Gel Formulation of Jamblang Leaf Extract (Syzygiumcumini L) Skeel and Antioxidant Activity
Department of Fharmacy, Faculty of Mathematics and Natural Science, Universitas Tulang Bawang Lampung, Bamdar Lampung Indonesia.
Corresponding Author E-mail: samsuar@utb.ac.id
DOI : http://dx.doi.org/10.13005/ojc/360521
Article Received on : 23-07-2020
Article Accepted on : 07-10-2020
In this paper we reported gel formulation that containing jamblang leaf extract (Syzygium cumini L) and activity test as antioxidant. The jamblang leaf plant contained polyphenols compound which function as antioxidants. The purpose of this study was to determine the antioxidant activity of gel formulation that containing jamblang leaf extract. Extraction of jamblang leaves were formulated with three varation of concentration and gel basis as negative controls. The gel formulation were evaluated for organoleptic observations, homogeneity, pH, viscosity, dispersion test, adhesion test, accelerated stability test for 4 weeks storage, and measurement of antioxidant activity was carried out using the DPPH method with wavelength of 519 nm. The results of gel formulation IC50 value shows the ability of a substance to inhibit 50% DPPH radical of 47.735 ppm. The gel extract of jamblang leaf has a very strong antioxidant activity.
KEYWORDS:Antioxidant; Gel Formulation; IC50; Jamblang Leaf
Download this article as:| Copy the following to cite this article: Samsuar, Hanifa D. Gel Formulation of Jamblang Leaf Extract (Syzygiumcumini L) Skeel and Antioxidant Activity. Orient J Chem 2020;36(5). |
| Copy the following to cite this URL: Samsuar, Hanifa D. Gel Formulation of Jamblang Leaf Extract (Syzygiumcumini L) Skeel and Antioxidant Activity. Orient J Chem 2020;36(5). Available from: https://bit.ly/3m6tHOc |
Introduction
Free radicals are defined as an atom or molecule that has one or more unpaired electrons in its outer orbitals, is very reactive and unstable1. Free radicals can come from cigarette smoke, fried or burned foods, excessive sun exposure, motor vehicle fumes, certain drugs, poisons and air pollution2. Excessive free radicals can trigger a variety of degenerative diseases, such as cancer and heart disease (cardiovascular)3. The emergence of degenerative diseases by free radicals can be inhibited or prevented by antioxidant compounds. Therefore, the body needs an important substance that is antioxidants to capture free radicals so that it cannot induce other diseases4.
Antioxidants are chemical compounds that can contribute one or more electrons to free radicals, so that free radicals can be suppressed5. However, the human body does not have excessive amounts of antioxidants, so if there is excessive radical exposure, the body needs antioxidants from outside the body 6. Based on the source, there are two kinds of antioxidants, namely natural antioxidants and synthetic antioxidants. Natural antioxidants are usually more desirable, due to better safety and wider benefits in the field of food, health and cosmetics. Natural antioxidants are found in most foods and agricultural products, including vegetables, fruits and plant extracts 7.
Jamblang plants are reported to contain chemical compounds including alkaloids, flavonoids, resins, tannins, and essential oils 8. Flavonoid glycoside, quercetin, myricetin 3-O-4 acetyl-L-rhamnopyranoside,triterpenoids and tannins are found in jamblang leaves 9. All of these plant contents are used for medicinal purposes. Research in India shows that the stems, leaves and fruit of jamblang plants have antioxidant, anti-inflammatory, anthelmintic, anticancer, antibacterial, and antidiabetic activities10. Gel is a semisolid dosage form that contains a solution of a single active ingredient or a mixture with a carrier of hydrophilic or hydrophobic compounds11. Gel formulation have several advantages including non-sticky, easily applied, easily washed, and does not leave an oily layer on the skin, thereby reducing the risk of inflammation due to accumulation of oil in the pores 12.
Based on the description above, the researchers are interested in further investigating the antioxidant activity of jamblang leaves which are formulated in gel formulation, where the antioxidant testing is carried out using free radical scavenging methods using DPPH.
