Assessment of Salts Effect in Sugar - Aqueous System
Om Kumari1, Braj Mohan1, Vikesh Kumar2 and Sailendra Kumar*
1Department of Chemistry, K.K. P.G. College Etawah, U.P. (India) 206001.
2Department of Chemistry, Awadhesh Pratap Singh University, Rewa, Madhya Pradesh- 486003, India.
*Department of Chemistry, S.G.S.P.G. College, Sidhi, M.P. (India) 486661.
Corresponding Author E-mail: mbraj7524@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360525
Article Received on : 07-08-2020
Article Accepted on : 20-10-2020
In the present study, the preferential salvation of salts in sugar- aqueous systems has been considered. It is carried out by using conductometric observation of analytical grade sugar and plantation white sugar aqueous system with salts viz. CaCl2, MgCl2, KCl, NaCl. It shows that the conductivity is in a linear relationship with the electrolytes and non-sugar present in both analytical grade sugar and plantation white sugar over a range of 5 to 25 W/V percent. The optimum range of concentration found to be for both the sugars is about 20%. Encouraging results could be obtained in the determination of sugars i.e. non-electrolytes and electrolytes in aqueous sugar solution products. The present study shows valid technological interest to understand the Maillard reaction due to the adoption of MgCl2 salt in place of sulphite. These sugars–salts complexes are responsible for the formation of molasses which leads to substantial loss of sugar of around ten percent of the total sugar present in cane.
KEYWORDS:Analytical Grade Sugar; Conductivity; Electrolytes; Non-Sugars; Plantation White Sugar; Salts
Download this article as:| Copy the following to cite this article: Kumari O, Mohan B, Kumar V, Kumar S. Assessment of Salts Effect in Sugar - Aqueous System. Orient J Chem 2020;36(5). |
| Copy the following to cite this URL: Kumari O, Mohan B, Kumar V, Kumar S. Assessment of Salts Effect in Sugar - Aqueous System. Orient J Chem 2020;36(5). Available from: https://bit.ly/34wMD2Y |
Introduction
Assessment of sugar quality is carried out by using conventional parameters like Brix, Pol, and Purity. These conventional parameters are calibrated in terms of pure sucrose solution, ignoring non-sugars substances having importance during cane sugar manufacture. Apart from these parameters, conductivity has a direct bearing on the quantity of non-sugars 1–3. A lower conducting sugar has a more saleable appeal than one which is higher conductivity due to inclusion of impurities in the form of mineral constituent’s, organic non-sugars & insoluble impurities present in cane juice (Table 1), or may form during sugar processing like carbonation, phosphotation, and sulphitation 4. In India, plantation white sugar is directly produced from cane juice having many impurities. These impurities like inorganic salts do affect solubility and crystallization rate of sucrose 5. The complexities of interactions between water, sugar, and eventually impurities in supersaturated solutions have been demonstrated earlier6-8. Knowledge of composition regarding these non-sugars as impurities with special reference to selected salts (CaCl2, MgCl2, KCl, NaCl) is used in this study through the conductivity method, because they affect sugar manufacturing process such as sucrose hydrolysis & sugar crystallization9-11. The development of a new approach for inhibiting the Maillard reaction employing additive such asMgCl2 in sucrose in place of sulphite has a valid technological interest. The effect of cations which is produced from CaCl2, MgCl2, KCl, NaCl, can be analyzed through electrolytes- non-electrolytes interaction, which modulate the kinetic of Maillard reaction12.
In recent years, Nuclear Magnetic Resonance is one of the useful techniques for analyzing the water mobility in food products 13.NMR techniques require lots of technical skill. Initially, the mobility of water in the sucrose solution used to be checked by conductivity method 14. The study of the interaction of salts with sucrose solution by the proposed conductivity method is explained based on ion–solvent, solvent–solvent, and ion-ion interaction, present in solution 15-18.
