Vanadyl Acetylacetonate-Copper (II) Trifluoro Methane Sulfonate Catalyzed Eco-friendly Synthesis of Substituted Benzimidazoles in Aqueous Media
Department of Chemistry,Charuchandra College, 22, Lake Road, Kolkata-700029, India
Corresponding Author E-mail : samiran14cd@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/360413
Article Received on : 30 June 2020
Article Accepted on : 31 July 2020
Article Published : 06 Aug 2020
Various substituted benzimidazoles have been successfully synthesized in aqueous medium by developing VO(acac)2–Cu(OTf)2 catalytic system. A green synthetic protocol has been created in presence of water and cetyltrimethyl ammonium bromide (CTAB) system in an organic solvent free condition. This chemoselective cyclocondensation cumoxidation process occurred in aqueous media. In this suitable method easily synthesized 2-Substituted benzimidazoles with good yields and no 1,2-disubstituted by-products were noticed. Excellent yields, environmentally benign and mild reaction condition, easy purification of the desired products are the main attractive features of this newly devised method.

Aqueous Medium; Benzimidazoles; Cyclocondensation; Catalysis; NMR Spectroscopy
Download this article as:| Copy the following to cite this article: Halder S. Vanadyl Acetylacetonate-Copper (II) Trifluoro Methane Sulfonate Catalyzed Eco-friendly Synthesis of Substituted Benzimidazoles in Aqueous Media. Orient J Chem 2020;36(4). |
| Copy the following to cite this URL: Halder S. Vanadyl Acetylacetonate-Copper (II) Trifluoro Methane Sulfonate Catalyzed Eco-friendly Synthesis of Substituted Benzimidazoles in Aqueous Media. Orient J Chem 2020;36(4). Available from: https://bit.ly/3i8aU38 |
Introduction
Benzimidazoles and their analogues are tremendous useful organic compounds, which shows an important function in organic, bioorganic and medicinal chemistry.1 Benzimidazoles and many of its analogues are widely known as their antimicrobial, anticancer,2,3 antifungal,4,5 antiparasitic,6 antiviral,7 anti-inflammatory,8 and antihistaminic9 activities. On the other hand, at present environment friendly synthetic processes have been extremely encouraging for organic synthesis.10 Consequently toxic, hazardous, volatile, carcinogenic and flammable organic solvents are being replaced by water11a or phase-transfer catalysts.11b Therefore, synthetic methodologies for the generation of biologically relevant 2-substituted benzimidazoles scaffolds in water media are very much desired and this is the major challenge for synthetic organic chemists.
The literature reports two general synthetic routes for the production of benzimidazoles. One method is the condensation of carboxylic acid with o-phenylenediamines .12-15 strong acid,16-19 PPA,19-21 high temperature and microwaves21,22 are also used to carry out these reactions. Some of the process are eventuate by using hydrochloric acid or phosphoric acid in refluxing at 250 oC.12 There is a second route to synthesize benzimidazoles by condensation which is usually take place between aldehydes and ortho-phenylenediamines.9,23-26 Nevertheless, most of the reported routes are some limitations manly low yields, high reaction temperatures, drastic reagents used, purification difficulties and formation of by-products. Under acid medium and thermal conditions formed 1,2-disubstituted by-products (4, Scheme 1) along with the desired product (3, Scheme 1).25,26 However, metal catalysed synthesis of 2-substituted benzimidazoles are inspiring.12
Gogoi and Konwar have reported a green protocol toward synthesis of benzimidazoles under anaerobic conditions at 90 oC using combination of reagents I2/KI/K2CO3/H2O.27 A rapid synthetic protocol was developed by Du and Wang using PhI(OAc)2 as the oxidant.28 Maleki and co-workers has synthesized tri- and tetra-substituted imidazole derivatives using silica-coated NiFe2O4 nanoparticles.29 Maleki have introduced an one-pot Synthesis of Polysubstituted Imidazoles Catalyzed by an Ionic liquid.30 Erfaninia and co-worker have reported a novel and effective solid nanoporous basic catalyst, ED‐UiO‐66 for the synthesis of 2‐aminithiophenes.31 Tayebee has demonstrated one‐pot four‐component condensation of an aromatic aldehyde with an enolisable ketone and acetyl chloride in the presence of H7SiV3W9O40 in acetonitrile for the synthesis of β‐acetamido ketones.32 Tayebee and co-workers have synthesis 3-substituted indoles using silica-supported H3PW12O40 (HPW) solid acid catalysts in solvent less condition.33 Tayebee has shown synthesis of 3,4‐dihydropyrimidin‐2‐(1H)‐ones by condensation of different substituted benzaldehydes with ethyl acetoacetate and urea using an effective heterogeneous nano catalyst under solvent‐free conditions.34
Herein, I have been developed a suitable pathway for synthesis of substituted benzimidazoles by applying metal catalysts VO(acac)2 and Cu(OTf)2 in water as a reaction media to the development of sustainable chemistry.35 I have used common phase transfer catalyst, cetyltrimethyl ammonium bromide (CTAB) for carry forward this reaction in water.