Materials and Methods
Materials
All reagents used were AR grade. carbopol, propyleneglycol (H8O2C3), triethanolamine (C3H15NO3), methyl paraben (C8H8O3), aquadest, DPPH , ethanol 80%, jamblang (Syzigium cumini L.) skeel leaves Instrumentation: oven (memmert), blender, mess 40, vaccum rotary evaporator, pH meter, Viscometer (Haake 550), scatter power testing device, calipers, UV-vis 1800 spectrophotometer (Shimadzu),
Test Material
The jamblang leaves that are obtained are separated from the branches, then cleaned from the dirt attached to the leaves, washed with running water, drained. Then the jamblang leaves are chopped and weighed as much as 3.5 kg, then dried in a drying cupboard until they are dry (marked when crumbly brittle). Furthermore, dried leaves are added as dry weight. Dry simplicia is blended to powder and then sieved in mess 40. The sieves are stored in a tightly closed container at room temperature to prevent the effects of moisture and other impurities. 13,14
Jamblang Leaf Extract
The jamblang leaf ethanol extract was done by maceration using 80% ethanol 14. As much as 800 grams of Simplicia powder of jamblang leaves are put into a dark glass container, then add 80% ethanol until the simplicia is completely submerged. Covered and left for 3 days protected from light while stirring frequently, then after 3 days filtered and squeezed. The pulp is re-macerated with 80% ethanol, transferred into a particular vessel, left in a cool place, protected from light for 3 days, the maceration process is carried out until a clear search solution. The ethanol maserate obtained was evaporated using a rotary evaporator at ± 40oC until a thick extract was obtained 13
Gel Formulation
Gel formulation is made in 3 variation which are distinguished by the concentration of jamblang leaf extract. Each gel contains 5%, 10%, and 15% jamblang leaf extract in the same base composition. The gel formulation to be made in this study can be seen in Table 1.
Table 1: 100 gram Jamblang Leaf Extract Gel Formulation Formulation
|
No |
Material |
Formula (%) |
||||
|
F0 |
F1 |
F2 |
F3 |
F4 |
||
|
1 |
Ekstrak daun jamblang |
0 |
0 |
5 |
10 |
15 |
|
2 |
Carbopol |
0,5 |
0 |
0,5 |
0,5 |
0,5 |
|
3 |
Trietanolamin |
0,5 |
0 |
0,5 |
0,5 |
0,5 |
|
4 |
Propilenglikol |
10 |
0 |
10 |
10 |
10 |
|
5 |
Metil paraben |
0,2 |
0 |
0,2 |
0,2 |
0,2 |
|
6 |
Haematococcus pluvialis extrac |
0 |
1 |
0 |
0 |
0 |
|
7 |
Aquadest ad |
100 |
30 |
100 |
100 |
100 |
Gel Formulation Preparations
The ingredients needed weigh according to Table 1 and Carbopol is developed by sprinkling on hot aquadest with a temperature of more than 80oC, leaving for 24 hours for the carbopol to expand properly, then stirring. Add TEA little by little, then stir until a gel base is formed (ingredient 1). Add a portion of propylenglycol to ingredient 1 (ingredient 2). Dissolve methylparaben with a portion of propylenglycol, then mix into phase 2 to homogeneous (phase 3). For F2, F3, F4 enter jamblang leaf extract according to the amount of each formula. Stir all ingredients until the gel is homogeneous. For F0, set the base in a pot. For F1, used antioxidant gel circulating in the market that already has a marketing authorization (used as a positive control in the antioxidant test). Enter the gel preparation into the appropriate container 15
Evaluation of Gel Formulation
Organoleptic Test
Organoleptic test includes checking the consistency of color, odor and form of gel preparations to determine the physical condition of the gel preparations. Each gel preparation (F0, F2, F3, and F4) was placed in a tightly closed pot container, then observed changes in shape, color and odor during 4 weeks storage. Changes in color and odor are an indication of the instability of the preparation16
Homogeneity Test
Each gel preparation (F0, F2, F3, and F4) weighed as much as 5 grams was applied sufficiently on the slide, then closed with the slide. It was observed whether the preparations showed homogeneous arrangement12
PH test
Determination of the pH of the preparation is done by using a pH meter that has been calibrated using buffer solution pH 7 and pH 4. Weigh 1 gram of the gel preparation that will be examined (F0, F2, F3, and F4) put into a beaker glass, then dissolve it with 10 ml aquadest. The pH meter electrode into the solution, then look and record the pH value that appears on the pH meter. The above method is repeated for each formula. Preparations made should have a pH that matches the pH of the skin, which is 4.5 – 6.5 12
Stickiness Test
0.5 gram gel (F0, F2, F3, and F4) is applied over a glass object and then covered with another glass object. The glass object is pressed with a weight of 1 kg for 5 minutes, then the glass object is mounted on the test equipment, note the time of release of the two glass objects. Adhesion test was repeated 3 times on day 1 and day 28 after making gel.