Multivalent electrolytes in aqueous sugar solution constitute an almost unexplored field. So the conductivity analyses of these inorganic non-sugar electrolytes in analytical grade sugar, as well as Indian sugar, have been carried out by taking 5 to 25% w/v concentration.
The aim of the present investigation is to find out the actual concentration of both analytical grade sugar as well as plantation white sugar where deviation in linearity occurs in the presence of selected salts. Study reveals that deviation in linearity for conductivity values occurs at around 22.5% w/v of sugar solution.
Materials and Method
The Shimadzu AW 320 balance was used for the weightiest of sugars and inorganic salts. Analytical grade sugar was purchased from Merck (Mumbai) India, and white sugar was obtained from Indian Sugar manufacturers with different grain sizes.
A glass cell (Vol. 30 cm3) with a platinum electrode was used for conductivity measurement (µS/cm). Two sets of experiments were performed, one at isothermal condition (298.15K), and the other at experimental temperature (298.15 K-373.15K). The temperature of the supercooled solution was controlled by ethylene water bath. Aqueous KCl solution (0.01M) was used for cell calibration. Double distilled water was used to prepared sugar solution.
The experiment results were given as the mean of five parallel trail and measurements. Analysis of variance and Duncan’s multiple range tests were employed to statistically analyze all results. A statistical data analysis software system (Stat Soft, Inc. version 6.2001) was used for analysis. P value<0.05 was regarded as significant.
Results and Discussion
Various impurities such as salts (Table 1), dextran, and invert sugars affect the sugar crystallization rate. Although, a detailed mechanism of impurity transfer during crystallization is not discussed in the literature.
In the first series of experiments, the blank value i.e. the solution containing the same concentration of salts (0.01M) as in the above experiment and with no sugar was observed. The variations of concentration of salts were, however, limited by the solubility of the salt under examination. However, it was in the range of 0.0025–0.01 molar concentration. Fig 1 shows the percent rise in conductivity values of NaCl & KCl is around 73 %, whereas in the case ofCaCl2, MgCl2 it is to be 76%.
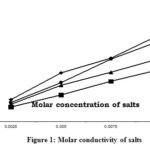 |
Figure 1: Molar conductivity of salts |
Table 1: Salts Concentration in Cane Juice
|
Components (cationic) |
Salts |
Concentration (% Brix) |
Molar Concentration |
|
K2O |
KCl |
0.67-1.21 |
0.6316 |
|
Na2O |
NaCl |
0.01-0.03 |
0.0068 |
|
CaO |
CaCl2 |
0.24-0.48 |
0.2016 |
|
MgO |
MgCl2 |
0.10-0.39 |
0.096 |
In the second series of experiments, the effect of inorganic salts on the electrical conductivity of analytical grade sugar as well as plantation white sugars was carried out. For this purpose, standard solutions of the mentioned salts were prepared for making the molar concentration of salts. It may be pointed out that all the inorganic salts examined viz. CaCl2, MgCl2, KCl, NaCl) were found as inhibiting the electrical conductivity of the sucrose solution. For a known concentration of sugar, the influence of salts on the electrical conductivity shows an increasing trend in both the case i.e. analytical reagent as well as plantation white sugar.
A comparative study was carried out to assess the conductivity of various alkaline earth metals in distilled water is summarized in Figure 1. Only CaCl2 was found to generate a sufficient amount of electrical conductivity to be monitored by electrical conductivity meter of 0.01 molar concentration ranges in analytical grade sugar as well as plantation white sugar at room temperature, and salts like MgCl2 (Figure.2). Further, to this, from Figure 2, one can get the following series indicating the effect of inorganic salt components on the electrical conductivity of sugars i.e. Ca ++ > Mg++.