Result and Discussion
For standardize the reaction p-bromobenzaldehyde (1 mmol) and 1,2-phenylenediamine (1.2 mmol) were taken as a model reaction. Initially, I have studied the reaction in different condition using o-phenylenediamines (1a) with 4-bromobenzaldehyde (2a) for the synthesis of 2-(4′-bromophenyl)-1H-benzimidazole (3a, Scheme 1) in organic solvent and water and the results were summarized in Table 1. After extensive study I have found VO(acac)2 as an useful catalyst which convert aryl aldehyde and o-phenylenediamines to corresponding 2-substituted benzimidazoles in organic media (entry 1, Table 1). However, in presence of 20 mol % Cu(OTf)2 as a cocatalyst improves the reaction rate and yield and catalytic load. (entry 6, Table 1). The reaction is progress in presence of stoichiometric amount of VO(acac)2 with long reaction time and low yield. However, due to improvement of the reaction rate and yield I have used Cu(OTf)2 as a cocatalyst along with VO(acac)2. I am very surprised to see that, introduction of 20 mol% of the cocatalyst reduces drastically the catalytic load of VO(acac)2 from the stoichiometric amount to 20 mol%. Cu(OTf)2 plays an important role to increase the electrophilicity of aldehyde group by coordination with aldehydic oxygen atom and consequently imine formation takes place very fast. Therefore, VO(acac)2-Cu(OTf)2 are used to examine the synthesis of 2-substituted benzimidazole derivatives in water media. It was observed that in protic solvent like water the catalyst was unable to work (entry 7, Table 1). Then I have study this reaction in with commercially available materials like CTAB cationic nature, SDS anionic nature and neutral amphiphilic surfactant Tween 80 (entries 8-10, Table 1). All the results reveal that CTAB is one of the most superior phase transfer catalyst for this method (entry 8, Table 1). Then different amount of CTAB was used to investigate the optimization of the reaction condition in water. (entries 8, 11 and 12, Table 1). When I have added 30 mol% of CTAB best result was found. Therefore, 30 mol% CTAB was sufficient for this benign synthetic method. Furthermore, without using any catalyst the reaction does not possible in CTAB water (entry 13, Table 1). The main envisioned of my work is to synthesis the 2-substituted-1H– benzimidazole by using environment benign synthetic protocol.