Scattering Test
This test is carried out by weighing 0.5 grams of gel (F0, F2, F3, and F4), then placed on a 20×20 cm glass, then covered with another glass and adding weights on top of 50, 100, 150, and 200 grams of weighing children and leave for 1 minute. The diameter of the gel that spreads (by taking the average length of the diameter from several sides) is measured using a calipers. Each additional weight is waited for 1 minute then note the diameter of the gel spread. Tests carried out on the 1st day and 28th day after making the gel 12. Scattering power requirements are between 5-7 cm.
Viscosity Test
The viscosity test is carried out using a viscosity measuring device namely a 550 Haake viscometer, a viscomester is mounted on the clamp, then the rotor is mounted on the viskotester by locking it counterclockwise. Put 10 grams of gel preparation (F0, F2, F3, and F4) into the beaker, place the rotor in the middle of the sample not to stick to the side of the beaker, then turn on the appliance. When the rotor rotates the needle viscosity indicator automatically moves to the frame, after showing a stable number, the viscosity is read on the rotor after the measurement is turned off. The standard measurement for gel viscosity is 2000-4000 cps 16.
Gel Stock Stability Test
Stability tests were carried out on gel formulas (F0, F2, F3, and F4) which were evaluated as being declared stable formulas and meeting physical quality requirements. The stability test includes a physical evaluation of odor, color and shape. The stability test in this study used an accelerated stability test for 4 weeks by means of the cycling test, storage at low temperature, room temperature, and high temperature.
Antioxidant Activity Test
Making DPPH 100 ppm main solution
Weigh 10 mg of DPPH powder put into a 100 mL volumetric flask Add 80% ethanol p.a to the pumpkin boundary markings Pumpkin lid cover coated aluminum foil or avoided from sunlight.
Determinationof Wavelength (λ)
3.25 ml DPPH mother liquor is put into a 5 ml volumetric flask, dilute with 80% ethanol to the mark of the pumpkin boundary (obtained DPPH concentration of 65 ppm). Measure the absorbance at a wavelength of 450 – 550 nm. The maximum wavelength is the wavelength at which the sample solution has maximum absorption.
Antioxidant Test
Antioxidant test used DPPH with Weigh each 10 mg of gel preparations (F1, F2, F3, and F4), put in a 10 ml glass beaker. Dissolve using 5 ml of 80% ethanol, stir until homogeneous. Enter the solution into a 10 ml volumetric flask, add 80% ethanol to the pumpkin boundary mark, obtained a 1000 ppm mother solution. Pipette as much as 2 ml of the mother formula of the formula, put into a measuring flask added 2 mL DPPH 100 ppm homogeneously, then incubated at 37ºC for 30 minutes. Measure the absorbance of each solution using a UV-Vis Spectrophotometer at maximum wavelength17.
Results and Discussion
Determination is done to find out the truth of the plant taken, to avoid mistakes in gathering materials used for research. The process of determination is done by matching the morphological characteristics of the plants to be studied, namely tree trunks, twigs, and leaves. Determination results obtained data that the plants used in this study are jamblang plant species with the name of the Myrtaceae tribe, the genus Syzygium and the type Syzygium cumini (L) Skeel.
The Materials formulation used are fresh old jamblang leaves green because old jamblang leaves have stronger antioxidant activity6. The leaves are harvested during the day while undergoing photosynthesis because at that time the leaves have a high content of secondary metabolites. After that, it is sorted wet to separate the parts of plants or dirt that are attached to the leaves, then washed using running water and drained. Then the leaves are cut into small pieces and weighed and dried to reduce water content so as to prevent the growth of fungi, bacteria, and other organisms that can damage leaf components.
Drying is done for 20 days in an open space without direct sunlight to avoid damage to the content of chemical compounds in the dried material. Drying time can be ended if the dried leaves have begun to become brittle by grasping or kneading. Dry jamblang leaves are blended to powder obtained by weight of 800 grams, then stored in a tightly closed container at room temperature to prevent the influence of damp and other impurities.