 |
Figure 2: Sugar Vs Bivalent salts conductivit |
Further, from Figure 3 it is evident that the series indicates the effect of inorganic salt components on the electrical conductivity of analytical grade sugar solution as K+>Na+. Similar data were obtained at the same concentration of plantation white sugar solution having slightly higher values than analytical grade sugar due to more impurities adhering in plantation white sugars. The observation for KCl and NaCl are represented graphically in Figure 3. On the other hand, the complex formed between sugar and univalent metallic salts like KCl and NaCl etc are unimolecular types. This is of marked importance and suggests that in a mixture of a sugar and univalent metallic salts complex formation takes place as a result of one molecule of sugar and one molecule of salt. The complexes may be represented as C12H22O11, KCl & C12H22O11 NaCl etc in accordance with the data obtained in crystallization and phase rule studies.
 |
Figure 3: Sugar Vs Univalent salts conductivit |
Exact measurement of inorganic salts as an impurity is mentioned at a known concentration of sugar solution in the present analysis by conductivity method to specify the salts generating higher effect on crystal growth as found in the order of Ca ++ > Mg++ > K+ > Na+.
Further to this, Fig 2 & 3 shows that the deviation in linearity of conductivity values of both the sugars occurs at 20 gram/volume concentration, whereas at higher concentration than 20% gram/volume linearity deviates. It is due to that in sugar–salts system a micellar colloid is formed and volatiles substances are entrapped. By observing electrical conductivity of sugar solution it suggests that the micellar colloid beyond this concentration forms at a maximum degree hence our observation for 22.5 % w/v proves to be appropriate. So the analysis would be helpful within this concentration. The observed results are having good agreement with previous work 19-20. Similar work on calcium chloride, affecting the sugar crystal growth is already mentioned in literature3 showing that impurity act on the properties of the solution is controlled by conductivity measurement.
Impurity measurement in sugar solution during batch crystallization, conductivity analysis would be helpful, and more importantly the pH which is practically ignored in this analysis due to some obvious reason as mentioned earlier 1-2. Literature also reveals that the conductivity of the solution depends upon several variables such as salts, and temperature.
The elucidating part of this study reveals that the complex formation occurs between sugar and inorganic salts causes a dip in electrical conductivity value. The charge transfer reaction occurs in sugar–water–salts system. Finally a similar picture of ion–water interactions has been reached by a quite different approach21. This can be more or less than the exchange time for molecules in pure water. The values of this ratio for the above-analyzed salts are given in Table 2.
Table 2: Hydration Number of Salts
|
Constituents (cationic) |
Average time |
Hydration Number |
|
Na+ |
1.46 |
7-9 |
|
K+ |
0.65 |
4 |
|
Mg++ |
86.3 |
12-14 |
|
Ca++ |
2.16 |
9-12 |
Moreover, it is based on a study that the ion association would be less in case of univalent anion, and this generally true; there is no evidence of ion pairing in the halides of sodium and potassium hence record lesser conductivity value in comparison with alkaline earth metals i.e. calcium and magnesium. Here the metal of higher atomic number shows more ion pairing, as in the halide. It is interesting to note that ion pairing is directly proportional to the atomic radius. Data is available in the literature for the group II metals, and with the increase in cationic charges, the formation of ion pairs in dilute solution becomes the rule rather than the exceptions (Table 2).
Conclusion
The present studyreveals that the conductivity parameter can be utilized to measure the impurity in sugar solution in terms of its concentration at around 20% W/V. The conductivity value can be assumed as a function of impurity concentration (C impurity) and sugar concentration (C sucrose) as a similar finding is available in literature. The Maillard reaction due to the adoption of magnesium chloride and not sulphite is another fact-finding interpretation of the present study.
References
- Kumar, V.; Sandeep, K. M.; Sanyal, P. Conductometric studies and its significance during cane sugar manufacture. Su. Tec., 2009, 11(4), 324-329.
CrossRef - Kumar, V.; Sandeep, K. M.; Sanyal, P. Conductometric studies and its significance during cane sugar manufacture. Su. Tec., 2009, 11(4), 324-329.
