |
4T1g(F) → 4A2g |
|
|
670 |
14925 (ѵ1) |
4T1g(F) → 4T2g |
|
|
2 |
330 |
30303 (ѵ2) |
1A1g → 1B1g |
|
420 |
23809 (ѵ1) |
1A1g → 1A2g |
|
|
3 |
340 |
29411(ѵ2) |
2B1g → 2B2g |
|
420 |
23809 (ѵ1) |
2B1g → 2A1g |
|
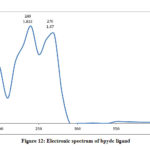 |
Figure 12: Electronic spectrum of bpdc ligand |
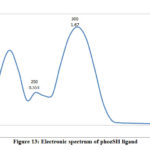 |
Figure 13: Electronic spectrum of phozSH ligand |
![Figure 14. Electronic spectrum of [Co(κ2-bpydc)(κ1-phozS)(H2O)3]Cl2 (a) UV. (b) Vis. Region](http://www.orientjchem.org/wp-content/uploads/2020/09/Vol36No5_New_Rez_Fig14-150x150.jpg) |
Figure 14. Electronic spectrum of [Co(κ2-bpdc)(κ1-phozS)(H2O)3]Cl2 (a) UV. (b) Vis. Region |
![Figure 15: Electronic spectrum of [Ni(κ2-bpdc)(κ1-phozSH)(H2O)3]: (a) UV. (b) Vis. Region](http://www.orientjchem.org/wp-content/uploads/2020/09/Vol36No5_New_Reza_Fig15-150x150.jpg) |
Figure 15: Electronic spectrum of [Ni(κ2-bpdc)( κ1-phozSH)(H2O)3] |
![Figure 16: Electronic spectrum of [Cu(κ2-bpydc)(κ1-phozS)(H2O)3]Cl2: (a) UV. (b) Vis. Region](http://www.orientjchem.org/wp-content/uploads/2020/09/Vol36No5_New_Rez_Fig16-150x150.jpg) |
Figure 16: Electronic spectrum of [Cu(κ2-bpdc)( κ1-phozSH)(H2O)3] |
Molar Conductivity for the Synthesized Complexes
The molar conductivities of the synthesized complexes (1-3) were taken for (10-3 M) solution in DMSO at (25 °C). It was deduced that all the synthesized complexes are non-electrolyte as demonstrated in Table 3.
Table 3: Molar conductivity (cm2. ohm-1. mol-1) of (10-3 M) solution in DMSO for the synthesized complexes+
| No. | Complexes | Molar conductivity (cm2. ohm-1. mol-1) | |
| 1 | [Co(κ2-bpdc)(κ1-phozSH)(H2O)3] | 17 | |
| 2 | [Ni(κ2-bpdc)(κ1-phozSH)(H2O)3] | 25 | |
| 3 | [Cu(κ2-bpdc)(κ1-phozSH)(H2O)3] | 20 | |
Antioxidant Assay (DPPH free Radical Scavenging Activity)
The metal complexes were monitored for free radical scavenging activity by the 2,2-diphenyl-1- picrylhydrazyl (DPPH) method.
The different concentrations of the tested complexes (25, 50, 75 ppm) and standard vitamin-C were received in separate test tubes, and by addition of DMSO solvent the volume of each tube was settled to 2 milliliter. To all sample solution tubes in DMSO, a methanolic solution of DPPH (2 milliliter). The tubes were permitted to stand for 30 min. The control experiment was fulfilled by the same method but without the addition of test samples. The absorbance was taken at 517 nm. Radical scavenging activity was calculated by the subsequent formula.30, 31

The results of free radical scavenger activity of the tested compounds at various concentrations are exhibited in Fig. 17. All complexes 1–3 showed comparable or slightly lower activity than the standard, ascorbic acid.
Co(II), Cu(II) complexes have demonstrated a good free radical scavenging activity with (IC50= 0.920, 5.075 𝛍 mol dm-1) were the most effective than Ni(II) complex, Fig. 18. Whereas Nicelectrolytekel(II) complex with (IC50 = 31.683 𝛍 mol dm-1) has showed less activity. The metal complexes of Copper(II), Cobalt(II) were displayed higher scavenging activity than the standard, while Ni(II) complex has lower activity than ascorbic acid. The synthesized complexes scavenged the DPPH radical in a concentration dependent manner.