Maceration is done by inserting the jamblang leaf simplicia into a dark colored container / bottle with the aim of protecting the solution from direct light exposure and preventing catalysis reactions by light, such as discoloration, then macerated with 80% ethanol for 9 days. The maceration method was chosen because of its simple implementation, low operating costs, and the process without heating so that the active substances contained in the material are not easily damaged [13]. While the reason for using 80% ethanol solvent is because the material used is simplicia, so water is needed to wet the simplicia so that the cells are open and the solvent is easier to enter to attract the compounds contained in the simplicia. In addition, 80% ethanol is very effective to be used as a finder because ethanol-water mixture can attract active ingredients in optimal amounts. From the maceration process, a maserat of 10 liters is obtained, the maserate obtained is then concentrated using a rotary evaporator at 40 ° C to separate the extract from the liquid with accelerated heating due to pumpkin rotation so that the liquid can evaporate below its normal boiling point so that the compound content at extract is not damaged.
Jamblang leaf extract is made into three formulas with various extract concentrations, namely 5%, 10%, and 15%. The color produced by the gel formulation containing the extract in it is a typical brownish green color of jamblang leaf extract, while for the negative control formula it produces clear gel, as shown in Figure 1 below
 |
Figure 1: Jamblang Leaves extract Gel |
The preparation of gel formulations is carried out using a gel forming material that is hydrogel carbohydrate (water-based gel) in which carbopol is dispersed into a heated aquadest to a temperature of ± 80 ° C, this aims to accelerate the formation of gel mass, then trietolamine is added as an alkaline agent carbopol, which is a base compound which will increase the pH of the carbopol gel mass which is initially at pH 2.5-3.0 to pH 6.0-11.0 which in this pH range carbopol forms a thicker gel mass. The gel mass formed is then added with propylenglycol as a humectant because most of the gel preparations contain water which is very susceptible to microbial growth, so methyl paraben is added as a preservative. Methyl paraben was chosen because it can dissolve in water and its activity as a preservative ranges from pH 4.0 to 8.0 so that it matches the pH range of carbopol 17.
The results of the gel formulation were left in place for 24 hours after making the preparations stable, then tests were performed which included organoleptic, homogeneity, viscosity, pH, dispersal, adhesion and antioxidant activity tests. Organoleptic test results can be seen in table 2 below
Table 2: Organoleptic Jamblang Leaf Extract Gel
|
F |
Shape |
Smell |
Color |
|
F0 |
Gel |
Odorless |
Clear |
|
F2 |
Gel |
Distinctive extract |
Brownish green |
|
F3 |
Gel |
Typical extract + |
Brownish green |
|
F4 |
Gel |
Typical extract ++ |
Brownish green |
Organoleptic preparations on F2, F3, and F4 are influenced by the concentration of the extract added. The higher the concentration of the extract produces a darker brownish green color and a stronger characteristic odor, whereas in formula 0 it is clear and has no odor because it does not contain extracts.
Homogeneity test results of the four preparations showed good results because there were no coarse grains as shown in Figure 2 below
 |
Figure 2: Gel homogeneity |
The test is carried out by observing the granules found in the gel preparation after being applied to the glass plate. Based on Indonesian Pharmacopoeia, the homogeneous gel is characterized by the absence of granules at the time of application. In this homogeneity test the four preparations showed good results because in all four preparations there were no coarse grains that were seen so that they met the requirements of a good gel 14.
The results of pH testing showed that the gel base had a pH value of 6.9 (F0), whereas gel preparations containing extracts (F2, F3, and F4) had lower pH compared to preparations which did not contain extracts. The pH value of the gel preparations can be seen in table 3.
Table 3: pH of Gel preparations
|
Sample |
pH |
SNI |
|
F0 |
6,9 |
4,5-8,0 |
|
F2 |
6,3 |
|
|
F3 |
5,8 |
|
|
F4 |
5,5 |
In table 3, it can be seen that the gel base (F0) has a higher pH value than other formulas, this is due to the base component of triethanolamine which is alkaline, while the pH value of the gel formulations F2, F3, and F4, decreases, this can influenced by the addition of extracts that are increasing where the pH of the jamblang leaf extract is acidic, 3.9. The higher the concentration of the extract, the pH value of the formulation will decrease, but still within the range of SNI requirements, namely 4.5-8.0. The pH value of the gel preparation will affect the quality of the gel preparation, it should not be too acidic because it will cause irritation and it should not be too alkaline because it will cause scaly skin 11.