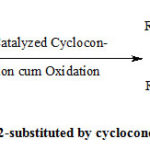 |
Scheme 1: Synthesis of 2-substituted by cyclocondensation cum oxidation |
Table 1: Development of reaction toward 2-substituted benzimidazoles
|
Entry |
Reagents |
Reaction Condition |
Observation |
Yield(%)a |
|
1 |
VO(acac)2c |
CH2Cl2, rt, 30 h |
75% conversion |
60 |
|
2 |
VO(acac)2,b In(OTf)3c |
CH2Cl2, rt, 24 h |
50% conversion |
40 |
|
3 |
VO(acac)2,b Agfb |
CH2Cl2, rt, 30 h |
50% conversion |
40 |
|
4 |
VO(acac)2,c Yb(OTf)3b |
CH2Cl2, rt, 24 h |
75% conversion |
60 |
|
5 |
VO(acac)2,c Cu(OTf)2b |
CH2Cl2, rt, 15 h |
100% |
85 |
|
6 |
VO(acac)2,bCu(OTf)2b |
CH2Cl2, rt, 15 h |
100% |
85 |
|
7 |
VO(acac)2,b Cu(OTf)2b |
H2O, rt, 24 h |
No Reaction |
– |
|
8 |
VO(acac)2,b Cu(OTf)2b |
CTABb, H2O, rt, 24 h |
80% |
70 |
|
9 |
VO(acac)2,b Cu(OTf)2b |
SDSd, H2O, rt, 24 h |
20% conversion |
10 |
|
10 |
VO(acac)2,b Cu(OTf)2b |
Tween 40d, H2O, rt, 24 h |
40% conversion |
25 |
|
11 |
VO(acac)2,b Cu(OTf)2b |
CTABd, H2O, rt, 20 h |
100% |
82 |
|
12 |
VO(acac)2,b Cu(OTf)2b |
CTABe, H2O, rt, 20 h |
100% |
82 |
|
13 |
– |
CTABd, H2O, rt, 24 h |
No Reaction |
– |
With this result, I have studied the catalytic system VO(acac)2-Cu(OTf)2 with various o-aromaticdiamines (1) and aromatic aldehyde (2) in CTAB water media forming the desired substituted benzimidazole derivatives (3, Scheme 2) and the results were depicted in table 2. Electron donating and withdrawing both groups attached to aromatic aldehyde were investigated. Six different o-aromaticdiamines having donating and withdrawing groups attached to aromatic ring were also examined of this new synthetic protocol. Surprisingly I have found that all of the substrates are reacted smoothly under the reaction conditions with excellent yields (71%–88%).
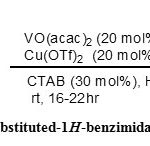 |
Scheme 2: Synthesis of 2-substituted-1H-benzimidazoles in aqueous medium |
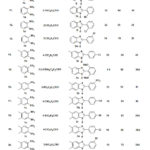 |
Table 2: Different 2-substituted-1 H-benzimidazole synthesis |
Benzimidazoles bearing either electron-donating (entries 5, 7-10, 15, 21, 22, 24 and 25, Table 2) or electron-withdrawing substituents (entries 1-4, 11-14 and 16-20, Table 2) were successfully produced in this benign synthetic procedure. A series of aromatic aldehydes bearing either electron-donating or electron-withdrawing groups on aromatic ring were investigated. There are no clear effect on the yield and reaction time with different substitution group attached on the aromatic ring. However, aldehydes containing strong electron withdrawing groups in the aromatic ring such as 4-nitrobenzaldehyde and 3-nitrobenzaldehyde gave the products with excellent yield (entries 3 and 16, Table 2). In case of aldehydes containing strong electron donating methoxy group in the aromatic ring (entries 15, 21 and 22-25, Table 2) gave the desired benzimidazoles with good yield. Aldehydes containing strong electron with drawing groups in the aromatic ring such as 4-cyano benzaldehyde, 4-bromo benzaldehyde, 4-chloro benzaldehyde and 3-bromo benzaldehyde gave the products with excellent yield and compared to take lower time than 3-nitrobenzaldehyde and 4-nitrobenzaldehyde (entries 14 and 17-20, Table 2) to complete the reaction. In reaction with heteroaromaticaldehyde such as furfural, with o-phenylenediamines and the corresponding product 3z was obtained with excellent yields (entry 26, Table 2). The o-aromaticdiamines with electron rich substituents like methyl and dimethyl (1b and 1c, entries 14-25, Table 2) and electron-deficient substituents like dichloro, acid and benzoyl (1d, 1e and 1f, entries 27, 28 and 29-30, Table 2) also consider and they are consistent to follow the cyclocondensation cum oxidation protocol for the synthesis of benzimidazole derivatives with good yield.