Table 4: Absorbance of compounds at different concentration at 517 nm
|
Com. No. |
Compound |
Concentration (𝛍g/ml) |
||
|
25 |
50 |
75 |
||
|
Ascorbic acid (AA) |
0.857 |
0.837 |
0.816 |
|
|
Com. 1 |
[Cu(κ2-bpdc)(κ1-phozS)(H2O)3]Cl2 |
0.791 |
0.679 |
0.571 |
|
Com. 2 |
[Ni(κ2-bpdc)(κ1-phozS)(H2O)3]Cl2 |
0.884 |
0.860 |
0.824 |
|
Com. 3 |
[Co(κ2-bpdc)(κ1-phozS)(H2O)3]Cl2 |
0.722 |
0.589 |
0.413 |
Table 5 : Antioxidant activity of the metal complexes at different concentration using DPPH assay
|
Com. No. |
Compound |
Concentration (𝛍g/ml) |
||
|
25 |
50 |
75 |
||
|
Ascorbic acid (AA) |
51.195 |
52.334 |
53.53 |
|
|
Com. 1 |
[Cu(κ2-bpdc)(κ1-phozS)(H2O)3]Cl2 |
54.954 |
61.332 |
67.482 |
|
Com. 2 |
[Ni(κ2-bpdc)(κ1-phozS)(H2O)3]Cl2 |
49.658 |
51.025 |
53.075 |
|
Com. 3 |
[Co(κ2-bpdc)(κ1-phozS)(H2O)3]Cl2 |
58.883 |
66.457 |
76.48 |
Table 6: Superoxide dismutase activity of the prepared complexes
|
Com. No. |
Complexes |
IC50 (𝛍 mol dm-1) |
|
Com. 1 |
[Cu(κ2-bpdc)(κ1-phozS)(H2O)3]Cl2 |
5.075818 |
|
Com. 2 |
[Ni(κ2-bpdc)(κ1-phozS)(H2O)3]Cl2 |
31.683 |
|
Com. 3 |
[Cu(κ2-bpdc)( κ1-phozSH)(H2O)3] |
0.92 |
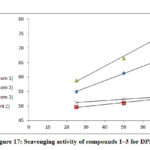 |
Figure 17: Scavenging activity of compounds 1–3 for DPPH |
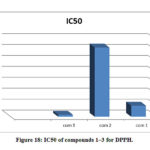 |
Figure 18: IC50 of compounds 1–3 for DPPH. |
Conclusion
This work comprises synthesis of new mixed ligand Cobalt(II), Nickel(II) and Copper(II) complexes with 2-Mercapto-5-phenyl-1,3,4-oxadiazoleand 2,2′-bipyridine-3,3′-dicarboxylic acid ligands. Based upon Infrared, UV-Visible, 1H-NMR, 13C-NMR and (CHNS) analysis, we concluded that all the prepared complexes have an octahedral shape in which (bpdc) ligand coordinated to the metals through oxygen atoms, whereas the phozSH ligand connected to the metals via oxadiazole nitrogen atom. In accordance with the molar conductivity data, it has been recommended that all complexes are non-electrolyte.Finally, on the basis of Antioxidant assay,in which metal complexes were screened for free radical scavenging activity by the 2,2-diphenyl-1- picrylhydrazyl (DPPH) method, it can be conclude that all the Cobalt(II), Nickel(II) and Copper(II) complexes have a good free radical scavenging activity.
Acknowledgement
We would like to thank the University of Salahaddin, College of Education for partial support of this work..
Conflict of Interest
There is no conflict of interest.
Funding Source
There is no funding source.
References
- Da Silva, A.; De Silva, M.; Carvalho, C.; Antunes, O.; Herreeea, J.; Brinn, I.; Mangrich, A. Inorganica chimica acta. 1999, 292, 1-6.
CrossRef - Subhi, A.; Ilham, N.; Lamaan, J.; Ayad, H. Transition metal chemistry 2002, 27, 191-195.
CrossRef - Toma, A.; Hapau, D.; Vlase, L.; Mogosan, C.; Zaharia, V. Clujul Medical 2013, 86, 34-39.
- Kumar, K.; Jayaroopa, P.; Kumar, G. International Journal of Chem Tech Research. 2012, 4, 1782-1791.