The results of the adhesion test measurements on preparations F0, F2, F3, and F4 on the 28th day decreased compared to the first day. Test data for the gel adhesion can be seen in table 4.
Table 4: Testing of Sticky Gels
|
Storage Time |
Stickiness (seconds) |
|||
|
F0 |
F2 |
F3 |
F4 |
|
|
Day 1 |
1,9 |
2,90 |
2,61 |
1,94 |
|
Day 28 |
1,6 |
2,55 |
1,95 |
1,45 |
Based on Table 4, it can be seen that the addition of extracts on preparations F2, F3, and F4 can affect the adhesion of the gel preparation, this can be influenced by the content of the compounds contained in the extract that causes the gel’s consistency to decrease so that the adhesion of the gel has decreased. The storage time in each formula can also affect the adhesion of the gel, this can be caused by the humectants used on the gel base which are less effective at maintaining the viscosity of the gel, causing macromolecules that are bound through dipole-dipole interactions to shorten and the molecular weight to be smaller so causes the gel adhesion to decrease. A good semi-solid adhesion requirement is more than 1 second 16. The gel dispersion test data can be seen in table 6 below.
Table 6: Testing Spread Gel
|
Storage Time |
Scattering Power (cm) |
|||
|
F0 |
F2 |
F3 |
F4 |
|
|
Day 1 |
5,41 |
5,65 |
6,14 |
6,21 |
|
Day 28 |
5,65 |
6,17 |
6,46 |
6,66 |
The addition of extracts on preparations F2, F3, and F4 can affect the spreadability of gel preparations, this can be influenced by the content of tannin compounds contained in the extract that can cause the gel to undergo hydrolysis so that the spreadability of the gel has increased. The storage time in each formula can also affect the gel dispersion, this can be caused by the humectants used on the gel base are less effective in maintaining the stability of the gel so that the gel spread increases, but it still meets the requirements for good semi-solid dispersion that is 5 -7 cm.
Observations were made by inserting 10 grams of sample into a cup and then mounted on the viskotester until the rotor limit was immersed, after that the program was set and the viskotester was run then the viscosity value of the sample would be seen. The results of the viscosity of jamblang leaf extract gel can be seen in table 7 below.
Table 7: Viscosity of Gel preparations
|
Sample |
Viscosity (cps) |
Requirements |
|
F0 |
3500 |
2000-4000 cps |
|
F2 |
2680 |
|
|
F3 |
2490 |
|
|
F4 |
2220 |
Based on Table 7 it can be seen that the viscosity value of each gel preparation is different, this can occur because the effect of adding different extracts in each formula is that the more extracts added the smaller the viscosity produced. However, the viscosity of gel preparations is still in the range of requirements for a good gel viscosity of 2000-4000 cps.
The stability tests includes a physical evaluation of odor, color and shape is carried out for 6 cycles where each cycle is stored at 4 ° C for 24 hours, then transferred to room temperature to normal temperature and then transferred to an oven at 40 ° C for 24 hours The results of stability test which a physical evaluation Tabel 8 below .
Table 8: Cycling Test
|
Sample |
Parameter |
Info |
||
|
Shape |
Color |
Smell |
||
|
F0 |
Gel |
White |
No smell |
Stable |
|
F2 |
Gel |
Brownish green |
Typical |
Stable |
|
F3 |
Gel |
Brownish green |
Typical |
Stable |
|
F4 |
Gel |
Brownish green |
Typical |
Stable |
Based on Table 8 after storage carried out as many as 6 cycles all samples showed no organoleptic changes at temperatures of 4oC ± 2oC, 28oC ± 2oC, and 40oC ± 2oC marked with semisolid gel dosage form, typical brownish green color, and characteristic odor of extract.
Next is an evaluation of organoleptic storage at low temperature, room temperature, and high temperature.