Proposed Mechanism for Synthesis of 2-aryl-1H-benzimidazoles by VO(acac)2-Cu(OTf)2 (3):
The plausible mechanistic pathway of this chemoselective cyclocondensation cum oxidation of aldehydes with o-phenylenediamines to the corresponding 2-substituted benzimidazoles is depicted in Scheme 3. First, Cu(OTf)2 coordinates with aldehydic oxygen atom to increase the electrophilicity of aldehyde group then condensation product imine intermediate (A) was obtained by the reaction with o-aromaticdiamines shown in the mechanism.44 A yellow colour spot appeared in TLC within an hour from start of the reaction in lieu of the substrates. Then cyclisation took place with continuous staring of the reaction mixture to produce another intermediate benzimidazoline (B) and it was readily oxidised to generate desired benzimidazole derivative catalysed by VO(acac)2. VO(acac)2 is a mild oxidant and probably the Vanadium(V)45 as the active species for this oxidation process and its generation is highly accelerated in the presences of O2.46
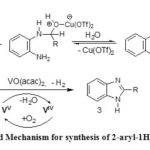 |
Scheme 3: Proposed Mechanism for synthesis of 2-aryl-1H-benzimidazoles |
Experimental
All materials and solvents were purchased from Marck and sigma Aldrich Chemical. VO(acac)2 was synthesized from literature. Perkin-Elmer spectrometer was used to record IR spectra. Bruker 300 MHz instrument was used to record 1H and 13C NMR spectra and used TMS as an internal standard. CDCl3 and DMSO-d6 were used as NMR solvents. Chemical shifts were recorded with respect to internal standard TMS. Melting point of the synthesized compound was detected with HRS Ambula Electrothermal apparatus. Reactions were monitored on glass sheets pre-coated thin layer chromatography with silica gel.
Experimental Procedure
Aromatic aldehyde (2, 1 mmol), ortho-phenylenediamines (1, 1.1 mmol) and CTAB (30 mol%) were mixed and taken in a round bottomed flask. Then added 5 ml water in to it and stirred at room temperature. 20 mol% VO(acac)2 and similar amount Cu(OTf)2 were added in to it. After 16-22 hrs the reaction was completed, examined by silica coated TLC. A brown spot appeared in an iodine chamber. 25 mL ethyl acetate three times was used to extract the residue from reaction mixture. Then 30 mL brine solution was used to wash two times the organic portion. It was dried over Na2SO4 and rotary evaporator was used to concentrate. Crude product was crystallized directly from hot ethanol to prepare pure compound (3a-z and 3a′-d′) in the yield of 71-88%. Thus the reaction of ortho-phenylenediamine (1a, 118 mg, 1.1 mmol) and 4-bromobenzaldehyde (2a, 186 mg, 1 mmol) afforded 2-(4-bromophenyl)-1H-benzimidazole (3a) with 88% yield (240 mg, 0.88 mmol). Benzimidazole derivative 3a and other derivatives were prepared by using the above methods easily. All known compounds (3a-z and 3a′-d′) were characterized by comparing the literature melting point values and also by FT-IR, 1H and 13C NMR data with the authentic known samples.
Selected Characterization Data for Synthesized Compounds
2-(4-bromo-phenyl)-1H-benzimidazole (3a)
Yellow solid, M.P: 296 oC [Lit47c 299-300 oC], FT-IR (KBr, cm-1): 962, 1013, 1114, 1275, 1321, 1428, 1592, 2785, 2856, 2935, 3051, 1H NMR (300 MHz, DMSO-d6): d 7.20 (2H, d), 7.49 (1H, d), 7.64 (1H, d), 7.73 (2H, d), 8.10 (2H, d), 12.97 (1H, s), 13C NMR (75 MHz, DMSO-d6): d 121.9, 123.2, 127.8, 128.7, 131.1, 150.4
Elemental analysis calculated for C13H19N2Br: C: 57.17, H: 3.32, N: 10.26; Found: C: 57.23, H: 3.23, N: 10.37%.