CrossRef - Ferreia, A.; Faria, N.; Rocha, F. The Effect of crystal surface roughness on impurity adsorption. Cry. Res.Tech., 2009, 44(5), 521-533.
CrossRef - Prasad, M.; Singh, K. Solid state conductance in sucrose and its allied crystalline products. J. Ind. Chem. Soc., 2005, 82(5), 437-440.
- Mantovani, G.; Fagioli, F. Change in the shape of sucrose crystal in the presence of some non sucrose substance. Z. Zuckerind., 1965, 15, 690-692.
- Mathlouthi, M.; Genotelle, J. Role of water in sucrose crystallization. Car. Poly., 1989, 37, 335-342.
CrossRef - Mathlouthi, M.; Ashok, L.; Cholli, Jack, L. Koenig Spectroscopic study of the structure of sucrose in the amorphous state and in aqueous solution. Car. Res., 1986, 141(1), 1-9.
CrossRef - Van Hook, A. Growth of sucrose crystals-A Review. Su. Tech. Rev., 1981, 8, 41-79.
- Santagapita, P. R.; Buera, M. P. Chemical and physical stability of disaccharides as affected by the presence of MgCl2 . In M. P. Buera.; J, Weltichances.; P. J. Lillford.; H. R. Corti.; (Eds.).Water properties of food pharmaceutical, and biological materials. CRC Press–Taylor and Francis., 2006, 9, 663-669.
- Santagapita, P. R.; Buera, M. P. Electrolyte effects on amorphous and super cooled sugar system. J. Non – Crystal. Sol., 2008, 354, 1760-1767.
CrossRef - Longinotti, M. P.; Mazzobre, M. F.; Buera, M. P.; Corti, H. R. Effect of salts on the properties of aqueous sugar system in relation to biomaterial stablisation.1 Water sorption behavior and ice crystallization/melting. Cryobio., 2002, 43(3), 199-210.
CrossRef - Acevodo, N.; Schebor, C.; Buera, M. P. Water solids interactions, matrix structural properties and the rate of non enzymatic browning. J. Food. Eng., 2006, 77, 1108-1115.
CrossRef - Schmidt, S. J.; Barbosa–Canovas, G. V.; Fontana, A. J.; Schmidt, S. J.; Labuza, (Eds) T. P. Water mobility in foods and Water activity in foods. Fundamental and applications Ames. IA: Blackwell Publishing., 2007, 47, 103-108.
- Lindenbaum, S. Water structure promotion by large organic anions. J. Phys. Chem., 1970, 74, 3027-3028.
CrossRef - Vishnu & Singh, A. K. Conductance studies on the interaction of sucrose with some multivalent ions in aqueous solution. Car. Res., 1978, 60(1), 19-27.
CrossRef - Vishnu and Singh, A. K. Conductance studies on the interaction of sodium corboxylates with sucrose in water and formamide. Car. Res., 1977, 54, 149-157.
CrossRef - Eggleston, G.; Vercellotti, J.; Edye, L.; Clarke, M. Effect of salts on the initials thermal degradation of concentrated aqueous solution of sucrose. J. Car. Chem., 1996, 15, 81-94.
CrossRef - Eggleston, G.; Amorim, H. Reason for the chemical destruction of sugars during the processing of sugarcane for raw sugar and fuel alcohol production. Int. Su. J., 2006, 108(1289), 271-282.
- Gillett, T. R. Conductometric Measurement of Ash in White Sugars. Anal. Chem., 1949, 21(9), 1081-1084.
CrossRef - Sanyal, P.; Nigam, R. B.; Gupta, S. K.; Mitra, S. K. Use of conductivity data for quality assurance in Plantation white sugar factory.Proc.65th Conv. Sug. Tech. Assoc. India., 2004, M61–M67.
- Samoilov, O.Y. A new approach to the study of hydration of ions in aqueous solutions. Discus. Farad. Soc., 1957, 24,141-146.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