Table 9: Organoleptic Gel Preparations in Stability Tests
|
Sample |
Temperature |
Organoleptic (Shape, color, odor) Week to – |
|||
|
1 |
2 |
3 |
4 |
||
|
F0 |
4oC ±2oC |
Cgdns |
Cgdns |
cgdns |
cgdns |
|
28oC ± 2oC |
Cgdns |
Cgdns |
cgdns |
cgdns |
|
|
40oC ±2oC |
Cgdns |
Cgdns |
cgdns |
cgdns |
|
|
F2 |
4oC ± 2oC |
Bggco |
Bggco |
bggco |
bggco |
|
28oC ± 2oC |
Bggco |
Bggco |
bggco |
bggco |
|
|
40oC± 2oC |
Bggco |
Bggco |
bggco |
bggco |
|
|
F3 |
4oC ± 2oC |
Bggco |
Bggco |
bggco |
bggco |
|
28oC ± 2oC |
Bggco |
Bggco |
bggco |
bggco |
|
|
40oC ± 2oC |
Bggco |
Bggco |
bggco |
bggco |
|
|
F4 |
4oC ± 2oC |
Bggco |
Bggco |
bggco |
bggco |
|
28oC ± 2oC |
Bggco |
Bggco |
bggco |
bggco |
|
|
40oC± 2oC |
Bggco |
Bggco |
bggco |
bggco |
|
Based on table 9 after storage for 4 weeks, all samples showed no organoleptic changes at 4oC ± 2oC, 28oC ± 2oC, and 40oC ± 2oC marked with the form of semisolid gel preparations, the typical brownish green color, the characteristic odor of jamblang leaf extract.
The results of observations of changes in pH at storage conditions temperature 4oC ± 2oC, 28oC ± 2oC, and 40oC ± 2oC every week. the results of testing the pH of the preparation decreased during storage time but not too significant. A decrease in pH can occur due to changes in the quality of each formulation composition which is affected by storage temperature, causing the pH of the preparation to become acidic.
Viscosity test results at 4oC ± 2oC, 28oC ± 2oC, and 40oC ± 2oC can be seen that each storage temperature viscosity has decreased after storage is carried out for 4 weeks. The highest decrease occurred at 40oC ± 2oC, and the lowest decrease at 4oC ± 2oC.
Figure 3: Viscosity Graph on Stability Test
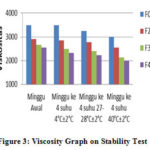 |
Figure 3: Viscosity Graph on Stability Test |
This shows that the temperature affects the viscosity, the higher the storage temperature, the viscosity decreases. Besides the storage time can also affect the value of its viscosity, the longer the storage time, the viscosity decreases.
Following are the results of testing the antioxidants F2, F3 and F4:
Table 10: Testing for Antioxidants F2, F3, and F4
|
Concentration (ppm) |
Absorbansi |
% Inhibisi |
IC50* (ppm) |
Intensity |
|
50 |
0,069 |
84,598 |
47,735 |
Very strong |
|
100 |
0,066 |
85,267 |
||
|
150 |
0,056 |
87,5 |
Antioxidant testing was carried out on formula 2, formula 3, and formula 4 after stability testing. Antioxidant testing was carried out using the DPPH method (2,2-diphenyl-1-picrylhydrazyl) because this method has a high sensitivity advantage, can analyze samples in a short period of time, and is simple to measure the antioxidant capacity of a sample. In addition, the DPPH method is a stable synthetic free radical that can represent the real free radical. The parameter of antioxidant activity is characterized by a change in the intensity of the purple DPPH to yellow due to the reaction of the DPPH molecule with hydrogen atoms released by the compound molecules in the gel preparation which forms the compound 2,2-diphenyl-1-picrylhydrazine.
DPPH absorbance measurements were measured using a UV VIS spectrophotometer with ethanol blank p.a at a wavelength of 400-550 nm, the maximum wavelength obtained from this study was 519 nm. Then the gel preparation is measured and absorbance is obtained to determine the percent inhibition. Percent inhibition is used to determine the percentage of inhibition of an ingredient that is carried out against free radical compounds. Percent of inhibition of each formulation were F2 = 84.598%, F3 = 85.267%, and F4 = 87.723%. The percent inhibition results are then substituted in a linear equation and expressed as IC50. Obtained a linear equation y = 0.526x + 24.891 where x is the amount of antioxidants needed to reduce or reduce the DPPH concentration by 50%, x obtained is 47.735 ppm. The higher the concentration of the sample the smaller the absorbance of DPPH due to the DPPH radical capture and the smaller IC50 value produced. The smaller the IC50 value, the stronger its antioxidant activity.