2-(4-methoxyphenyl)-1H-benzo[d]imidazole-5-carboxylic acid (3b′)
Grey solid, M.P: 120 oC [Lit44 118 ºC], FT-IR (KBr, cm-1): 830, 1018, 1184, 1264, 1396, 1507, 1610, 1687, 1901, 2372, 2575, 2932, 3392.
1H NMR (300 MHz, DMSO-d6): d 3.78 (3H, s), 6.96 (2H, d), 7.54 (1H, d), 7.83 (1H, d), 8.09 (2H, d), 8.19 (1H, s). 13C NMR (75 MHz, DMSO-d6): d 54.0, 112.8, 113.0, 115.5, 120.2, 122.6, 123.6, 127.3, 137.1, 140.7, 152.4, 160.1, 167.0, 171.2, Elemental analysis calculated for C15H12N2O3: C: 57.17, H: 3.32, N: 10.26; Found: C: 57.23, H: 3.23, N: 10.37%.
Conclusion
The work reported in this paper is an environmentally benign synthetic protocol for the synthesis of substituted benzimidazoles. Herein I report VO(acac)2-Cu(OTf)2 catalyzed a green chemical approach for the synthesis of above derivatives using various o-phenylenediamines and different aromatic aldehydes in presence of CTAB water system. Different kinds of derivatives of 2-substituted benzimidazoles are easily synthesized in this echo friendly reaction condition with good yield and easy purification process.
Acknowledgement
I am very much grateful to Dr. Hari Sadhan Ghosh, Dept. of Chemistry, Surendranath College for his valuable suggestions. I am also thankful to Professor Dilip K. Maiti, Department of Chemistry, Calcutta University for providing me laboratory facilities.
References
- (a) Arienti, K. L.; Brunmark, A.; Axe, F. U.; Mc Clure, K.; Lee, A.; Blevitt, J.; Neff, D. K.; Huang, L.; Crawford, S.; Pandit, C. R.; Karlsson, L.; Breitenbucher, J. G. J. Med. Chem. 2005, 48, 1873-1885. (b) Shrader, W. D.; Kolesnikov, A.; Burgess-Henry, J.; Rai, R.; Hendrix, J.; Hu, H.; Torkelson, S.; Ton, T.; Young, W. B.; Katz, B. A.; Yu, C.; Tang, J.; Cabuslay, R.; Sanford, E.; Janc, J. W.; Sprengeler, P. A. Bioorg. Med. Chem. Lett. 2006, 16, 1596-1600. (c) Sann, C. L.; Baron, A.; Mann, J.; van den Berg, H.; Gunaratnam, M.; Neidle, S. Org. Biomol. Chem. 2006, 4, 1305-1312.
- Ahmed, A. El R.; Hassan, Y. A.; Mini-Reviews in Medicinal Chemistry. 2013, 13, 399.
- Soni, B.; Ranawat, M. S.; Bhandari, A.; Sharma, R.; International Journal of Drug Research and Technology. 2012, 2, 479.
- Maxwell W. A.; Brody, G.; Appl. Environ. Microbiol. 1971, 21, 944.
- Ayhan-Kılcıgil, G.; Altanlar, N.; Turk. J. Chem. 2006, 30, 223.
- Navarrete-Vázquez, G.; Cedillo, R.; Hernández-Campos, A.; Yépez, L.; Hernández-Luis, F.; Valdez, J.; Morales, R.; Cortés, R.; Hernández, M.; Castillo, R., Bioorg. Med. Chem. Lett. 2001, 11, 187.
- Cheng, J.; Xie, J.; Luo, X.; Bioorg. Med. Chem. Lett. 2005, 15, 267.
- Chen, G.; Liu, Z.; Zhang, Y.; Shan, X.; Jiang, L.; Zhao, Y.; He, W.; Feng, Z.; Yang, S.; Liang G.; ACS Med. Chem. Lett. 2013, 4, 69.