- Husain, A.; Ajmal, M. Acta. pharmaceutica. 2009, 59, 223-233.
CrossRef - Kerur, S.; Alagawadi, K.; Zhu, H.; Manvi, F. World Journal of Pharmacy and Pharmaceutical Sciences. 2014, 3, 573-585.
- Pattan, S.; Rabara, P.; Pattan, J.; Bukitagar, A.; Wakale, V.; Musmade, D. Indian journal of chemistry. Section B, Organic including medicinal. 2009, 48, 1453.
- Somashekhar, M.; Sonnad, B.; Tare, B.; Heralagi, R.; Lokapure, S. International Journal of Medicine and Pharmaceutical Research. 2014, 2, 560-569.
- Kumar, S.Turkish Journal of Chemistry. 2011, 35, 99-108.
- Jha, K.; Samad, A.; Kumar, Y.; Shaharyar, M.; Khosa, R.; Jain, J.; Bansal, S. Iranian Journal of Pharmaceutical Research. 2009, 8, 163-167.
- Deshmukh, R.; Jha, A.; Thakur, A.; Dewangan, D. International Journal of Research in Pharmaceutical and Biochemical. Sciences. 2011, 2, 215-219.
- Rajasekaran, S.; Rao, G.; Chatterjee, A. International Journal of Drug Development and Research. 2012, 4, 303-309.
- Adimule, V.; Medapa, S.; Kumar, L.; RAao, P. Int. J. Drug Dev. and Res. 2014, 6, 188-195.
- V Gina, V.; Mariana, C.; Coralia, B.; Crina, K.,; Lumini¸M.; Constantin, G.; Catalin, M.; Larisa, C.; Rodica, O.; Mihaela, B. Molecules, 2018, 23, 10.
- Rehab, A.; Zainab, A. Al- Mustansiriya J. Sci. 2009, 20, 23.
- Raghad, H.; Emad, Y.; Ahmed A. Springer Plus 2013, 2, 3.
- Buttrus, N.; Mohamed, S. Research Journal of Chemical Sciences 2013, 3, 56.
- Venu, K.; Siva, P.; Ashok, P.; Rameshbabu, K.; Sreeramulu, J. Journal of Chemical and Pharmaceutical Research 2013, 5, 52.
- Dina, A. Journal of Al-Nahrain University 2011, 14, 37.
- Mahasin, A.; Sahar, I.; Souad, A. Journal of Al-Nahrain University 2015, 18, 30.
- Chandraleka, S.; Chandramohan, G. African journal of pure and applied chemistry 2014, 8, 167.
- Saravana, P.; Tharmaraj, P.; Muthuraj, V.; Umadevi, M. International Journal Of Engineering And Science 2013, 2, 16-25.
- Kitagawa, S.; Munakata, M. Inorganic Chemistry 1981, 20, 2261-2267.
CrossRef - Venu, K.; Siva, P.; Ashok, P.; Rameshbabu, K.; Sreeramulu, J. Journal of Chemical and Pharmaceutical Research 2013, 5, 50-59.
- Gudasi, K.; Patil, M.; Vadavi, R.; Shenoy, R.; Patil, S. J. Serb. Chem. Soc. 2007, 72, 357–366.
CrossRef - Ibraheem, H.; Adel, H.; Ahmed, A.; Salih, N.; Salimon, J.; Graisa, A.; Farina, Y.; Yousif, E. Journal of Al-Nahrain University 2010, 13, 43-47.
CrossRef - Panagiotis, N.; Dafnopoulos, K.; Tortopidis, C.; Koumbis, A.; Koffa, M.; Psomas, G.; Fylaktakidou, K. Journal of Photochemistry & Photobiology, B: Biology 2016, 158, 30–38.
CrossRef - Hoda, A.; Abdel-Nasser, M.; Mutlak S. Int. J. Electrochem. Sci. 2013, 8, 9406.
- Sutton, D. Electronic spectra of Transition metal complexes: an Introductory text. McGraw-Hill. 1968, 73-193.
- Subarani, R.; Metilda, P. International Journal of Scientific & Engineering Research 2017, 8, 337.
- Karekal, M.; Bennikallu, H. Journal of Saudi Chemical Society 2017, 21, 202.

This work is licensed under a Creative Commons Attribution 4.0 International License.