Based on table 10 the lowest concentration of jamblang leaf extract added to the gel preparation can reduce free radicals by more than 50% DPPH, which means the gel formula with the jamblang leaf extract content has very strong antioxidant activity.
Conclusion
Based on research that has been done it can be concluded that:
Jamblang (Syzigium cumini L) Skeel extract can be formulated as an antioxidant gel preparation
Formulation of jamblang leaf extract (Syzigium cumini L) Skeel has very strong antioxidant activity
Suggestion
Further research needs to be done on the use of jamblang leaf extract in antioxidant gel preparations with purer compounds such as fractions and isolation
Further research needs to be done on the use of jamblang leaf extract in antioxidant gel preparations with the addition of fragrance or color modification and irritation testing of preparations.
Bibliography
- Muchtadi. “Antioksidan Dan Kiat Sehat Di Usia Produktif”. Bandung: Alfabeta (2013).
- Trianda, B., “Uji Aktivitas Antioksidan Dan Kombinasi Ekstrak Etanol Rimpang Temugiring (Curcuma Heyneana) Dan Daun Pugun Tanoh (Curangafelterrae) Menggunakan Metode DPPH,” Fakultas. Farmasi. Universitas. Sumatera Utara (2016).
- Euis, R.Y., “Pengantar Radikal Bebas dan Antioksidan”. Yogyakarta: Deepublish. (2018).
- Ratnayani, “Kadar Total Senyawa Fenolat Pada Madu Randu Dan Madu Kelengkeng Serta Uji Aktivitas Antiradikal Bebas dengan Metode DPPH.,” Jurnal Kimia, vol. 6, no. 2, p. 164. (2012).
- Suhartono, “Oxygen Toxicity by Radiation and Effect of Glutamic Piruvat Transamine (GPT) Activity Rat Plasma After Vitamine C Treatmen”. Yogyakarta,” Int. Semin. Environ. Chem. Toxicol.(2012).
- Rohdiana, D., “Aktivitas Daya Tangkap Radikal Polifenol Dalam Daun Teh,” Maj. J. Indones., vol. 12, no. 1, pp. 53–58. (2001).
- Rohman, A., “Lipid : Sifat Fisika-Kimia dan Analisisnya”. Yogyakarta: Pustaka Pelajar (2016).
- Arifin, H., “Standarisasi Ekstrak Etanol Daun Eugenia Cumini Merr,” J. Sains Teknol. Farm., vol. 11, no. 12, p. 88 (2006).
- Ayyanar and Babu, “Syzygium Cumini (L) Skeel A Revier of Phytochemical Constituent and Traditional Uses,” Asian Pacific J. Trop. Biomed., vol. 2, no. 3, pp. 240–246 (2012).
CrossRef - Haroon, “Comparative Analysis of Antioxidant Profiles of Bark, Leaves and Seeds of Syzigium Cumini (Indian Blacberry),” Int. J. Res. Granthaalayah, vol. 3, no. 5 (2015).
- Tranggono, R.L.F., Ilmu Pengetahuan Kosmetik. Jakarta: Gramedia Pustaka Utama. (2007).
- Lieberman, R. and Banker, “Disperse System. Pharmaceutical Dosage Forms. New York,” Marcell Dekker Inc, vol. 2. (1989).
- Department kesehatan RI & DP., Parameter Standar Umum Ekstrak Tumbuhan Obat. Departemen Kesehatan R.I. (2000).
- Department Kesehatan., Farmakope Indonesia Edisi III. Jakarta: Department Kesehatan Republik Indonesia (1979).
- Sharon and Yuliet, “Formulasi Krim Antioksidan Ekstrak Etanol Bawang Hutan,” J. Nat. Sci., pp. 111–122 (2013).
- Voight, R.,Buku Pelajaran Teknologi Farmasi. Yogyakarta: Universitas Gajah Mada Press (1994).
- Burgess C M. 2005, “Cosmetic Dermatology. Germany,” Springer – Verleg Berlin Heidelberg (2005).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