- Terzioğlu, N.; van Rijn, R. M.; Bakker, R. A.; De Esch, I. J. P.; Leurs, R.; Bioorg. Med. Chem. Lett. 2004, 14, 5251.
- (a) Anastas, P. T.; Warner, J. C. Green Chemistry, Theory and Practice; Oxford University Press: Oxford, 1998; (b) Clark, J.; Macquarrie, D. M. A. Handbook of Green Chemistry; Blackwell: Oxford, 2002.
- (a) Grieco, P. A. Organic Synthesis in Water; Blackie Academic and Professional: London, 1998. (b) Vasudevan, V. N.; Rajender, S. V. Green Chem. 2001, 3, 146.
- (a) Philips, M. A. J. Chem. Soc. 1928, 2393. (b) Hein, D. W.; Alheim, R. J.; Leavitt, J. J. J. Am. Chem. Soc. 1957, 79, 427-429. (c) Huang, W.; Scarborough, R. M. Tetrahedron Lett. 1999, 40, 2665-2668. (d) Blatch, A. J.; Chetina, O. V.; Howard, J. A. K.; Patrick, L. G. F.; Smethurst, C. A.; Whiting, A. Org. Biol. Chem. 2006, 4, 3297-3302.
- (a) Wu, Z.; Rea, P.; Wickam, G. Tetrahedron Lett. 2000, 41, 9871-9874. (b) Tumelty, D.; Cao, K.; Holmes, C. P. Org. Lett. 2001, 3, 83-86.
- Renard, G.; Lerner, D. A. New J. Chem. 2007, 31, 1417-1420.
- Dudd, L. M.; Venardou, E. V.; Garcia-Verdugo, E.; Licence, P.; Blake, A. J.; Wilson, C.; Poliakoff, M. Green Chem. 2003, 5, 187-192.
- Preston, P. N.; Chem. Rev. 1974, 74, 279.
- Phillips, M. A.; J. Chem. Soc. 1928, 2393.
- Ören, I.Y.; Yalçın, I.; Şener, E.A.; Uçartürk, N.; Eur. J. Med. Chem. 2003, 39, 291.
- Grimmet, M. R.; Best Synthetic Methods-Key Systems and Functional Groups, Imidazole and Benzimidazole Synthesis, Academic Press: San Diego, 1997.
- Chatterjee, S.; Wolski, J.; J. Indian Chem. Soc. 1966, 43, 660.
- Lu, J.; Yang, B.; Bai, Y.; Synth. Commun. 2002, 32, 3703.
- Wang, Y.; Sarris, K.; Sauer, D. R.; Djuric, S. W.; Tetrahedron Lett. 2006, 47, 4823.
- Cheng, J.; Xiu, N. Y.; Li, X. B.; Luo, X. J.; Synth. Commun. 2005, 35, 2395.
- (a) Srivastava, R.G.; Venkataramani, P. S. Synth. Commun. 1988, 18, 1537-1544. (b) Shen, M.; Driver, T. G. Org. Lett. 2008, 10, 3367-3370. (c) Bahrami, K.; Khodaei, M. M.; Naali, F. J. Org. Chem. 2008, 73, 6835-6837.
- (a) Sun, P.; Hu, Z. J. Heterocycl. Chem. 2006, 43, 773-775. (b) Salehi, P.; Dabiri, M.; Zolfigol, M. A.; Otokesh, S.; Baghbanzadeh, M. Tetrahedron Lett. 2006, 47, 2557-2560.
- (a) Evindar, G.; Batey, R. A. Org. Lett. 2003, 5, 133-136. (b) Brain, C. T.; Steer, J. T. J. Org. Chem. 2003, 68, 6814-6816. (c) Ooyama, Y.; Nakamura T.; Yoshida, K. New J. Chem. 2005, 29, 447-456.
- Gogoi, P.; Konwar, D. Tetrahedron Lett. 2006, 47, 79-82.
- Du, L.-H., Wang, Y.-G. Synthesis 2007, 05, 675-678.
- Maleki, B.; Eshghi, H.;Khojastehnezhad, A.; Tayebee, R.; Ashrafi, S. S.; Kahoo, G. E.; Moeinpour, Farid. RSC Advances. 2015, 5, 64850-64857.
- Maleki, B.; Kahoo, G. E.; Tayebee, R. Organic Preparations and Procedures International. 2015, 47, 461-472.
- Erfaninia, N.; Tayebee, R.; Dusek, M.; Amini, M. M. Applied Organometallic Chemistry. 2018, 32, e4307.
- Tayebee, R.; Pejhan, A. Applied Organometallic Chemistry. 2020, 34, e5350.
- Tayebee, R.; Lee, A. F., Frattini, L.; Rostami, S. Catalysts. 2019, 9, 409-416.
- Tayebee, R.; Abdizadeh, M. F.; Erfaninia, N.; Amiri, A.; Baghayeri, M.;Kakhki, R. M.; Maleki, B.; Esmaili, E. Applied Organometallic Chemistry. 2019, 33, e4959.
- (a) Otto, S.; Engberts, J. B. F. N.; Pure Appl. Chem. 2000, 72, 1365. (b) Ribe, S.; Wipf, P.; Chem. Commun. 2001, 299. (c) Li, C. J.; Chem. Rev. 2005, 105, 3095. (d) Narayan, S.; Muldoon, J.; Finn, M. G.; Fokin, V. V.; Kolb, H. C.; Sharpless, K. B.; Angew. Chem. Int. Ed. 2005, 44, 3275. (e) Li, C. J.; Chen, L.; Chem. Soc. Rev. 2006, 35, 68. (f) Lindström, U. M.; Andersson, F.; Angew. Chem., Int. Ed. 2006, 45, 548. (g) Hailes, H. C.; Org. Process Res. Dev. 2007, 11, 114.
- (a) Leroy, K. J. Org. Chem. 1964, 29, 3630-3632. (b) Schiffmann, R. J. Med. Chem. 2006, 49, 511-522. (c) Hubbard, J. W. Tetrahedron 2007, 63, 7077-7085. (d) Mukhopadhyay, C.; Tapaswi, P. Tetrahedron Lett. 2008, 49, 6237-6240.
- (a) Chakrabarty, M.; Karmakar, S.; Mukherji, A.; Arima, S.; Harigaya, Y. Heterocycles 2006, 68, 967-974. (b) Ma, H.; Wang, Y.; Wang, J. Heterocycles. 2006, 68, 1669-1673. (c) Ma, H.; Han, X.; Wang, Y.; Wang, J. Heterocycles 2007, 71, 1821-1825
- Shen, M.; Driver, T. G. Org. Lett. 2008, 10, 3367-3370.
- Du, L.-H.; Wang, Y.-G. Synthesis. 2007, 5, 675.
- (a) Eren, B.; Bekdemir. Y. Quim. Nova. 2014, 37, 643-647. (b) Cohen, V.I. Journal of Heterocyclic Chemistry. 1979, 16, 13-16.
- Songnian, L.; Lihu, Y. Tetrahedron Lett. 2005, 46, 4315.
- Sondhi, S. M.; Singh, N.; Kumar, A.; Lozach, O.; Meijer, L. Bioorg. Med. Chem. 2006, 14, 3758.
- Savall, B. M. Tetrahedron Lett. 2008, 47, 6667-6669.
- Chari, M. A.; Mosaa, Z.-A.; Shobha, D.; Malayalama, S. International Journal of Organic Chemistry. 2013, 3, 243-250
- Sutradhar, M.; Mukherjee, G.; Drew, M. G. B.; Ghosh, S. Inorg. Chem. 2006, 45, 5150-5161.
- Maiti, D. K.; Halder, S.; Pandit, P.; Chatterjee, N.; Joarder, D. D.; Pramanik, N.; Saima,Y.; Patra, A.; Maiti. P. K. J. Org. Chem. 2009, 74, 8086-8097.

This work is licensed under a Creative Commons Attribution 4.0 International License.










